Abstract
Background: Accumulating evidence suggests that vitamin D is involved in the development of type 2 diabetes (T2D).
Objective: Our objective was to examine the relation between vitamin D status and incidence of T2D.
Design: We used a subsample of 1972 Framingham Offspring Study participants to develop a regression model to predict plasma 25-hydroxyvitamin D [25(OH)D] concentrations from age, sex, body mass index, month of blood sampling, total vitamin D intake, smoking status, and total energy intake. Using this model, we calculated the predicted 25(OH)D score for each nondiabetic participant at the cohort's fifth examination to assess the association between the predicted 25(OH)D score and incidence of T2D by using Cox proportional hazards models.
Results: A total of 133 T2D cases were identified over a 7-y average follow-up. In comparison with individuals in the lowest tertile of the predicted 25(OH)D score at baseline, those in the highest tertile had a 40% lower incidence of T2D after adjustment for age, sex, waist circumference, parental history of T2D, hypertension, low HDL cholesterol, elevated triglycerides, impaired fasting glucose, and Dietary Guidelines for Americans Adherence Index score (hazard ratio: 0.60; 95% CI: 0.37, 0.97; P for trend = 0.03).
Conclusions: Our findings suggest that higher vitamin D status is associated with decreased risk of T2D. Maintaining optimal 25(OH)D status may be a strategy to prevent the development of T2D.
INTRODUCTION
Vitamin D, a fat-soluble vitamin, plays a critical role in regulating plasma calcium concentration through effects on intestinal absorption and bone metabolism (1). Vitamin D is formed in the skin from 7-dehydrocholesterol during exposure to solar ultraviolet B (UVB) radiation (2). Although vitamin D can be derived from the diet, only a few foods naturally contain vitamin D, such as oily fish. However, in the United States and Canada, certain foods are fortified with vitamin D, such as milk, some cereals, bread, and orange juice (3). The circulating concentration of 25-hydroxyvitamin D [25(OH)D] is the common biomarker used to assess vitamin D status. Studies have shown that, in addition to vitamin D intake, 25(OH)D concentration is associated with age, sex, adiposity, latitude of residence, skin pigmentation, and season of blood sampling (4–7).
The relation between vitamin D status and bone health is well established, but there is accumulating evidence that vitamin D might have other functions, including involvement in the development of type 2 diabetes (T2D) (8). There are few prospective studies on the association between vitamin D status and risk of T2D, and evidence from these studies is limited by the use of vitamin D intake or a single measurement of 25(OH)D concentration as a surrogate for usual vitamin D status (9–11). Because vitamin D can be synthesized in human skin under sunlight exposure and thereby has seasonal variation, the use of vitamin D intake cannot capture an individual's overall vitamin D status, whereas a single measurement of 25(OH)D does not take into account the within-person variation across different seasons.
In this study we sought to test the hypothesis that vitamin D status is inversely associated with subsequent risk of T2D using a predicted 25(OH)D score, which was derived from known potential determinants of plasma 25(OH)D concentration and aimed to remove the seasonal effect on vitamin D status by holding season constant. This method of developing a predicted 25(OH)D score has been previously used to relate vitamin D status to cancer risk (12).
SUBJECTS AND METHODS
Study population
The Framingham Study was initiated in 1948 as a longitudinal, population-based study of cardiovascular disease. In 1971, 5135 offspring of original participants of the study and their spouses were recruited to participate in the Framingham Offspring Study. Members of the Framingham Offspring Study have returned, on average, every 4 y for a physical examination, questionnaires, laboratory tests, and assessment of cardiovascular and other risk factors (13). A total of 3799 cohort members participated in the fifth examination cycle (1991–1995). Valid food-frequency questionnaires (FFQs) were available for 3418 participants.
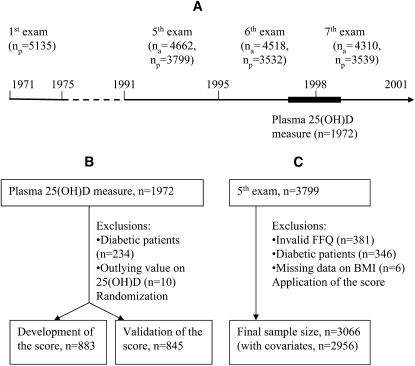
Participants were excluded from these analysis on the basis of previous diagnosis of T2D (n = 208), fasting glucose concentration ≥7.0 mmol/L or 2-h postchallenge glucose concentration ≥11.1 mmol/L (n = 134), or current use of insulin or oral hypoglycemic medication (n = 4). We also excluded those individuals without body mass index (BMI) data (n = 6). After exclusions, 3066 participants (1402 men and 1664 women) remained eligible for the present investigation. The participants were essentially all white. Participants were followed across the sixth (1996–1998) and seventh (1998–2001) offspring cohort examinations for a mean of 7 y for the development of incident T2D (Figure 1). The Institutional Review Boards for Human Research at Boston University and Tufts Medical Center approved the study protocols.
