Abstract
Background: Chronic inflammation and oxidative stress are common risk factors for atherosclerosis. Zinc is an essential micronutrient that can function as an antiinflammatory and antioxidative agent, and as such, it may have atheroprotective properties.
Objective: We hypothesized that zinc down-regulates the production of atherosclerosis-related cytokines/molecules in humans.
Design: To examine these effects, we conducted a randomized, double-blinded, placebo trial of zinc supplementation in elderly subjects. We recruited 40 healthy elderly subjects (aged 56–83 y) and randomly assigned them to 2 groups. One group was given an oral dose of 45 mg zinc/d as a gluconate for 6 mo. The other group was given a placebo. Cell culture models were conducted to study the mechanism of zinc as an atheroprotective agent.
Results: After 6 mo of supplementation, the intake of zinc, compared with intake of placebo, increased the concentrations of plasma zinc and decreased the concentrations of plasma high-sensitivity C-reactive protein (hsCRP), interleukin (IL)-6, macrophage chemoattractant protein 1 (MCP-1), vascular cell adhesion molecule 1 (VCAM-1), secretory phospholipase A2, and malondialdehyde and hydroxyalkenals (MDA+HAE) in elderly subjects. Regression analysis showed that changes in concentrations of plasma zinc were inversely associated with changes in concentrations of plasma hsCRP, MCP-1, VCAM-1, and MDA+HAE after 6 mo of supplementation. In cell culture studies, we showed that zinc decreased the generation of tumor necrosis factor-α, IL-1β, VCAM-1, and MDA+HAE and the activation of nuclear transcription factor κB and increased antiinflammatory proteins A20 and peroxisome proliferator–activated receptor-α in human monocytic leukemia THP-1 cells and human aortic endothelial cells compared with zinc-deficient cells.
Conclusion: These findings suggest that zinc may have a protective effect in atherosclerosis because of its antiinflammatory and antioxidant functions.
INTRODUCTION
Atherosclerosis is a slowly progressive chronic inflammatory disease characterized by focal arterial lesions that ultimately occlude the entire blood vessel and may lead to angina, myocardial infarction, or sudden death (1). Inflammation, oxidative stress, and/or endothelial dysfunction caused by classic risk factors such as age, sex, smoking, hypertension, diabetes, and obesity are involved in the development and progression of atherosclerosis (1–3). The strategy for protection against atherosclerosis has been a subject of intense research. The prevention and early management of atherosclerosis are still lacking because of the lack of a clear understanding of the causes of this process. The important role of zinc has been recognized in various biochemical and physiologic functions (4). Nutritional zinc deficiency is common in developing countries, and conditioned deficiency of zinc is present in many chronic diseases such as rheumatoid arthritis, diabetes, and cancers, which are associated with chronic inflammation and oxidative stress (4, 5). Studies (5–10) showed that zinc deficiency increases the concentration of inflammatory cytokines and oxidative stress and induces apoptosis and endothelial cell dysfunction.
Elderly subjects are susceptible to the development and progression of atherosclerosis. It is estimated that 30–40% of elderly subjects in the Detroit, Michigan, area have mild to moderate zinc deficiency (7). We previously observed that healthy elderly subjects had increased concentrations of plasma lipid peroxidation byproducts and endothelial cell adhesion molecules compared with concentrations in younger adults (5). Zinc was proposed to have an atheroprotective function because of its antiinflammatory, antioxidant, and other properties (9). We hypothesized that zinc supplementation would down-regulate the inflammatory biomarkers for atherosclerosis in humans. In this study, we examined 1) the effect of zinc supplementation on concentrations of plasma C-reactive protein (CRP), interleukin (IL)-6, macrophage chemoattractant protein 1 (MCP-1), vascular endothelial cell adhesion molecules, and oxidative stress markers in elderly subjects and 2) A20, peroxisome proliferator–activated receptor α (PPAR-α), and nuclear transcription factor κB (NF-κB) activation in human vascular endothelial cell lines and monocytic cell lines.
SUBJECTS AND METHODS
Human subject study
The protocol of this study was approved by the Human Investigation Committee (Wayne State University, Detroit, MI). Forty healthy, elderly subjects of both sexes (aged 56–83 y) and inclusive of all ethnic groups from St Patrick's Senior Citizen Center (Detroit, MI) were recruited to participate in a randomized, placebo-controlled trial of the efficacy of zinc supplementation on CRP, oxidative stress markers, inflammatory cytokines, and endothelial cell adhesion molecules in elderly subjects. A complete chart review of each elderly subject was done by the research nurse to determine his or her potential eligibility for the study. Exclusion criteria were described previously (7). We excluded those individuals who were self-supplementing zinc, noncompetent, or who did not understand and could not sign the informed consent. All of our elderly subjects were mobile without any other type of aid. The human subject study was conducted from September 2004 to December 2006.
Elderly subjects were randomly assigned to a zinc or placebo group in pairs. Briefly, subjects were assigned to the zinc or placebo group in pairs by the technician's blinded choice of 1 of 2 identical bottles labeled as the study drug (ie, not labeled as zinc or placebo): one bottle contained zinc pills, and the other bottle contained placebo pills. The order of assignment (ie, zinc or placebo) within pairs was random, and the persons caring for the subjects were blinded to the assignment. The zinc-supplemented group received 45 mg zinc for 6 mo. One capsule of zinc gluconate (15 mg elemental zinc)/d was taken 1 h before breakfast, and 2 capsules were taken before going to bed (≥2 h after dinner or last meal). The placebo group received the placebo capsules in the same manner as the zinc-supplemented group. Both zinc and placebo capsules were supplied by Labcatal Laboratories (Paris, France).
