Abstract
The mammalian brain is the most complex organ in the body. It controls all aspects of our bodily functions and interprets the world around us through our senses. It defines us as human beings through our memories and our ability to plan for the future. Crucial to all these functions is how the brain is wired in order to perform these tasks. The basic map of brain wiring occurs during embryonic and postnatal development through a series of precisely orchestrated developmental events regulated by specific molecular mechanisms. Below we review the most important features of mammalian brain wiring derived from work in both mammals and in nonmammalian species. These mechanisms are highly conserved throughout evolution, simply becoming more complex in the mammalian brain. This fascinating area of biology is uncovering the essence of what makes the mammalian brain able to perform the everyday tasks we take for granted, as well as those which give us the ability for extraordinary achievement.
Pioneer axons first set the stage for the general axonal map of the brain. Second, neuronal populations are generated and specified by the expression of transcription factors, with these neurons sending out axons that grow towards their final targets through the use of intermediate targets such as glial and corridor cells along the pathway. Target and synapse specificity in the region of the final target is coordinated both through molecular and activity-dependent mechanisms.
PATTERNING OF THE BRAIN IN RELATION TO PIONEERING AXONS
In the adult nervous system, axons originating from groups of neurons in nuclei, specific cellular layers, or ganglia preferentially associate, for at least some of their pathway, in well defined white matter tracts precisely distributed along the rostrocaudal and dorsoventral axes. This feature is attributed to the preferred growth of developing axons along pre-existing axonal tracts (Goodman and Shatz 1993). However, the first axons that appear in the developing brain grow in a largely axon-free environment, navigating superficially through undifferentiated neuroepithelial cells. These early axons are termed “pioneers,” and are thought to lay down the path followed by later growing axons (Easter et al. 1994). Later arriving axons tend to fasciculate with the pioneers through an established “scaffold” that provides a basic framework for “follower” axons. Time-lapse studies have shown that the growth cone morphology, behavior and actin dynamics of pioneer axons are distinct from those of the follower axons, that are less complex, grow at a higher speed through choice points, and have higher actin dynamics (Bak and Fraser 2003; Kulkarni et al. 2007). A role for pioneer axons in guiding followers (coming from the same nucleus or having a different origin) is primarily supported by experiments performed in fish embryos in which the pioneer axons were cut or ablated (Kuwada 1986; Chitnis and Kuwada 1991; Chitnis et al. 1992; Pike et al. 1992).
Only in the late 1980s was it rediscovered that, in all vertebrate species, the first axonal tracts develop in an extremely conserved and stereotyped spatio-temporal sequence (Fig. 1). At a very early stage, pioneer axons establish growth patterns that are retained later in development, such as the formation of longitudinal versus circumferential growth, attraction or repulsion from the midline, and rostral or caudal orientation. In vertebrates, despite some species variation (Easter et al. 1993; Chédotal et al. 1995; Hjorth and Key 2002; Barreiro-Iglesias et al. 2008), two to three ventral/basal longitudinal tracts form first, and extend from the forebrain and midbrain to the hindbrain; these include the tract of the postoptic commissure (TPOC), the medial longitudinal fasciculus (MLF), and the descending root of the mesencephalic nucleus of the trigeminal nerve (MesV). Dorso-ventral tracts form next with their axons later joining the longitudinal tracts, or crossing the midline to form commissures. In the last 20 years a major effort has been made to understand the mechanisms controlling the development of pioneer axons.
Figure 1.
Schematic representation of the early axonal scaffold in mouse and Xenopus. Abbreviations: MesV, descending tract of the mesencephalic nucleus of the trigeminal nerve; MLF, medial longitudinal fasciculus; TPOC, tract of the postoptic commissure; PC, posterior commissure; IV, trochlear nerve; MMT, mammilothalamic tract; SOT, supraoptic tract; AC, anterior commissure; DVDT, dorsoventral diencephalic tract; POC, postoptic commissure; mes, mesencephalon; die, diencephalon; tel, telencephalon; rhomb, rhombencephalon.