FIGURE 1.
A: Timeline for the Framingham Offspring cohort examinations. B: Participants included in the development and validation of predicted 25-hydroxyvitamin D [25(OH)D] score. C: Participants in the diabetic follow-up study. na = number alive at exam, np= number of participants at exam; FFQ, food-frequency questionnaire.
Dietary assessment
Usual dietary intakes for the previous year were assessed at each examination by using the 126-item semiquantitative Harvard FFQ (version 88GP) (14). The questionnaires were mailed to participants before the examination, and the participants were asked to bring the completed questionnaire with them to their scheduled appointment. The FFQ consists of a list of foods with a standardized serving size and a selection of 9 frequency categories ranging from never or <1 serving/mo to >6 servings/d. Nutrient intakes were calculated at the Harvard Channing Laboratory by multiplying the frequency of consumption of each unit of food from the FFQ by the nutrient content of the specified portion. Separate questions about use of vitamin and mineral supplements and type of breakfast cereal most commonly consumed were also included in the FFQ. In addition to assessing specific vitamin D supplement use, we used information on vitamin D content from multivitamins. FFQs with reported energy intakes <2.51 MJ/d (600 kcal/d) for men and women, >16.74 MJ/d (4000 kcal/d) for women, or >17.57 MJ/d (4200 kcal/d) for men or with >12 food items left blank were considered invalid. The FFQ has been shown to be valid for both nutrients and foods (14, 15). The FFQ has also been validated specifically for vitamin D intake in relation to plasma 25(OH)D (16), and the key dietary sources of vitamin D (including milk and dark fish) correlated well between the FFQ and diet records (17).
Lifestyle variables
Height, weight, and waist circumference were measured while the subjects were standing. BMI was calculated as weight in kilograms divided by the square of height in meters (kg/m2). A physical activity score was calculated from the number of self-reported hours spent doing specific activities that were categorized and weighted according to oxygen consumption required to perform them (MET-h/d) (18). Additional measurements included age, sex, current smoking (none, <20 cigarettes/d, ≥20 cigarettes/d), and alcohol consumption.
Laboratory measurements at the fifth examination cycle
Blood samples were drawn after the participants had fasted for ≥8 h for measurement of fasting glucose and lipid profile at the fifth examination cycle. A 75-g oral-glucose-tolerance test was administered according to World Health Organization standards to measure 2-h postchallenge glucose (19). Plasma glucose concentrations were measured in fresh specimens with a hexokinase reagent kit (A-gent glucose test; Abbott Laboratories Inc, South Pasadena, CA); the intraassay CV was <3%. Lipid measures included enzymatic measurement of triglyceride (20) and the measurement of the HDL-cholesterol fraction after precipitation of LDL and VLDL cholesterol with dextran sulfan magnesium (21). Blood pressure was measured twice after the participants sat for ≥5 min. Impaired fasting glucose was defined as fasting glucose ≥5.6 mmol/L; low concentration of HDL cholesterol was defined as <1.04 mmol/L in men or <1.29 mmol/L in women; elevated concentration of triglyceride was defined as ≥1.70 mmol/L; and hypertension was defined as blood pressure ≥130/85 mm Hg or current treatment of hypertension (22).
Ascertainment of incident T2D
Incident T2D was defined as a fasting plasma glucose concentration ≥7.0 mmol/L or use of insulin or oral hypoglycemic drug therapy at the sixth or seventh study examinations.
Statistical analysis
Development and validation of predicted 25(OH)D score
Plasma 25(OH)D concentration was measured by radioimmunoassay (Diasorin, Stillwater, MN) in a subsample of 1972 participants in the Framingham Offspring Study during the period of July 1997 to May 1999, which included the latter half of the sixth and the beginning of the seventh examination cycles. Using data from the subsample with plasma 25(OH)D measurements, we developed a multiple linear regression model to predict plasma 25(OH)D concentration [predicted 25(OH)D score] from potential determinants of vitamin D status. Because we planned to apply this predicted score to a nondiabetic sample, we excluded subjects with T2D (n = 234) from the 1972 participants for the development of the multiple linear regression model. We also excluded 10 subjects with influential outlying values (plasma 25(OH)D concentrations >112.5 nmol/L). After these exclusions, a total of 1728 subjects were available for the development of the predicted 25(OH)D score. We randomly selected ≈50% of the 1728 subjects (n = 883) for the development of the predicted 25(OH)D score. We used the remaining subjects (n = 845) in the subsample with 25(OH)D measurements to validate the predictive multiple linear model (Figure 1). Using multiple linear regression modeling, we related the measured 25(OH)D concentration to the following potential determinants of vitamin D status: age, sex, BMI, month of blood sampling, total vitamin D intake, physical activity score, smoking status, total energy intake, and alcohol consumption. To meet the assumption of a linear relation between our independent and dependent variables in our regression model, we transformed BMI using the reciprocal of BMI and total vitamin D intake using the square root of total vitamin D intake. We also examined all possible 2-way interactions between sex, BMI (reciprocal), and other potential determinants of vitamin D status. A potential determinant of vitamin D status was excluded from our final model if it was not a significant predictor of the plasma 25(OH)D concentrations in this subsample, and little additional variance in plasma 25(OH)D concentrations could be explained by adding it back into the model.