The Recommended Dietary Allowance for zinc (intake of 15 mg zinc/d for men and 12 mg zinc/d women) was established in 1974 for the first time. A daily intake of >50 mg zinc/d for 12 wk may induce copper deficiency (11, 12). We used 45 mg zinc/d as supplementation in elderly individuals for 1 y, and at this concentration, the copper status remained normal. This dose of zinc was effective in correcting immune dysfunction (7). Also, oxidative stress markers and the generation of inflammatory cytokines were decreased at this level of supplementation (7). The only adverse effect of oral zinc supplementation in amounts >50 mg zinc/d is copper deficiency, which can be easily corrected by supplementation of 2 mg copper/d. In a study conducted by the National Eye Institute (National Institutes of Health, Bethesda, MD), 80 mg zinc/d as an oxide was used to prevent blindness in patients with age-related macular degeneration, and for 10 y no substantial side effects were observed; in the study, 2 mg copper/d was also supplemented to prevent the deficiency of copper (13, 14).
In the current study, blood was drawn from each elderly subject before and after 6 mo of supplementation to evaluate the concentrations of plasma zinc, CRP, oxidative stress markers, inflammatory cytokines, and endothelial cell adhesion molecules. Plasma zinc was assayed by flameless atomic absorption spectrophotometry (7). Plasma lipid peroxidation byproducts, malondialdehyde and hydroxyalkenals (MDA+HAE), were measured by using a thiobarbituric acid lipid peroxidation assay kit (Oxford Biochemical Research, Oxford, MI); plasma high-sensitivity CRP (hsCRP) was assessed by enzyme-linked immunosorbent assay (Alpco Diagnostics, Windham, NH); plasma antioxidant power was measured by the ABEL antioxidant test kit (Knight Scientific, Plymouth, United Kingdom); plasma IL-6, MCP-1, secretory phospholipase A2 (sPLA), soluble vascular cell adhesion molecule (sVCAM)-1, soluble intercellular adhesion molecule (sICAM)-1, and soluble E-selectin (sE-selectin) were assayed by enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN) at baseline and at the end of 6 mo of supplementation. Inflammation generates oxidative stress by increasing reactive oxygen species (ROS), which results in the oxidation of biological molecules including LDL. Oxidized LDL activates NF-κB and up-regulates its downstream target genes, such as inflammatory cytokines, CRP, adhesion molecules, inducible nitric oxide synthase, cyclooxygenase 2, fibrinogen, and tissue factor. These cytokines and molecules attract more blood cells and platelets for coagulation, which initiate the development of atherosclerosis. Increasing evidence shows that CRP; inflammatory cytokines such as IL-6 and MCP-1; adhesion molecules such as VCAM, ICAM, and E-selectin; sPLA; and lipid peroxidation byproducts are associated with the development and progression of atherosclerosis (2, 15–22). Patients with atherosclerosis or cardiovascular disease risk factors have increased concentrations of these inflammatory cytokines or molecules. Therefore, we selected these markers to measure the effect of zinc supplementation in our study.
Cell culture study
To support the evidence that zinc may have an atheroprotective function, we used human aortic endothelial cells (HAECs), the human promyelocytic leukemia cell line HL-60, which differentiates into monocytes after stimulation, and the human monocytic leukemia cell line THP-1 to measure oxidative stress markers, inflammatory cytokines, A20, PPAR-α, and NF-κB activation under zinc-deficient and zinc-sufficient conditions. Zinc-deficient medium was prepared by removing zinc from fetal bovine serum (FBS) used with culture medium as described previously (23). We developed zinc-deficient medium to simulate in vivo zinc deficiency in humans so that differences between zinc-deficient and zinc-sufficient cells could be characterized in the cultured cells (23). For each cell line, we monitored the development of deficiency on a daily basis, such as cell growth and/or cellular zinc content, and established the optimum number of days required for culture in zinc-deficient medium to obtain zinc-deficient viable cells. Zinc-deficient (1 μmol Zn/L) and zinc-sufficient (15 μmol Zn/L, physiologic condition) media differed only in their zinc content.
HL-60 (CCL-240; American Type Culture Collection, Manassas, VA) cells were maintained in 10% normal FBS (without chelation)-RPMI-1640 medium, as described previously (23). THP-1 (TIB-202; American Type Culture Collection) cells were maintained in 10% normal FBS (without chelation)-RPMI-1640 medium supplemented with 10 mmol/L HEPES at 37°C. HAECs (Cascade Biologics, Portland, OR) were maintained in 2% normal FBS (without chelation)-medium 200 supplemented with 10 μg heparin/mL, 10 ng human epidermal growth factor/mL, 3 ng basic fibroblast growth factor/mL, and 1 μg hydrocortisone/mL (Cascade Biologics) at 37°C.
A total of 2 × 105 cells/mL were separated into 2 groups. One set of cells was transferred to zinc-deficient medium, and the other set was transferred to zinc-sufficient medium. The cells in either zinc-deficient or zinc-sufficient medium were incubated for 10 d (HL-60 cells), 8 d (THP-1 cells), and 6 d (HAECs) to develop zinc-deficient and zinc-sufficient conditions. The viability of those cells incubated in either zinc-deficient or zinc-sufficient medium was 83–86% for HAECs and 89–94% for HL-60 and THP-1 cells. There was no significant difference between zinc-deficient and zinc-sufficient cells (P > 0.05). After 24 h of stimulation with oxidized LDL (oxLDL, 50 μg/mL; Biomedical Technologies, Stoughton, MA), zinc-deficient and zinc-sufficient cells were harvested for Western blot analysis or NF-κB activation by electrophoretic mobility shift assay (EMSA) and reporter-gene luciferase assays. The media were collected for the assessment of lipid peroxidation byproducts and generation of inflammatory cytokines as described. The details of Western blot analysis, EMSA, gene transfection, and reporter-gene luciferase assays were described previously (24). All cell culture studies were conducted 3 times.