Classically, the embryonic neural tube has been subdivided longitudinally into three main vesicles, the prosencephalon, the mesencephalon, and the rhombencephalon, each separated by transverse constrictions. As development proceeds, new transverse segments, called prosomeres are added in the prosencephalon, with rhombomeres being added in the rhombencephalon (Lumsden and Keynes 1989; Puelles and Rubenstein 2003; Kiecker and Lumsden 2005). The delineation of the neuroepithelial domains relies upon morphological and molecular criteria, as each domain expresses a distinct combination of transcription factors and cell adhesion molecules, set up by specific morphogens such as Fgfs, Shh, and Wnts that are enriched at their boundaries. Interestingly, these domains form before the first axons appear (HH9 in chick and E8.5 in mouse) (Sechrist and Bronner-Fraser 1991; Easter et al. 1993). In fishes, birds, and rodents, there is a striking, albeit not absolute (Hjorth and Key 2001), correlation between the sites where pioneer axons grow and the boundaries of the neuroepithelial domains (Krauss et al. 1991; Figdor and Stern 1993; Macdonald et al. 1994). Therefore, in the hindbrain, the segmented pattern of motorneuron projections is dictated by their rhombomeric origin (Fig. 2) (Lumsden and Keynes 1989; Kiecker and Lumsden 2005).
Figure 2.
Flat-mount view of the HH21 chick rhombencephalon illustrating its early segmentation into 8 rhombomeres (r1-r8). The hox code specific for each rhombomere or odd- and even-numbered pair of rhombomeres is indicated by a color code. The different cranial motornuclei and nerve root are also represented. Abbreviations: IV, trochlear nucleus; V, trigeminal nucleus; VI, abducens nucleus; VII, facial nucleus; IX, glossopharyngeal nucleus; X, vagus nucleus; XII, hypoglossus nucleus; MHB midbrain hindbrain boundary; Mes, mesencephalon; FP, floorplate. Adapted from Kiecker and Lumsden, 2005.
This alignment suggests that the first axons recognize guidance cues distributed in a regionalized manner in the neuroepithelium. In support of this idea, the trajectory of several early axonal tracts is perturbed in fish and mouse mutants lacking the transcription factors Pax6, or the Pax2 homolog Noi (No-isthmus, pax2.1; Macdonald et al. 1994; Mastick et al. 1997; Wilson et al. 1997). Likewise, in zebrafish lacking cyclops (a nodal-related factor) the expression pattern of several transcription factors is altered and this is accompanied by a disorganization of early axonal tracts (Macdonald et al. 1994). As some of the downstream targets of these transcription factors are cell adhesion molecules such as cadherins and other axon guidance molecules (Nguyen Ba-Charvet et al. 1998; Andrews and Mastick 2003; Geisen et al. 2008), different cell surface properties may explain the selective preference of pioneer axons for some domains or, their exclusion from others.
In the forebrain, pioneering axon populations traverse boundaries, rather than forming in conjunction with them. For example, a transient and heterogenous layer of neurons populating the subplate of the neocortex (Ayoub and Kostovic 2009) pioneer the corticothalamic and thalamocortical trajectory forming the internal capsule. Disruption of the subplate causes defects in thalamocortical targeting (McConnell et al. 1989; Ghosh et al. 1990; Ghosh and Shatz 1992). Transient contacts formed between subplate neurons and thalamocortical axons are also required for their proper segregation and synaptic refinement (Kanold et al. 2003). Pioneering axons also form the first projections across the corpus callosum and are derived from the cingulate cortex, rather than the neocortex (Koester and O’Leary 1994; Rash and Richards 2001). Recent evidence for their involvement in corpus callosum formation implicates Npn1/Sema signaling in this process (Piper et al. 2009).