Application of predicted 25(OH)D score
We applied the multiple linear regression model to the entire cohort at the fifth examination cycle to calculate a predicted 25(OH)D score for each participant without T2D (n = 3066). Although 25(OH)D concentration is a good measure of contemporaneous vitamin D status, it may not be a good reflection of a person's typical status throughout the year because of the strong seasonal effect of sunlight exposure on 25(OH)D concentration. Therefore, to remove the seasonal effect on the predicted 25(OH)D score, we treated month of the blood sample as a confounder and calculated the score assuming that all blood samples were collected in the month of May. We chose the month of May because the 25(OH)D concentrations from samples drawn in May for the subsample of individuals used in the development and validation of the score were similar to the average 25(OH)D concentration observed for these individuals (Table 1).
TABLE 1.
Characteristics of study participants included in the development, validation, and application of the predicted 25-hydroxyvitamin D [25(OH)D] score1
| Characteristics | Sample for development of the score | Sample for validation of the score | Sample for application of the score |
| n | 805 | 769 | 3066 |
| Age (y) | 59.3 ± 9.32 | 59.5 ± 9.4 | 54.2 ± 9.7 |
| Women (%) | 55.5 | 51.8 | 54.3 |
| BMI (kg/m2) | 27.9 ± 5.2 | 27.8 ± 5.1 | 27.1 ± 4.7 |
| Smoking status (%) | |||
| Nonsmoker | 90.4 | 91.7 | 80.9 |
| <20 cigarettes/d | 7.1 | 6.0 | 6.8 |
| ≥20 cigarettes/d | 2.5 | 2.3 | 12.3 |
| Energy intake (kJ/d) | 7629 ± 2780 | 7750 ± 2794 | 7769 ± 2589 |
| Total vitamin D intake (μg/d) | 10.9 ± 7.9 | 10.5 ± 7.9 | 7.4 ± 6.0 |
| Dietary vitamin D intake (μg/d) | 5.6 ± 3.3 | 5.7 ± 3.4 | 4.9 ± 3.2 |
| Supplement vitamin D intake (μg/d) | 5.3 ± 6.9 | 4.8 ± 7.0 | 2.6 ± 4.9 |
| Plasma 25(OH)D (nmol/L) | 49.1 ± 18.3 | 49.3 ± 17.4 | — |
| Plasma 25(OH)D by month of examination (nmol/L)3 | |||
| January | 46.2 ± 17.9 (8) | 44.0 ± 14.5 (11) | — |
| February | 42.8 ± 14.5 (11) | 42.0 ± 14.9 (9) | — |
| March | 44.3 ± 16.4 (13) | 40.4 ± 15.1 (12) | — |
| April | 43.0 ± 20.2 (12) | 43.0 ± 17.2 (9) | — |
| May | 51.3 ± 22.3 (7) | 50.1 ± 20.1 (6) | — |
| June | 64.3 ± 23.3 (2) | 63.7 ± 15.9 (2) | — |
| July | 67.3 ± 16.7 (2) | 57.3 ± 10.4 (3) | — |
| August | 64.2 ± 18.2 (7) | 62.3 ± 16.5 (7) | — |
| September | 50.0 ± 14.7 (5) | 55.9 ± 16.6 (8) | — |
| October | 53.8 ± 15.0 (12) | 53.1 ± 16.4 (12) | — |
| November | 51.3 ± 16.8 (9) | 54.0 ± 16.4 (11) | — |
| December | 46.1 ± 15.5 (12) | 48.3 ± 16.5 (10) | — |
| Predicted 25(OH)D score (nmol/L) | 49.1 ± 9.3 | 49.1 ± 9.1 | 47.8 ± 6.8 |
No values were significantly different between the 25(OH)D score development and validation subsamples on the basis of t tests and chi-square tests. No statistical tests were performed to compare the development or validation subsamples to the application sample because of the lack of independence between these samples.