Statistical analyses
Demographic differences between the zinc and placebo groups were examined by t test (for age) and chi-square analyses (for sex and ethnicity). For other variables, t tests were used to compare group differences when variables were normally distributed. If distributions were not normal, group differences were compared by using a nonparametric Wilcoxon rank-sum test. The changes in laboratory markers before and after intervention within the zinc group and within the placebo group were compared by using a paired t test. Group comparisons between the changes in laboratory values (post- and presupplementation) between zinc and placebo groups were examined by a t test (2-tailed). P < 0.05 was considered to be statistically significant. All statistical analyses were conducted with JMP software (version 5.0; SAS Institute Inc, Cary, NC) on a Macintosh Powerbook G4 computer (Apple Computers, Cupertino, CA).
RESULTS
Elderly subject study
The demographic characteristics of participants in this study are shown in Table 1. Participants' mean (±SD) ages in placebo and zinc-supplementation groups were comparable (67 ± 7 y compared with 65 ± 7 y, respectively; P = 0.355; n = 40). The numbers of men and women in each group were similar. Ethnicity was also similar between both groups. After 6 mo of supplementation, there was no significant difference in plasma zinc concentrations before and after supplementation in the placebo group (P = 0.134; Table 2). However, zinc-supplemented elderly subjects had a significant increase in plasma zinc concentrations after 6 mo of supplementation (ΔP < 0.0001; Table 2). Δ>P was represented as P for differences (Pre compared with Post) between placebo and the zinc group (Student's t test).
TABLE 1.
Demographic characteristics of study participants
| Variable | Placebo group (n = 20) | Zinc group (n = 20) |
| Age (y) | 67 ± 71 | 65 ± 7 |
| Sex (n) | ||
| Men | 5 | 6 |
| Women | 15 | 14 |
| Ethnicity (n) | ||
| Black | 6 | 2 |
| White | 12 | 16 |
| Other | 2 | 2 |
| Use of medications (n) | 11 | 9 |
| Types of medications (n) | ||
| Antihypertensive2 | 7 | 5 |
| Gemfibrozil | 2 | 0 |
| Propafenone | 1 | 0 |
| Metformin | 1 | 1 |
| Glipizide | 1 | 1 |
| Glyburide/metformin | 1 | 0 |
| Theophyline | 1 | 0 |
| Levothyroxine | 1 | 1 |
| Omeprazole | 1 | 0 |
| Esomeprazole | 1 | 0 |
| Furosemide | 1 | 0 |
| Hydrochlorothiazide | 0 | 1 |
| Nabumetone | 1 | 0 |
Mean ± SD (all such values).
Includes terazosin, valsartan, metoprolol, amlodipine, triamterene/hydrochlorothiazide, nifedipine, lisinopril, and enalapril.
TABLE 2.
Changes in plasma zinc, oxidative stress markers, and inflammatory cytokines/molecules in zinc-supplemented (Zn supp) elderly subjects1
| Group | n | Pre | Post | P2 | Change3 | ΔP4 |
| Zinc (μmol/L) | ||||||
| Placebo | 20 | 92.0 ± 3.85 | 90.8 ± 5.0 | 0.134 | −1.17 ± 4.59 | <0.0001 |
| Zn supp | 20 | 91.9 ± 7.4 | 101.5 ± 9.2 | 0.00006 | 9.52 ± 8.88 | |
| MDA+HAE (μmol/L) | ||||||
| Placebo | 14 | 1.66 ± 0.37 | 1.68 ± 0.35 | 0.357 | 0.019 ± 0.186 | 0.002 |
| Zn supp | 14 | 1.59 ± 0.40 | 1.29 ± 0.26 | 0.0011 | −0.30 ± 0.30 | |
| Antioxidant power (U/mL) | ||||||
| Placebo | 20 | 6.6 ± 2.2 | 6.5 ± 1.7 | 0.417 | −0.11 ± 2.32 | 0.0258 |
| Zn supp | 20 | 6.0 ± 1.6 | 7.6 ± 1.9 | 0.0001 | 1.56 ± 2.23 | |
| hsCRP (μg/L) | ||||||
| Placebo | 20 | 2.14 ± 1.71 | 2.49 ± 1.94 | 0.149 | 0.36 ± 1.45 | 0.0298 |
| Zn supp | 20 | 2.46 ± 1.91 | 1.90 ± 1.51 | 0.015 | −0.55 ± 1.05 | |
| IL-6 (pg/mL) | ||||||
| Placebo | 20 | 5.42 ± 3.47 | 7.15 ± 4.56 | 0.026 | 1.74 ± 3.76 | 0.0031 |
| Zn supp | 20 | 8.34 ± 7.13 | 5.44 ± 4.85 | 0.013 | −2.94 ± 5.46 | |
| MCP-1 (pg/mL) | ||||||
| Placebo | 20 | 496.5 ± 154.0 | 570.4 ± 205.4 | 0.011 | 74.1 ± 133.3 | 0.0113 |
| Zn supp | 20 | 531.5 ± 142.7 | 506.8 ± 131.0 | 0.136 | −24.25 ± 97.2 | |
| sPLA (U/mL) | ||||||
| Placebo | 20 | 76.0 ± 25.8 | 100.6 ± 28.8 | 0.001 | 24.6 ± 30.9 | 0.006 |
| Zn supp | 20 | 73.3 ± 34.6 | 70.0 ± 32.2 | 0.314 | −3.23 ± 29.5 | |
| sVCAM-1 (ng/mL) | ||||||
| Placebo | 20 | 2102.9 ± 415.1 | 2297.6 ± 358.2 | 0.0024 | 194.7 ± 273.1 | <0.0001 |
| Zn supp | 20 | 2208.0 ± 345.6 | 2035.0 ± 267.8 | 0.001 | −171.5 ± 218.5 | |
| sICAM-1 (ng/mL) | ||||||
| Placebo | 20 | 321.1 ± 89.1 | 302.7 ± 105.6 | 0.307 | −18.40 ± 160.4 | 0.830 |
| Zn supp | 20 | 301.3 ± 68.9 | 292.4 ± 75.6 | 0.365 | −8.90 ± 113.6 | |
| sE-selectin (ng/mL) | ||||||
| Placebo | 20 | 54.7 ± 8.3 | 57.5 ± 7.3 | 0.023 | 2.73 ± 5.70 | 0.0068 |
| Zn supp | 20 | 57.2 ± 9.7 | 51.6 ± 8.6 | 0.023 | −5.65 ± 11.7 |
Pre, baseline; Post, after 6 mo; MDA+HAE, malondialdehyde and hydroxyalkenals; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; MCP-1, macrophage chemoattractant protein 1; sPLA, secretory phospholipase A2; sVCAM-1, soluble vascular cell adhesion molecule 1; sICAM-1, soluble intercellular adhesion molecule 1; sE-selectin, soluble E-selectin. There were no significant differences in plasma zinc and all biomarkers between placebo and Zn supp groups before supplementation (Pre).