Several studies suggest that homeobox transcription factors may themselves directly act as short-range secreted guidance factors for pioneer axons, with new evidence demonstrating the uptake and retrograde transport of transcription factors to neighboring cells (reviewed in Prochiantz and Joliot 2003 and Brunet et al. 2007). The rostro-caudal distribution of retinal axons in the mesencephalon follows a gradient of the homeobox transcription factor Engrailed established by Fgf8 (Itasaki and Nakamura 1996; Chen et al. 2009) diffusing from the midbrain/hindbrain boundary. Engrailed activity may involve its downstream targets, ephrinA ligands, but it was recently shown that Engrailed can be secreted by tectal cells and internalized by retinal axons, inducing their turning (Brunet et al. 2005). Likewise, some tracts extend along domains expressing a high level of Otx2 (Nguyen Ba-Charvet et al. 1998), a transcription factor that can also act non cell autonomously during brain development (Sugiyama et al. 2008). Directly testing the non cell autonomous role of homeobox-containing transcription factors in early axonal tract development, will require blocking their secretion or internalization in vivo.
Other recent studies have shown that pioneer axons largely respond to the same set of guidance molecules as later born axons. In zebrafish, Sema3D initially repels MLF axons from the forebrain and attracts anterior commissural axons toward the midline; however at a later stage, it promotes the fasciculation of these tracts by influencing the cell surface level of L1-CAM (Wolman et al. 2004; Wolman et al. 2007). In chick, Sema3F/neuropilin-2 repulsion also plays a role in restraining the growth of chick trochlear motor axons at the midbrain/hindbrain boundary (Watanabe et al. 2004). In chick and mice, caudally projecting longitudinal axons of the MLF and MesV express Robo receptors and grow between domains expressing high levels of Slit ligands (Molle et al. 2004; Farmer et al. 2008; Kastenhuber et al. 2009). In anamniotes, Slit/Robo signaling controls the fasciculation of TPOC axons that also extend over a Netrin-rich region in the basal forebrain (Wilson and Key 2006; Devine and Key 2008).
The factors providing rostro-caudal directionality in the brain are still unknown but several morphogens such as Shh, Wnt, and Fgf may contribute. Fgf8 regulates the patterning of pioneer axons in the forebrain (Shanmugalingam et al. 2000) and attracts trochlear axons along the midbrain/hindbrain boundary (Irving et al. 2002).
MECHANISMS OF AXON GUIDANCE IN THE BRAIN
The incredible complexity of the mammalian brain, and the targeting and growth of axons over long distances, requires a unique strategy for enabling brain wiring to occur during development. This is achieved through the use of intermediate targets. To accomplish axon navigation over long distances, the system is broken down into smaller, more manageable, guidance decisions through the use of intermediate targets consisting of glial cells or intermediate guidepost cells. In the hindbrain and midbrain this occurs through the floorplate, a transient, glial-like structure at the ventral midline of the brain. However, important differences exist between the forebrain and hindbrain. Whereas in the forebrain commissures are restricted to a limited number of locations, commissural axons are widespread throughout the hindbrain and spinal cord and tend to coalesce/fasciculate in well defined tracts only after they have crossed the midline and adopted a longitudinal growth mode. This important difference is likely to be related to the presence of floorplate cells that extend from the caudal tip of the spinal cord to the hypothalamus (Fig. 3) and play a major role in patterning axonal connections at this CNS level through the secretion of chemoattractants and chemorepellents. By contrast, commissural axons in the forebrain appear to be channeled through very specific locations.
Figure 3.
Commissural and longitudinal projections in the forebrain. Both glial and neuronal structures are associated with axonal tracts in the brain. (A–C) depict commissural tracts in schematics of horizontal sections from dorsal to ventral. (A and B) are schematics of the brain, whereas C is a ventral view of the head. Associated with the corpus callosum (blue tract in A) are the glial wedge and indusium griseum glia and the sling cells. Glia are also associated with the hippocampal commissure (purple tract in A) the anterior commissure (green tract in B) and the optic chiasm (red crossing fibers in C). In (D), longitudinal tracts are shown, including the corticothalamic, thalamocortical, cortico-collicular and corticospinal tacts that all pass through the internal capsule. Associated with the internal capsule are the corridor cells. The lateral olfactory tract (LOT) is also shown in D, together with the LOT cells. All schematics are of sections of mouse brain or head at embryonic day 18.