Mean ± SD (all such values).
Percentages are shown in parentheses and denote the relative frequency of the participants examined in each month.
Subjects were followed from the fifth to the sixth and seventh examinations. The date of diagnosis was the examination visit date at which a new case of T2D was identified. We used hazard ratios (HRs) from Cox proportional hazards models to estimate relative risks and 95% CIs for incident T2D across the tertile category of the predicted 25(OH)D score. P values for trend were calculated by treating the median of the predicted 25(OH)D score in each tertile category as a continuous independent variable in the hazards models. The following risk factors, which have been previously shown to predict incidence of T2D in the Framingham Offspring Cohort (23), were included as covariates in the regression models: age, sex, parental history of T2D, hypertension, low concentration of HDL cholesterol, elevated concentration of triglycerides, and impaired fasting glucose. Given that BMI was included in the prediction model, we included waist circumference as a covariate in place of BMI. We also included the Dietary Guideline for Americans Adherence Index (DGAI) as a covariate to determine whether any association with vitamin D was a consequence of overall diet quality (24). Because of missing information for some covariates, our sample size for these analyses was 2956.
Proportional hazards assumptions were tested by modeling interaction terms between each of the variables and person-years of follow-up. None of the interaction terms was statistically significant (ie, P > 0.05), indicating that the HR for the variables was reasonably constant over time. On the basis of observations from an earlier study (8), we examined whether calcium intake from foods and supplements was an effect modifier of the association between the predicted 25(OH)D score and incident T2D. The interaction term was not statistically significant. Statistical analysis was performed using Statistical Analysis Systems statistical software package, version 9.1 (SAS Institute, Cary, NC).
RESULTS
Participant characteristics
Characteristics of the study participants used in the development, validation, and application of the 25(OH)D score are shown in Table 1. The development and validation samples were comparable on all characteristics considered; none of the characteristics considered were significantly different between these 2 groups. Although no formal statistical comparison was made because of lack of independence between samples, participants in the application sample were younger than participants in the subsamples used to develop and validate the score, as would be expected based on the difference between the dates of the study examinations, and they also appeared to have lower vitamin D intakes and were more likely to be heavy smokers.
Development and validation of predicted 25(OH)D score
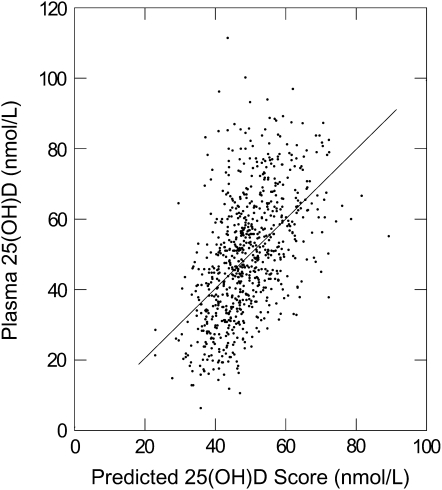
Our final predictive model included age, sex, reciprocal of the BMI, total energy intake, square root of total vitamin D intake, smoking status, and month of blood sampling as independent predictors of the 25(OH)D score. Physical activity and alcohol consumption were not significant independent predictors of 25(OH)D concentrations (P > 0.05), and together they explained only 0.03% of the additional variance in plasma 25(OH)D concentration. Therefore, we excluded them from the final model. No interaction was found between sex, BMI, and other variables in the model. After excluding those with missing values for the predictor variables, 805 subjects were left in the final model, and the model r2 was 25.75% (Table 2). We then calculated the predicted 25(OH)D score for 769 of the 845 remaining participants with actual plasma 25(OH)D measurements and complete data on the predictor variables. In this validation subset, the correlation coefficient between the predicted 25(OH)D score (mean: 49.1 nmol/L) and the actual plasma 25(OH)D measurement (mean: 49.3 nmol/L) was 0.51 (P < 0.001). The slope relating actual to predicted 25(OH)D in the validation sample was 0.99 (SE = 0.06, P < 0.001). The regression constant was 0.77 (SE = 2.79, P = 0.80) (Figure 2).
TABLE 2.