Pre compared with Post values (paired t test).
Post minus Pre values.
P for differences (Pre compared with Post) between placebo and zinc groups (t test).
Mean ± SD (all such values).
Plasma antioxidant powers (represented by ascorbate equivalent units, U/mL) presupplementation compared with postsupplementation with the placebo were not significantly different (P = 0.417; Table 2). Plasma mean (±SD) antioxidant powers before supplementation compared with those aftersupplementation with zinc were 6.0 ± 1.6 and 7.6 ± 1.9 U/mL, respectively (n = 20; P = 0.0001). Concentrations of plasma MDA+HAE before supplementation compared with those after supplementation with the placebo were not significantly different (n = 14; P =0.357; Table 2). Mean (±SD) concentrations of plasma MDA+HAE before supplementation compared with those after supplementation with zinc were 1.59 ± 0.40 and 1.29 ± 0.26 μmol/L, respectively (n = 14; P = 0.0011). We observed a significant change (post- minus presupplementation) in lipid peroxidation products and a significant increase in antioxidant power in the zinc-supplemented group (ΔP < 0.05; Table 2).
Concentrations of plasma hsCRP before supplementation compared with those after supplementation with the placebo were not significantly different (P = 0.419; Table 2). Mean (±SD) concentrations of plasma hsCRP before supplementation compared with those after supplementation with zinc were 2.46 ± 1.91 and 1.90 ± 1.51 μmol/L (P = 0.015), respectively. Compared with the placebo group, zinc-supplemented elderly subjects showed a significant change (post- minus presupplementation) in plasma hsCRP concentrations after 6 mo of supplementation (ΔP = 0.015; Table 2). Plasma IL-6 concentrations before supplementation compared with those after supplementation with the placebo significantly increased (P = 0.026). Plasma IL-6 concentrations before supplementation compared with those after supplementation with zinc decreased significantly (P = 0.013). The changes (post- minus presupplementation) in plasma IL-6 concentrations in zinc-supplemented elderly subjects after 6 mo of supplementation was significant compared with those in subjects in the placebo group (ΔP = 0.0016; Table 2). Similarly, zinc-supplemented elderly subjects had a significant change in concentrations of plasma MCP-1, sPLA, sVCAM-1, and sE-selectin after 6 mo of supplementation compared with those in the placebo group (ΔP < 0.05; Table 2). However, there was no significant change in plasma sICAM-1 concentration between zinc-supplemented and placebo groups after 6 mo of supplementation (n = 20; ΔP = 0.415; Table 2). This may have been due to the high SD, which indicated large variability in the test and small numbers of cases in our study.
Gemfibrozil has antiinflammatory effect, which might affect the results of the above markers in our study. If we removed the elderly subjects who were taking gemfibrozil from our data pool, specifically 2 subjects from the placebo group, the differences between the zinc and placebo groups with respect to markers for oxidative stress, inflammation, and adhesion molecules remained highly significant (data not shown).
We also performed correlation analyses of the differences (post- minus presupplementation) in plasma zinc concentrations and other laboratory markers in elderly subjects. The results show that the change in plasma zinc concentrations in elderly subjects was inversely correlated with the change in concentrations of plasma hsCRP (r = −0.33; n = 40; P = 0.039), VCAM-1 (r = −0.45; n = 40; P = 0.0036), MCP-1 (r = −0.40; n = 40; P = 0.01), and MDA+HAE (r = −0.41; n = 28; P = 0.029) after 6 mo of supplementation.
Cell culture study
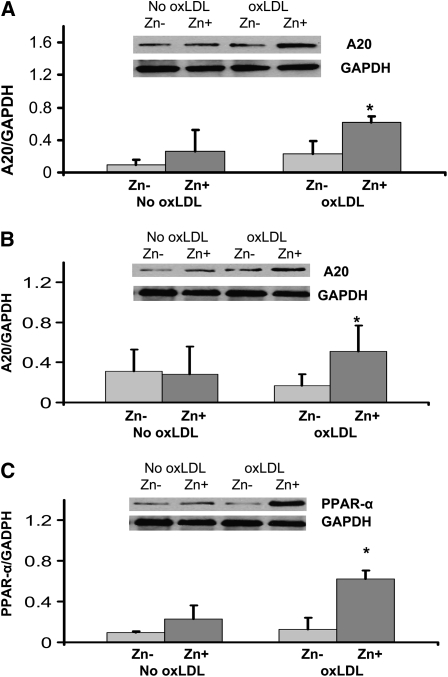
The effect of zinc on inflammatory cytokines and oxidative stress markers in HL-60 and THP-1 cells and HAECs is shown in Table 3. The data indicate that zinc significantly decreased the generation of tumor necrosis factor-α (TNF-α), IL-1β, VCAM-1, and MDA+HAE in HL-60 and THP-1 cells and HAECs after incubation with oxLDL for 24 h compared with zinc-deficient cells (P < 0.05). Zinc increased A20 and PPAR-α proteins in oxLDL-stimulated THP-1 cells and HAECs compared with zinc-deficient cells (Figure 1; P < 0.05; n = 3).