In the forebrain there is no floorplate structure but additional, transient midline glial populations are present that secrete similar guidance molecules to the floorplate in more caudal regions of the nervous system. Midline glial populations are associated with every commissural and decussating projection in the brain (Silver et al. 1993). These glial populations are known as the “palisade” (optic chiasm) (Marcus et al. 1995), “tunnels” (anterior commissure and fornix) (Pires-Neto et al. 1998; Braga-de-Souza and Lent 2004; Lent et al. 2005), “wedge” and “indusium griseum glia” (corpus callosum) (Shu and Richards 2001; Shu et al. 2003b) (Fig. 3). Molecules expressed by these glial populations include Slits (Erskine et al. 2000; Plump et al. 2002; Shu et al. 2003c), Wnts (Keeble and Cooper 2006), Ephrins (Mendes et al. 2006; Williams et al., 2003), Draxin (Islam et al. 2009), and chondroitin sulphate proteoglycans (Braga-de-Souza and Lent 2004). The development of these glial populations is regulated by transcription factors such as Nfi genes (Shu et al. 2003a; Steele-Perkins et al. 2005; Barry et al. 2008) and fibroblast growth factor signaling (Smith et al. 2006). Like the floor plate, each of these populations is transient and only present during development of the axon tracts with which they are associated.
In addition to transient glial populations in the brain, a number of transient neuronal populations have also been identified that act as “guidepost cells” or “corridor cells” for axons. In the developing olfactory system, the major efferent projection from the olfactory bulb is the lateral olfactory tract (LOT), which contains guidepost cells, known as LOT cells (Tomioka et al. 2000; Figure 3). LOT cells migrate tangentially and ventrally from the neocortex to reside within the lateral forebrain where the LOT will later form. The migration of these cells is directed by Netrin and Sema3F (Kawasaki et al. 2006; Ito et al. 2008) and the development of the LOT axons depends on the presence of these cells. Slits secreted from the septum, and several secreted semaphorins, also play a key role in LOT positioning (de Castro et al. 1999; Nguyen Ba-Charvet et al. 1999; Nguyen-Ba-Charvet et al. 2002; Fouquet et al. 2007). In the telencephalon, several other migratory populations of neurons are implicated in axon guidance. The subcallosal sling cells (Silver et al. 1982; Shu et al. 2003b) and neurons within the corpus callosum (Riederer et al. 2004; Niquille et al. 2009) guide axons of the corpus callosum, and “corridor” cells in the internal capsule guide thalamocortical axons into the cortex through their expression of neuregulin (Lopez-Bendito et al. 2006) (Fig. 3). Similarly, early born neurons at the optic chiasm are required for the guidance of retinal ganglion cell axons at the midline (Marcus and Mason 1995; Sretavan et al. 1995).
A driving hypothesis in the field of axon guidance has been that axonal growth cones are guided by molecular gradients within the developing nervous system. Although several in vitro assays have allowed this hypothesis to be tested (reviewed in Pujic et al. 2009), the exact parameters required for axonal chemotaxis are still being uncovered (Mortimer et al. 2009). What is established is that throughout the neuraxis both attractive and repulsive guidance mechanisms operate to guide axons. In the forebrain, midbrain and spinal cord, Draxin acts as a repellent (Islam et al. 2009; Naser et al. 2009) expressed by the roof plate in the spinal cord and the glial wedge in the forebrain. Molecules of the Neuropilin and Semaphorin families mediate guidance through both attraction and repulsion and play an important role in the guidance and positioning of the corpus callosum and anterior commissure (Falk et al. 2005; Niquille et al. 2009; Piper et al. 2009; Hatanaka et al., 2009). Netrin1 acts as an attractant for corticofugal (Metin et al. 1997; Richards et al. 1997) and thalamocortical pathways (Braisted et al. 2000). These tracts, as well as many of the other commissural projections in the brain, are affected in both Netrin1 and DCC mutant mice (Serafini et al. 1996; Fazeli et al. 1997). In the visual system Netrin1 guides axons at the optic disk to enter the optic nerve (Deiner et al. 1997). Slits have been shown to act as chemorepulsive signals for decussating axons at the optic chiasm (Erskine et al. 2000; Plump et al. 2002) as well as callosal axons (Shu and Richards 2001; Bagri et al. 2002; Shu et al. 2003c) but their role in mediating the guidance of other forebrain commissural projections has not been thoroughly investigated. In a number of systems Slits and their receptors, Robos, have also been shown to regulate the fasciculation of axon tracts. As described earlier, the formation of pioneering axon tracts in the brain allows for later arriving axons to use the pioneers for guidance by fasciculating with these axons. Fasciculation occurs through axon–axon interactions and may be mediated by cell adhesion molecules (CAMs), such as NCAM, L1-CAM or TAG-1 or through receptor homophilic interactions between axons mediated by Robo or Eph receptors.