Predictors of 25-hydroxyvitamin D score (n = 805)
| Predictors | Estimate | P |
| Intercept | 10.57 | 0.127 |
| Age (y) | −0.02 | 0.790 |
| Sex (male vs female) | 3.61 | 0.003 |
| BMI (kg/m2)1 | 395.23 | <0.001 |
| Total energy intake (kJ/d) | −0.0007 | <0.001 |
| Smoking | ||
| Nonsmoker | 11.45 | 0.002 |
| <20 cigarettes/d | 11.00 | 0.009 |
| ≥20 cigarettes/d | Reference | |
| Total vitamin D intake (μg/d)1 | 4.85 | <0.001 |
| Month of blood sampling | ||
| January | 1.67 | 0.52 |
| February | −4.05 | 0.09 |
| March | −1.88 | 0.41 |
| April | −3.31 | 0.16 |
| May | 5.50 | 0.05 |
| June | 19.13 | <0.001 |
| July | 19.22 | <0.001 |
| August | 19.04 | <0.001 |
| September | 4.12 | 0.16 |
| October | 6.51 | 0.005 |
| November | 4.99 | 0.04 |
| December | Reference |
BMI was entered into the regression model as its reciprocal and total vitamin D intake as its square root to meet the linearity assumptions.
FIGURE 2.
Relation between actual and predicted 25-hydroxyvitamin D [25(OH)D] concentrations in the validation sample (n = 769).
Application of predicted 25(OH)D score
Using the multiple linear regression model, we calculated the predicted 25(OH)D score for 3066 participants without T2D at the fifth examination cycle. The mean of the predicted score was 47.8 and ranged from 18.8 to 68.8 nmol/L. Among the 2956 participants with all covariate information, we identified 133 incident T2D cases from the fifth to seventh examination cycle. The association between the tertile categories of the predicted 25(OH)D score and the risk of T2D is shown in Table 3. After adjustment for risk factors for T2D, including waist circumference, diet quality (as assessed by the DGAI). parental history of T2D, hypertension, low concentrations of HDL cholesterol, elevated concentrations of triglyceride, and impaired fasting glucose, individuals in the middle and highest tertile had a 30% and a 40% reduction in risk of development of T2D over the 7-y follow-up compared with individuals in the lowest tertile of the predicted 25(OH)D score, respectively (P for trend = 0.03). When we further adjusted for other potential confounders that were also used to calculate the 25(OH)D predicted score, including age and sex, the association persisted.
TABLE 3.
Median 25-hydroxyvitamin D [25(OH)D] score, number of cases and person-years, and hazard ratios (95% CIs) for type 2 diabetes across tertile (T) categories of predicted 25(OH)D score
| Predicted 25(OH)D score tertile categories |
||||
| T1 | T2 | T3 | P for trend | |
| Predicted 25(OH)D score (nmol/L)1 | 42.3 (18.8–45.6) | 47.9 (45.7–50.4) | 54.3 (50.5–68.8) | |
| n | 987 | 983 | 986 | |
| No. of cases | 70 | 36 | 27 | |
| Person-years | 6359 | 6441 | 6430 | |
| Model 123 | 1.00 | 0.72 (0.48, 1.10) | 0.61 (0.38, 0.97) | 0.03 |
| Model 224 | 1.00 | 0.71 (0.46, 1.11) | 0.60 (0.37, 0.97) | 0.03 |
Values are medians; ranges in parentheses.
Values are hazard ratios; 95% CIs in parentheses.
Adjusted for waist circumference, parental history of diabetes, hypertension (≥130/85 mm Hg), low concentrations of HDL cholesterol (<1.04 mmol/L in men or <1.29 mmol/L in women), elevated concentrations of triglycerides (≥1.70 mmol/L), impaired fasting glucose (≥5.6 mmol/L), and Dietary Guidelines for Americans Adherence Index (n = 2956).
Adjusted as in model 1 and further adjusted for age and sex (n = 2956).
DISCUSSION
We used a subsample of participants with plasma 25(OH)D measurements to develop a model using known and suspected determinants of vitamin D status to predict 25(OH)D status in the larger cohort. This approach has been successfully used in other cohorts to examine the relation between predicted 25(OH)D concentrations and cancer risk (12). Consistent with the literature, we found that plasma 25(OH)D concentration was positively associated with total vitamin D intake and inversely associated with BMI (4, 25, 26). Men had higher plasma 25(OH)D than women, and smokers had lower 25(OH)D than nonsmokers (27, 28). Unlike previous studies (12, 26), physical activity, presumably a proxy of outdoor exposure to sunlight, was not a significant predictor for plasma 25(OH)D concentrations. Physical activity may not relate to UVB exposure in our sample because of the latitude of residence and the fact that only 11% of our 25(OH)D measurements were taken during the summer months (June, July, or August) when sunlight exposure is most effective for 25(OH)D production in the northeastern United States (29); we also did not measure factors such as clothing, use of sunscreen, and duration spent outdoors that affect the actual sunlight exposure during outdoor physical activity. In addition, we could not obtain information on winter residence for our study participants at the time of their study examinations.