TABLE 3.
Effect of zinc on tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), vascular cell adhesion molecule 1 (VCAM-1), and malondialdehyde and hydroxyalkenals (MDA+HAE) in HL-60 and THP-1 cells and human aortic endothelial cells (HAECs)1
| No stimulation |
oxLDL stimulation |
|||||
| Zn− | Zn+ | P2 | Zn− | Zn+ | P2 | |
| HL-60 cells | ||||||
| TNF-α (pg/mL) | 26.5 ± 25.4 | 12.1 ± 12.1 | 0.21 | 317.2 ± 119.7 | 152.7 ± 96.4 | 0.007 |
| IL-1β (pg/mL) | 1.4 ± 1.2 | 0.8 ± 0.7 | 0.52 | 3.9 ± 1.4 | 1.3 ± 0.5 | 0.042 |
| VCAM-1 (pg/mL) | 18.3 ± 4.7 | 14.2 ± 1.7 | 0.073 | 69.9 ± 2.8 | 32.1 ± 4.3 | 0.006 |
| MDA+HAE (μmol/L) | 2.6 ± 0.7 | 1.5 ± 0.6 | 0.001 | 5.6 ± 1.4 | 2.3 ± 0.5 | 0.046 |
| THP-1 cells | ||||||
| TNF-α (pg/mL) | 32.2 ± 15.1 | 23.8 ± 12.0 | 0.022 | 181.2 ± 13.9 | 121.0 ± 17.9 | 0.027 |
| IL-1β (pg/mL) | 1.5 ± 0.1 | 0.9 ± 0.4 | 0.027 | 4.4 ± 0.7 | 1.7 ± 0.9 | 0.004 |
| MDA+HAE (μmol/L) | 1.4 ± 0.6 | 1.0 ± 0.5 | 0.013 | 4.5 ± 0.6 | 2.0 ± 0.7 | 0.004 |
| HAECs | ||||||
| TNF-α (pg/mL) | 8.0 ± 6.6 | 4.2 ± 5.0 | 0.06 | 22.6 ± 2.3 | 13.6 ± 2.1 | 0.034 |
| IL-1β (pg/mL) | 5.8 ± 5.7 | 2.8 ± 2.7 | 0.11 | 13.1 ± 4.8 | 6.2 ± 1.8 | 0.028 |
| VCAM-1 (ng/mL) | 3.5 ± 1.5 | 4.5 ± 2.1 | 0.247 | 23.8 ± 4.7 | 13.8 ± 2.1 | 0.016 |
| MDA+HAE (μmol/L) | 1.16 ± 0.36 | 1.02 ± 0.20 | 0.18 | 3.33 ± 1.02 | 1.45 ± 0.77 | 0.028 |
All values are means ± SDs. Zn−, zinc deficient; Zn+, zinc sufficient; oxLDL, oxidized LDL.
For differences between Zn− (1 μmol Zn/L) and Zn+ (15 μmol Zn/L) cell groups (Student's t test; n = 3).
FIGURE 1.
Effect of zinc on A20 and peroxisome proliferator–activated receptor α (PPAR-α) in THP-1 cells (A) and human aortic endothelial cells (HAECs) (B and C) after oxidized LDL (oxLDL) stimulation. The cells were incubated either in zinc-deficient (Zn−, 1 μmol/L) or zinc-sufficient (Zn+, 15 μmol/L) medium for 8 d (for THP-1) and for 6 d (for HAECs), followed by 24 h of stimulation with 50 μg oxLDL/mL. A20 and PPAR-α proteins were measured by Western blot analysis. *P < 0.05 for Zn− compared with Zn+ (n = 3). GADPH, glyceraldehyde 3-phosphate dehydrogenase.
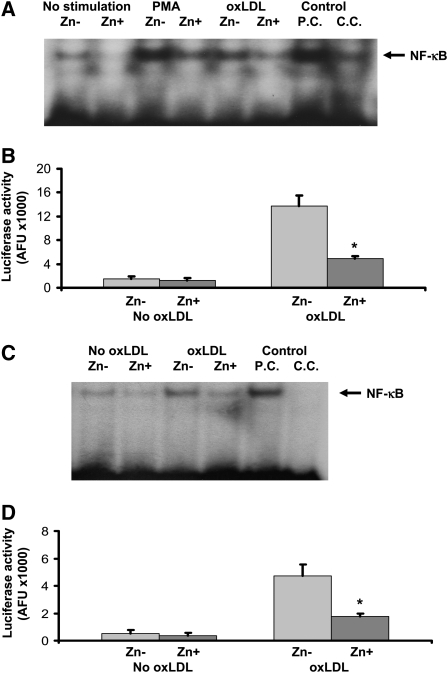
The effect of zinc on NF-κB activation in THP-1 cells and HAECs is shown in Figure 2. There was no significant difference in NF-κB activation by either NF-κB-driven luciferase reporter gene assay or EMSA assay between the nonstimulated THP-1 cells and HAECs incubated in zinc-deficient and zinc-sufficient media (P > 0.05). However, after 24 h of oxLDL stimulation, zinc-sufficient THP-1 cells and HAECs showed a significant decrease in NF-κB activation compared with zinc-deficient cells (P < 0.05).