Despite the obvious neurological relevance, the mechanisms controlling the growth of axonal projections from neurons using biogenic amine neurotransmitters such as cathecholamines (noradrenaline and dopamine), acetylcholine, or Serotonin (5-hydroxytryptamine or5-HT) has been largely neglected. Recent studies on the ontogeny of these systems in zebrafish will allow the use of genetic methods to answer this question (McLean and Fetcho 2004; Kastenhuber et al. 2009; Lillesaar et al. 2009). In rodents, the growth of dopaminergic axons from the midbrain toward the forebrain appears to be guided by classic secreted axon guidance molecules (Fig. 4) (see Van den Heuvel and Pasterkamp 2008 for a review). Dopaminergic neurons express Robo and neuropilin receptors (Nakamura et al. 2000; Marillat et al. 2002; Hernandez-Montiel et al. 2008) and respond to several Semaphorins and Slit proteins. The rostral growth of dopaminergic axons is influenced by a repulsive gradient of Sema3F, regulated by Fgf8 originating at the midbrain/hindbrain boundary (Nakamura et al. 2000; Kolk et al., 2009; Yamauchi et al. 2009). These axons are also guided by the attractive activity of Sema3A and Sema3C produced in the diencephalon and striatum (Hernandez-Montiel et al. 2008; Yamauchi et al. 2009) and Sema 3F, acting through Npn2, in the medial prefrontal cortex (Kolk et al., 2009). Dopaminergic axons also respond in vitro to floorplate explants, and Netrin1 and Slit proteins (Lin et al. 2005). Finally, dopaminergic projections that are primarily ipsilateral, defasciculate and cross the midline in Slit1/Slit2 double-knockout mice (Bagri et al. 2002).
Figure 4.
Development of dopaminergic projections in the mouse embryo. Dopaminergic axons originate from the substantia nigra (SN) and ventral tegmental area (VTA) in the midbrain and innervate the striatum and cortex. (A) and (B) show the development of this tract at E11 and P0, respectively. They grow rostrally under the repulsive action of Sema3F secreted from the midbrain/hindbrain boundary (MHB, C; C is an enlargement of the boxed area in [A]). The gradient of Sema3F is controlled by Fgf8. Secreted repellents from the mesencephalon and diencephalon/thalamus (B, C) maintain the dopaminergic axons ventrally, whereas factors secreted from the striatum attract them. (D) Dopaminergic axons mostly project ipsilaterally and are maintained away from the midline by Slits and other repellents. Abbreviations: os, optic stalk; tel, telencephalon; die, diencephalon; mes, mesencephalon; rhomb, rhombencephalon; Stri, striatum; Thal, thalamus. Modified from Yamauchi et al., 2009 and Van den Heuvel and Pasterkamp, 2008.
Axonal projections typically form in a stereotyped manner. However, the remarkable plasticity of the brain was highlighted recently in a study on Sema6A mutant mice (Little et al. 2009). As the majority of mouse models with mutations in axonal guidance genes die at birth, there has been little opportunity to investigate what happens to mis-targeted axons in the adult animal. However in the Sema6A mutant, which does survive to adulthood, some thalamic axons from the lateral geniculate nucleus project ectopically into the neocortex via the upper layers. Even though the primary visual area is decreased, the ectopic projections remain in the adult (Little et al. 2009). This finding illustrates the plasticity of axonal targeting and the ability to retain functional ectopic projections into adulthood.