By applying this model to the larger cohort, we found that the predicted 25(OH)D score was inversely associated the incidence of T2D after adjustment for established risk factors for T2D and insulin resistance, including waist circumference, parental history of T2D, hypertension, low HDL-cholesterol concentrations, elevated triglyceride concentrations, impaired fasting glucose, diet quality, age, and sex. The appropriateness of adjustments for age and sex, both components of the predicted score, is a potential limitation of the current study. Failure to adjust for these variables might result in residual confounding of the relation between the 25(OH)D score and T2D risk because age and sex are also established risk factors for T2D. However, such adjustment may also result in overadjustment, because these 2 variables are constituents of the predicted 25(OH)D score. Consequently, we presented the association without and with this adjustment. We found that the inverse association between predicted 25(OH)D and risk of T2D was not appreciably attenuated after further adjustment for age and sex. Therefore, the observed association between predicted 25(OH)D and T2D risk does not appear to be a consequence of confounding by age and sex.
The absolute values of the predicted 25(OH)D score should also be used cautiously because previous research has shown large differences in 25(OH)D concentrations using different assays or even using the same assay in different laboratories (30). However, we have only used our predicted score to rank participants and not to classify participants’ vitamin D adequacy.
The fact that we were only able to predict 26% of the variance in 25(OH)D concentrations using our score in the validation sample limits our ability to accurately classify participants’ vitamin D status. This is likely to result in a reduction in the strength of any true association between vitamin D status and diabetes incidence. However, the fact that our regression slope and constant relating the actual and predicted scores in the validation sample were very close to 1 and 0, respectively, suggests that the error in our estimates is largely random error and that, on average, we were able to classify vitamin D status.
Finally, the predicted 25(OH)D score, which was developed for a population from a northern latitude (Massachusetts), may not be generalized to other populations in which sunlight exposure could be the main contributor of vitamin D status. However, this limitation strengthens the internal validity of our finding because the residual confounding by sunlight exposure, which was not measured in our sample, will be reduced. As we mentioned before, only 11% of our blood samples used to measure plasma 25(OH)D concentrations were collected in the summer months when sunlight exposure is most effective for vitamin D production at higher latitudes.
Our findings are consistent with the few studies that have examined the prospective relation between vitamin D intake or serum 25(OH)D concentration and the risk of T2D. Pittas et al (9) found that women with a daily total calcium and total vitamin D intake >1200 mg and 20 μg, respectively, had a 33% lower risk of developing T2D than those with a daily calcium and vitamin D intake <600 mg and 10 μg. This study was limited by the use of dietary vitamin D intake data as the exposure variable. A study from Finland found a significant inverse association between serum 25(OH)D concentrations and subsequent risk of T2D, but the association was attenuated and not statistically significant in the multivariate analysis (11). In a second Finnish study, Knekt et al (31) found that men in the highest serum 25(OH)D quartile had a >70% lower risk of T2D than those in the lowest quartile after adjustment for potential confounders. However, no association was found among women. Although the use of 25(OH)D concentrations in blood has the advantage of accounting for all sources of variability in vitamin D status, these latter studies were limited by the use of a single measurement, which may not adequately reflect an individual's typical status because 25(OH)D concentrations can fluctuate dramatically from one season to another and consequently attenuate the observed association between 25(OH)D and T2D risk. In contrast, our predicted 25(OH)D score is not subject to seasonal variation because we adjusted the score to a single month (May) to remove the seasonal variation when we calculated the individual scores.
A recent report from the Women's Health Initiative randomized trial reported no effect of calcium plus vitamin D supplementation (1000 mg calcium and 10 μg vitamin D daily) on incident T2D among postmenopausal women (11). The null result from this trial cannot exclude the possibility that vitamin D might play roles in the development of T2D because the dose of vitamin D in this trial was relatively low and higher doses may be required to modify the risk of T2D. Indeed, in another trial originally designed for bone-related outcomes, daily supplementation with calcium (500 mg) and vitamin D (17.5 μg) attenuated increases in glycemia and insulin resistance in adults with impaired fasting glucose (32).
Vitamin D has been related to both pancreatic β cell dysfunction (33–39) and insulin resistance (39, 40), the 2 main defects that determine the development of T2D. However, the underlying mechanisms by which vitamin D may affect β cell function and insulin resistance remain unclear. One plausible mechanism is that poor vitamin D status induces higher parathyroid hormone (PTH) concentrations. The elevated PTH in turn inhibits insulin synthesis and secretion in β cells (41) and induces insulin resistance in target cells (42, 43) by regulating the intracellular free calcium concentrations. Studies have showed that increased PTH concentrations were associated with impaired glucose tolerance and decreased insulin sensitivity (44, 45). Another potential mechanism is stimulation of the expression of the insulin receptor by 1,25-dihydroxyvitamin D, thereby enhancing insulin responsiveness for glucose transport (46, 47).