FIGURE 2.
A and B: Effect of zinc on nuclear transcription factor κB (NF-κB) activation in THP-1 cells after oxidized LDL (oxLDL) or phorbol myristate acetate (PMA) stimulation. Zinc-deficient (Zn−) THP-1 cells and zinc-sufficient (Zn+) THP-1 cells were used for the measurement of NF-κB activation by electrophoretic mobility shift assay (EMSA; A) and luciferase reporter gene assay (B). C and D: Effect of zinc on NF-κB activation in human aortic endothelial cells (HAECs) after oxLDL stimulation. Zn− HAECs and Zn+ HAECs were used for the measurement of NF-κB activation by EMSA (C) and luciferase reporter gene assay (D). *P < 0.05 for Zn− compared with Zn+ (n = 3). AFU, arbitrary fluorescent unit/β-galactosidase U/100 μg protein; P.C., positive control; C.C., competition control.
DISCUSSION
The change in plasma zinc concentrations was small (9 .5 μM) in the supplemented group. However, some of the changes in inflammatory markers were large. These data suggest that the changes in inflammatory markers were more sensitive to zinc status than changes of plasma zinc concentrations. Monocytes/macrophages that produce these inflammatory markers are sensitive to zinc status, and thus changes in inflammatory markers may be a better indicator of zinc status than plasma zinc concentrations. It is well known that plasma zinc concentration is not a sensitive indicator for zinc status in humans. The intracellular zinc concentration, such as the lymphocyte zinc concentration, is a better indicator of zinc status as we previously reported (4, 6).
A high CRP concentration is a risk factor that is independent of traditional risk factors, such as total cholesterol, HDL cholesterol, age, smoking, body mass index, and hypertension (2). CRP is a widely used and easily measured marker for the presence of atherosclerosis itself and for its clinical course, complication, and prognosis (2). In prospective studies, healthy men and women with increased baseline concentrations of CRP were at risk of future coronary artery disease (2). In the current study, we observed that the supplementation of 45 mg zinc/d decreased plasma hsCRP concentrations in elderly subjects compared with elderly subjects who took a placebo. To our knowledge, this is the first documentation to show the down-regulation of plasma CRP concentrations by zinc supplementation in human subjects.
The increased production of ROS and the activation of redox-dependent signaling cascades are critically involved in atherosclerosis (25). ROS itself can initiate NF-κB–mediated transcriptional activation of inflammatory genes (26), thereby potentially acting as independent triggers of atherosclerosis. Zinc deficiency increases oxidative stress, and zinc supplementation decreases oxidative stress in cell culture models, animal models, and humans (5, 7, 10, 27). In this study, we confirmed that zinc supplementation decreased oxidative stress in elderly subjects and human vascular endothelial and monocytic cells. Thus, decreased oxidative stress by zinc may decrease the modification of LDL oxidation and exhibit an atheroprotective effect.
In this study, we also showed that the concentrations of IL-6, sVCAM, and sE-selectin changed bidirectionally negatively in the placebo group and positively in the zinc-supplemented group. Some biomarkers such as MCP-1 and sPLA2 were significantly changed in the placebo group but not in the zinc group. CRP and MDA+HAE concentrations and the antioxidant power were significantly changed in the zinc group but not in the placebo group. However, the mechanism by which these changes accrued is not clear.
NF-κB is one of the major immune response transcription factors involved in the initiation and development of atherosclerosis (26). Zinc plays an important role in NF-κB activation. However, the regulation of NF-κB activation by zinc appears to be cell specific. We and other investigators (26, 28) reported that zinc is required for NF-κB DNA binding in purified or recombinant NF-κB p50 protein or T helper cell lines. However, additional studies (10, 29) showed that zinc decreases lipopolysaccharide-, ROS-, or TNF-α–induced NF-κB activation in endothelial cells and cancer cells. Prasad et al (5) also reported that normal healthy volunteers who were supplemented with 45 mg zinc/d, compared with placebo-treated normal healthy volunteers, had a significant decrease in TNF-α and IL-1β messenger RNAs and TNF-α-induced NF-κB DNA binding in isolated peripheral mononuclear cells, and zinc up-regulated the expression of A20 in HL-60 cells. In the current study, we observed that zinc decreased oxLDL-induced generation of TNF-α, IL-1β, and VCAM-1, oxidative stress markers, and activation of NF-κB and increased A20 and PPAR-α proteins in human monocytic and vascular endothelial cells. Although the molecular mechanism of down-regulation of NF-κB activation by zinc has not been defined, zinc was proposed to inhibit NF-κB activation via A20 (5), a zinc finger–transactivating factor that plays an important role in reducing IL-1β– and TNF-α–induced NF-κB activation (30). A20 was originally reported to protect cells from TNF-α–induced cytotoxicity by inhibiting the activation of NF-κB, which leads to decreased IL-1β and TNF-α signaling in endothelial cells (30). It was reported that A20 inhibits NF-κB signaling by TNF-α and IL-1β via TNF receptor–associated factor pathways in endothelial cells (28–31).
The PPAR-α and -γ of nuclear receptors, the mediators for lipoprotein metabolism, inflammation, and glucose homeostasis, were shown to play an important protective role in the development and progression of atherosclerosis (32). The mechanisms by which zinc has atheroprotective function may be due to its antiinflammatory effect by down-regulation of atherosclerosis-related NF-κB activation via negative cross-talk in the nuclear DNA binding level (33). The activation of PPAR-α and -γ and the down-regulation of inflammatory cytokines and endothelial cell adhesion molecules in endothelial cells were reported to be zinc dependent (34). In the current study, zinc-sufficient HAEC cells showed an increase in PPAR-α concentrations compared with zinc-deficient HAEC cells, which suggested that zinc increases the expression of PPAR-α protein, which may contribute to the down-regulation of inflammatory cytokines and adhesion molecules. Thus, the decrease in atherosclerosis-related inflammatory cytokines/molecules including endothelial cell adhesion molecules and oxidative stress biomarkers may be due to the down-regulation of NF-κB activation by zinc via A20 and PPAR signaling pathways.