CONTRALATERAL AND IPSILATERAL TRACTS IN THE BRAIN
The mammalian brain is wired based on functional specificity, with different regions of the brain that subserve the same functional modality being connected. The establishment of this functional specificity is controlled by gene expression early in development, followed by later, activity-dependent mechanisms of refinement.
In the forebrain, recent studies suggest that transcriptional control of axon specificity occurs as neurons are born in the ventricular and subventricular zones (VZ and SVZ, respectively) of the neocortex. Neocortical connectivity involves both VZ and SVZ projection neurons, and interneurons, born in the ventral forebrain and cortical hem (reviewed in Pierani and Wassef 2009). Projection neurons wire the brain over long distances and provide connectivity between different regions of the brain specializing in the same sensory-motor modality. How projection neurons find their targets within the brain is an important question in neurobiology. Recent insight has come from studies identifying transcription factors that impart layer and connection specificity to projection neurons in the cortex (reviewed in Molyneaux et al. 2007 and Leone et al. 2008). Some of the most significant experiments in this regard involve the manipulation of gene expression, resulting in the re-specification of axons to a different projection neuron type. For example Ctip2, Fezf2, and Sox5 specify subcerebral projections and Satb2 specifies callosal projections in this system (Molyneaux et al. 2005; Alcamo et al. 2008; Arlotta et al. 2008; Britanova et al. 2008; Chen et al. 2008; Kwan et al. 2008; Lai et al. 2008). In addition to these studies, transcription factors also regulate regional differences in the human brain (Johnson et al., 2009), as well as axon pathway specificity in other tracts including the retino-tectal system and thalamocortical projection, and also the spinal cord motor- and sensory-neuron projections (reviewed in Polleux et al. 2007). Likewise, in the hindbrain and spinal cord, transcription factors such as homeodomain containing proteins act upstream of many axon guidance receptors and ligands (see Chédotal and Rijli 2009 for a review).
In addition to layer and cell-type specification, axonal guidance molecules within the brain play a crucial role in determining axon tract development. The formation of both ipsilaterally and contralaterally projecting axon tracts allows the brain to integrate sensory and motor information from the environment from both sides of the body and to perform the appropriate behavioral responses. Similarly, evolutionarily conserved molecules, such as Slits, IgCAMs, Netrins, Semaphorins and Ephrins regulate axon tract formation in the mammalian brain. There are examples of molecules from each guidance family that regulate the formation of both ipsilateral and contralateral projections: thus the initial direction of growth may not be specified by axonal guidance molecules, but rather by transcriptional regulation. These transcription factors could regulate the expression of receptors at specific times in development to allow the axon to be guided by extrinsic cues (e.g., in the visual system) (Petros et al. 2008), but as yet it is unclear how transcriptional regulation is able to impart axon tract and guidance specificity within most systems of the brain (Chédotal and Rijli 2009).
HOW NEURONS LOCATE AND SYNAPSE WITH THEIR TARGET IN THE MAMMALIAN BRAIN
In vivo, eye rotation, ablation (e.g., in the visual system, reviewed in Goodhill and Richards 1999) and axon rerouting (reviewed in Sur and Rubenstein 2005) have been used to investigate the role of activity-dependent mechanisms in axonal guidance and the refinements of map formation. Our understanding of both molecular and activity-dependent mechanisms are based on these experimental paradigms.
As far as axon guidance is concerned, not all neurons are born equal. Making synapses on the proper target cells is a problem of extreme complexity depending on the type of neuron. At one extreme, this is not an issue for neurons that do not have an axon, such as most amacrine cells in the retina and granule cells in the olfactory bulb. At the other extreme, it is a particularly challenging task for axons forming point-to-point connections with a unique distant target cell(s). Moreover, the distribution of most axon terminals on their target neuron is not random, but restricted to specific subcellular compartments such as the cell body, dendrite, spines and axon. Here we summarize the different molecular mechanisms that control the final targeting of some neuronal classes.