Our findings advance the hypothesis that vitamin D status is inversely associated with T2D risk. To further our understanding of this relation, future studies should examine the relation of long-term estimates of vitamin D status and T2D risk, identify the optimal vitamin D intake or 25(OH)D concentration associated with low risk of T2D, and elucidate the underlying mechanisms of the relation between vitamin D and T2D.
Acknowledgments
We are grateful to the Framingham Study participants and staff for data collection. We thank Gail Rogers for data management.
The authors’ responsibilities were as follows—EL: study design, data preparation, statistical analyses, interpretation of results, and drafting of the manuscript; PFJ, JBM, AGP, CDE, and NMM: study design, interpretation of the results, and editing of the manuscript; and SLB: provision of plasma 25(OH)D data and editing of the manuscript. All authors approved the manuscript. The authors stated that they had no conflicts of interest.
REFERENCES
- 1.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 2004;80:1689S–96S [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81 [DOI] [PubMed] [Google Scholar]
- 3.Calvo MS, Whiting SJ, Barton CN. Vitamin D fortification in the United States and Canada: current status and data needs. Am J Clin Nutr 2004;80:1710S–6S [DOI] [PubMed] [Google Scholar]
- 4.Jacques PF, Felson DT, Tucker KL, et al. Plasma 25-hydroxyvitamin D and its determinants in an elderly population sample. Am J Clin Nutr 1997;66:929–36 [DOI] [PubMed] [Google Scholar]
- 5.Bolland MJ, Grey AB, Ames RW, et al. Determinants of vitamin D status in older men living in a subtropical climate. Osteoporos Int 2006;17:1742–8 [DOI] [PubMed] [Google Scholar]
- 6.Macdonald HM, Mavroeidi A, Barr RJ, Black AJ, Fraser WD, Reid DM. Vitamin D status in postmenopausal women living at higher latitudes in the UK in relation to bone health, overweight, sunlight exposure and dietary vitamin D. Bone 2008;42:996–1003 [DOI] [PubMed] [Google Scholar]
- 7.Shea MK, Benjamin EJ, Dupuis J, et al. Genetic and non-genetic correlates of vitamins K and D. Eur J Clin Nutr 2007;63:458–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab 2007;92:2017–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pittas AG, Dawson-Hughes B, Li T, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care 2006;29:650–6 [DOI] [PubMed] [Google Scholar]
- 10.de Boer IH, Tinker LF, Connelly S, et al. Calcium plus vitamin D supplementation and the risk of incident diabetes mellitus in the Womenaposs Health Initiative. Diabetes Care 2008;31:701–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattila C, Knekt P, Mannisto S, et al. Serum 25-hydroxyvitamin D concentration and subsequent risk of type 2 diabetes. Diabetes Care 2007;30:2569–70 [DOI] [PubMed] [Google Scholar]
- 12.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst 2006;98:451–9 [DOI] [PubMed] [Google Scholar]
- 13.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study: design and preliminary data. Prev Med 1975;4:518–25 [DOI] [PubMed] [Google Scholar]
- 14.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26 [DOI] [PubMed] [Google Scholar]
- 15.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 16.Jacques PF, Sulsky SI, Sadowski JA, Phillips JC, Rush D, Willett WC. Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am J Clin Nutr 1993;57:182–9 [DOI] [PubMed] [Google Scholar]
- 17.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–6 [DOI] [PubMed] [Google Scholar]
- 18.Kannel WB, Sorlie P. Some health benefits of physical activity. The Framingham Study. Arch Intern Med 1979;139:857–61 [PubMed] [Google Scholar]
- 19.WHO Expert Committee on Diabetes Mellitus Second report. World Health Organ Tech Rep Ser 1980;646:9–12 [PubMed] [Google Scholar]
- 20.McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin Chim Acta 1987;166:1–8 [DOI] [PubMed] [Google Scholar]
- 21.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem 1982;28:1379–88 [PubMed] [Google Scholar]
- 22.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–52 [DOI] [PubMed] [Google Scholar]
- 23.Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D'Agostino RB., Sr Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med 2007;167:1068–74 [DOI] [PubMed] [Google Scholar]