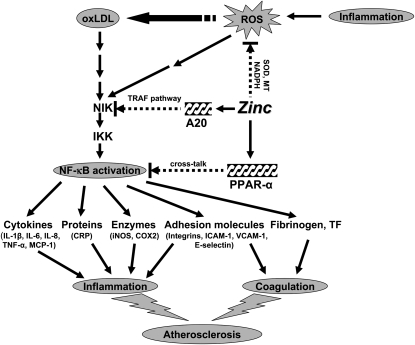
Our concept of the mechanism by which zinc may be beneficially regulating various pathways involved in the development of atherosclerosis is shown in Figure 3. Inflammation generates oxidative stress by increasing ROS, resulting in the oxidation of biological molecules including LDL. Oxidized LDL activates the NF-κB inducible kinase/IκB kinase/NF-κB signaling pathway and up-regulates its downstream target genes such as inflammatory cytokines, CRP, adhesion molecules, inducible nitric oxide synthase, cyclooxygenase 2, fibrinogen, and tissue factor. These cytokines and molecules attract more blood cells and platelets for coagulation, which initiate the development of atherosclerosis. In this study, we showed that zinc supplementation increased the plasma concentrations of antioxidant power and decreased the plasma concentrations of inflammatory cytokines/molecules and lipid peroxidation biomarkers in elderly subjects. Zinc decreased NF-κB activation and its target genes, such as TNF-α, IL-1β, and VCAM, and increased the expression of A20 and PPAR-α, the 2 zinc finger proteins with antiinflammatory properties, in HL-60 and THP-1 cells and HAECs after oxLDL stimulation. Thus, zinc decreased the expression of these cytokines and molecules by inhibition of NF-κB activation via A20 and PPAR-α pathways.
FIGURE 3.
Signaling pathway for zinc prevention of atherosclerosis in monocytes/macrophages and vascular endothelial cells: a proposed hypothesis. Reactive oxygen species (ROS) induced by many stimuli modif LDL into oxidized LDL (oxLDL) in macrophages and vascular endothelial cells. oxLDL or ROS can activate the apoptotic pathway via activation of proapoptotic enzymes and the nuclear transcription factor κB (NF-κB) pathway via NF-κB inducible kinase (NIK) activation, which eventually results in the development and progression of atherosclerosis. Zinc might have an atheroprotective function by the following mechanisms: 1) inhibition of ROS generation via metallothionein (MT), superoxide dismutase (SOD), and NADPH, and 2) down-regulation of atherosclerotic cytokines/molecules such as inflammatory cytokines, adhesion molecules, inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX2), fibrinogen, and tissue factor (TF) through inhibition of NF-κB activation by A20-mediating tumor necrosis factor (TNF)–receptor associated factor (TRAF) signaling and peroxisome proliferator–activated receptor α (PPAR-α)–mediating crosstalk signaling. The black arrows indicate up-regulation; arrows with a broken line indicate down-regulation or the inhibitory pathway. IKK, IκB kinase; IL, interleukin; MCP-1, macrophage chemoattractant protein 1; CRP, C-reactive protein; ICAM-1, intercell adhesion molecule 1; VCAM-1, vascular cell adhesion molecule 1.
In summary, this study showed that zinc increased antioxidant power and decreased CRP, inflammatory cytokines, adhesion molecules, and oxidative stress markers in elderly subjects after 6 mo of supplementation, and zinc decreased the generation of TNF-α, IL-1β, VCAM-1, and MDA+HAE, as well as NF-κB activation and increased A20 and PPAR-α in THP-1 cells and HAECs, which suggest that zinc may have an atheroprotective effect because of its antiinflammatory and antioxidant function.
Acknowledgments
The authors' responsibilities were as follows—BB: designed and conducted experiments; ASP: designed and obtained funding; FWJB: conducted zinc assay; JTF: conducted data analysis; DS: recruited subjects and withdrew blood; GWB and TS: conducted cytokine assays; and LJC: conducted clinical assessment of subjects. Labcatal Laboratories did not play any role in the design, implementation, analysis, or interpretation of the research data except for providing free zinc and placebo capsules. None of the authors reported financial conflicts of interest.
REFERENCES
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–95 [DOI] [PubMed] [Google Scholar]
- 2.da Luz PL, Bertini PJ, Favarato D. Noninvasive detection of coronary artery disease--challenges for prevention of disease and clinical events. Clinics 2005;60:415–28 [DOI] [PubMed] [Google Scholar]
- 3.Futterman LG, Lemberg L. Fifty percent of patients with coronary artery disease do not have any of the conventional risk factors. Am J Crit Care 1998;7:240–4 [PubMed] [Google Scholar]
- 4.Prasad AS. Zinc in human health: an update. J Trace Elem Exp Med 1998;11:63–87 [Google Scholar]
- 5.Prasad AS, Bao B, Beck FWJ, Kucuk O, Sarkar FH. Anti-oxidant effect of zinc humans. Free Radic Biol Med 2004;37:1182–90 [DOI] [PubMed] [Google Scholar]
- 6.Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr 1998;68:447S–63S [DOI] [PubMed] [Google Scholar]
- 7.Prasad AS, Beck FW, Bao B, et al. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc in generation of cytokines and oxidative stress. Am J Clin Nutr 2007;85:837–44 [DOI] [PubMed] [Google Scholar]
- 8.Prasad AS, Beck FW, Grabowsski SM, Kaplan J, Mathog RH. Zinc deficiency: changes in cytokine production and T-cell subpopulations in patients with head and neck cancer and in noncancer subjects. Proc Assoc Am Physicians 1997;109:68–77 [PubMed] [Google Scholar]
- 9.Hennig B, Toborek M, Mcclain CJ. Antiatherogenic properties of zinc: implications in endothelial cell metabolism. Nutrition 1996;12:711–7 [DOI] [PubMed] [Google Scholar]
- 10.Ho E, Courtemanche C, Ames BN. Zinc deficiency induces oxidative DNA damage and increases p53 expression in human lung fibroblasts. J Nutr 2003;133:2543–8 [DOI] [PubMed] [Google Scholar]
- 11.Prasad AS, Brewer GJ, Schoomaker EB, Rabbani P. Hypocupremia induced by zinc therapy in adults. JAMA 1978;240:2166–8 [PubMed] [Google Scholar]
- 12.Brewer GJ. Practical recommendations and new therapies for Wilson's disease. Drugs 1995;50:240–9 [DOI] [PubMed] [Google Scholar]
- 13.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta-carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 2001;119:1417–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clemons TE, Kurinij N, Sperduto RD; AREDS Research Group. Associations of mortality with ocular disorders and an intervention of high-dose antioxidants and zinc in the Age-Related Eye Disease Study: AREDS report no. 13. Arch Ophthalmol 2004;122:716–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836–43 [DOI] [PubMed] [Google Scholar]
- 16.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499–511 [DOI] [PubMed] [Google Scholar]
- 17.Touyz RM. Reactive oxygen species and angiotensin II signaling in vascular cells – implications in cardiovascular disease. Braz J Med Biol Res 2004;37:1263–73 [DOI] [PubMed] [Google Scholar]
- 18.Landmesser U, Harrison DG. Oxidant stress as a marker for cardiovascular events: Ox marks the spot. Circulation 2001;104:2638–40 [PubMed] [Google Scholar]
- 19.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation 2000;101:2149–53 [DOI] [PubMed] [Google Scholar]
- 20.Biasucci LM, Liuzzo G, Fantuzzi G, et al. Increasing levels of interleukin (IL)-1Ra and IL-6 during the first 2 days of hospitalization in unstable angina are associated with increased risk of in-hospital coronary events. Circulation 1999;99:2079–84 [DOI] [PubMed] [Google Scholar]
- 21.Mazzone A, Cusa C, Mazzucchelli I, et al. Cigarette smoking and hypertension influence nitric oxide release and plasma levels of adhesion molecules. Clin Chem Lab Med 2001;39:822–6 [DOI] [PubMed] [Google Scholar]
- 22.Ohta T, Saku K, Takata K, Adachi N. Soluble vascular cell-adhesion molecule-1 and soluble intercellular adhesion molecule-1 correlate with lipid and apolipoprotein risk factors for coronary artery disease in children. Eur J Pediatr 1999;158:592–8 [DOI] [PubMed] [Google Scholar]
- 23.Prasad AS, Beck FW, Endre L, Handschu W, Kukuruga M, Kumar G. Zinc deficiency affects cell cycle and deoxythymidine kinase gene expression in HUT-78 cells. J Lab Clin Med 1996;128:51–60 [DOI] [PubMed] [Google Scholar]
- 24.Prasad AS, Bao B, Beck FW, Sarkar FH. Zinc activates NF-κB in HUT 78 cells. J Lab Clin Med 2001;138:250–6 [DOI] [PubMed] [Google Scholar]
- 25.Schulman IH, Zhou MS, Raij L. Nitric oxide, angiotensin II, and reactive oxygen species in hypertension and atherogenesis. Curr Hypertens Rep 2005;7:61–7 [DOI] [PubMed] [Google Scholar]
- 26.Collins T, Cybulsky MI. NF-kappaB: pivotal mediator or innocent bystander in atherogenesis? J Clin Invest 2001;107:255–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bettger WJ. Zinc and selenium, site-specific versus general antioxidant. Can J Physiol Pharmacol 1993;71:721–4 [DOI] [PubMed] [Google Scholar]
- 28.Zabel U, Schreck R, Baeuerle PA. DNA binding of purified transcription factor NF-kappa B. Affinity, specificity, Zn2+ dependence, and differential half-site recognition. J Biol Chem 1991;266:252–60 [PubMed] [Google Scholar]
- 29.Connell P, Young VM, Toborek M, et al. Zinc attenuates tumor necrosis factor-mediated activation of transcription factors in endothelial cells. J Am Coll Nutr 1997;16:411–7 [DOI] [PubMed] [Google Scholar]
- 30.Jaattela M, Mouritzen H, Elling F, Bastholm L. A20 zinc finger protein inhibits TNF and IL-1 signaling. J Immun 1996;156:1166–73 [PubMed] [Google Scholar]
- 31.Song HY, Rothe M, Goeddel DV. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF1/TRAF2 and inhibits NF-κB activation. Proc Natl Acad Sci U S A 1996;93:6721–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blaschke F, Takata Y, Caglayan E, Law RE, Hsueh WA. Obesity, peroxisome proliferators-activated receptor, and atherosclerosis in type 2 diabetes. Arterioscler Thromb Vasc Biol 2006;26:28–40 [DOI] [PubMed] [Google Scholar]
- 33.Delerive P, De Bosscher K, Besnard S, et al. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J Biol Chem 1999;274:32048–54 [DOI] [PubMed] [Google Scholar]
- 34.Reiterer G, Toborek M, Hennig B. Peroxisome proliferator activated receptors alpha and gamma require zinc for their anti-inflammatory properties in porcine vascular endothelial cells. J Nutr 2004;134:1711–5 [DOI] [PubMed] [Google Scholar]