- 24.Fogli-Cawley JJ, Dwyer JT, Saltzman E, McCullough ML, Troy LM, Jacques PF. The 2005 Dietary Guidelines for Americans Adherence Index: development and application. J Nutr 2006;136:2908–15 [DOI] [PubMed] [Google Scholar]
- 25.Bischof MG, Heinze G, Vierhapper H. Vitamin D status and its relation to age and body mass index. Horm Res 2006;66:211–5 [DOI] [PubMed] [Google Scholar]
- 26.van Dam RM, Snijder MB, Dekker JM, et al. Potentially modifiable determinants of vitamin D status in an older population in the Netherlands: the Hoorn Study. Am J Clin Nutr 2007;85:755–61 [DOI] [PubMed] [Google Scholar]
- 27.Brot C, Jorgensen NR, Sorensen OH. The influence of smoking on vitamin D status and calcium metabolism. Eur J Clin Nutr 1999;53:920–6 [DOI] [PubMed] [Google Scholar]
- 28.Szulc P, Garnero P, Claustrat B, Marchand F, Duboeuf F, Delmas PD. Increased bone resorption in moderate smokers with low body weight: the Minos study. J Clin Endocrinol Metab 2002;87:666–74 [DOI] [PubMed] [Google Scholar]
- 29.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 2004;80(6 suppl):1678S–88S [DOI] [PubMed] [Google Scholar]
- 30.Lips P, Chapuy MC, Dawson-Hughes B, Pols HA, Holick MF. An international comparison of serum 25-hydroxyvitamin D measurements. Osteoporos Int 1999;9:394–7 [DOI] [PubMed] [Google Scholar]
- 31.Knekt P, Laaksonen M, Mattila C, et al. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology 2008;19:666–71 [DOI] [PubMed] [Google Scholar]
- 32.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care 2007;30:980–6 [DOI] [PubMed] [Google Scholar]
- 33.Cade C, Norman AW. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology 1986;119:84–90 [DOI] [PubMed] [Google Scholar]
- 34.Chertow BS, Sivitz WI, Baranetsky NG, Clark SA, Waite A, Deluca HF. Cellular mechanisms of insulin release: the effects of vitamin D deficiency and repletion on rat insulin secretion. Endocrinology 1983;113:1511–8 [DOI] [PubMed] [Google Scholar]
- 35.Clark SA, Stumpf WE, Sar M. Effect of 1,25 dihydroxyvitamin D3 on insulin secretion. Diabetes 1981;30:382–6 [DOI] [PubMed] [Google Scholar]
- 36.Labriji-Mestaghanmi H, Billaudel B, Garnier PE, Malaisse WJ, Sutter BC. Vitamin D and pancreatic islet function. I. Time course for changes in insulin secretion and content during vitamin D deprivation and repletion. J Endocrinol Invest 1988;11:577–84 [DOI] [PubMed] [Google Scholar]
- 37.Gedik O, Akalin S. Effects of vitamin D deficiency and repletion on insulin and glucagon secretion in man. Diabetologia 1986;29:142–5 [DOI] [PubMed] [Google Scholar]
- 38.Boucher BJ, Mannan N, Noonan K, Hales CN, Evans SJ. Glucose intolerance and impairment of insulin secretion in relation to vitamin D deficiency in East London Asians. Diabetologia 1995;38:1239–45 [DOI] [PubMed] [Google Scholar]
- 39.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr 2004;79:820–5 [DOI] [PubMed] [Google Scholar]
- 40.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004;27:2813–8 [DOI] [PubMed] [Google Scholar]
- 41.Lee S, Clark SA, Gill RK, Christakos S. 1,25-Dihydroxyvitamin D3 and pancreatic beta-cell function: vitamin D receptors, gene expression, and insulin secretion. Endocrinology 1994;134:1602–10 [DOI] [PubMed] [Google Scholar]
- 42.Naveh-Many T, Silver J. Vitamin D and the parathyroid. 2nd ed.London, United Kingdom: Elsevier, 2004 [Google Scholar]
- 43.Christakos S, Gabrielides C, Rhoten WB. Vitamin D-dependent calcium binding proteins: chemistry, distribution, functional considerations, and molecular biology. Endocr Rev 1989;10:3–26 [DOI] [PubMed] [Google Scholar]
- 44.Chiu KC, Chuang LM, Lee NP, et al. Insulin sensitivity is inversely correlated with plasma intact parathyroid hormone level. Metabolism 2000;49:1501–5 [DOI] [PubMed] [Google Scholar]
- 45.Reis JP, von Muhlen D, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care 2007;30:1549–55 [DOI] [PubMed] [Google Scholar]
- 46.Maestro B, Davila N, Carranza MC, Calle C. Identification of a vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol 2003;84:223–30 [DOI] [PubMed] [Google Scholar]
- 47.Maestro B, Campion J, Davila N, Calle C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J 2000;47:383–91 [DOI] [PubMed] [Google Scholar]