An initial, important distinction can be made between interneurons that will contact target cells in their immediate vicinity, and projection neurons whose targets can be millimeters away. Axons from different types of interneurons do not grow and synapse randomly but arborize in specific patterns and layers, as best exemplified in the cerebral cortex (Huang et al. 2007; Ascoli et al. 2008; Batista-Brito and Fishell 2009).
Recent studies have started to reveal that cell-adhesion molecules of the immunoglobulin superfamily (IgCAM) guide the axons of several classes of interneurons in the forebrain and hindbrain. In the molecular layer of the cerebellum, two types of GABAergic inhibitory interneurons, basket cells and stellate cells, innervate the same target, the Purkinje cell, which is the only output neuron of the cerebellar cortex (Sotelo 2008). Whereas stellate cell axons only innervate the smooth surface of Purkinje cell proximal dendrites, basket cells innervate the Purkinje cell soma and axon at the level of the axon initial segment, forming characteristic “pinceaux” formations (Sotelo 2008). Two proteins of the L1-CAM family of IgCAMs control the differential targeting of stellate and basket cells (Ango et al. 2004; Ango et al. 2008). Neurofascin 186 was shown to be expressed in a gradient on the Purkinje cell body and enriched at the axon initial segment, where it binds AnkyrinG (Ango et al. 2004). Basket cell axons fail to target properly to the axon initial segment when the NF186 gradient is abolished, such as in AnkyrinG knockout mice or following expression of a dominant-negative form of neurofascin in Purkinje cells (Fig. 5). Homophilic interactions between the processes of Bergmann glia and stellate cell axons appear to guide these axons to the Purkinje cell dendrites (Ango et al. 2008). This involves close homolog of L1 (CHL1), because in CHL1 knockout mice, stellate cell axons fail to properly innervate the Purkinje cell dendrites. In the retina, homophilic interactions involving DsCAMs and Sidekick IgCAMs coordinate the precise wiring of subsets of bipolar neurons, amacrine cells and retinal ganglion cells in the inner plexiform layer (Yamagata and Sanes 2008).
Figure 5.
Development of basket cell axons. (A) original drawing by Santiago Ramon y Cajal of a cerebellar basket cell (B) labeled by Golgi staining demonstrating its characteristic axonal arbors (a), the “pinceaux” formations, around the Purkinje cell body and axon. (B) Basket cell axons synapse preferentially on the Purkinje cell axon initial segment (AIS) under the influence of a gradient of Neurofascin 186, stabilized by Ankyrin G. In Ankyrin G knockout mice, the gradient of Neurofascin is abolished and basket cell axons do not synapse preferentially on the AIS. Abbreviations: ML, molecular layer; PCL, Purkinke cell layer; GCL, granule cell layer. A, Cajal drawing. Original conserved at the Instituto Cajal (CSIC), Madrid (Spain). B is adapted from Huang et al., 2007.
Guiding long projection axons is also quite a variable challenge depending on the type of neurons. Thalamic axons are guided to specific regions and layers of cortex through the expression of molecules such as ephrin/Eph (Uziel et al. 2005) but are also sorted in the internal capsule prior to entering the cortex through both ephrin/Eph signaling and Netrin1 (Dufour et al. 2003; Powell et al. 2008). Corticospinal axons, which constitute the longest axonal projections in the nervous system, are guided by the expression of multiple guidance molecules, including the expression of a Wnt gradient that directs them posteriorly (Liu et al. 2005; Canty and Murphy 2008).
The topography of aminergic projections is rather loose. Cholinergic neurons from the basal forebrain (medial septum, diagonal band of Broca, substantia inominata, and globus pallidus) extensively innervate the cerebral cortex (Gould et al. 1991). Midbrain dopaminergic neurons from the substantia nigra and ventral tegmental area project primarily to the striatum and neocortex. The density of dopaminergic inputs varies between cortical layers and areas but there is no clear specificity. This is also the case for noradrenegic projections from the locus coeruleus and serotonergic projections from the brainstem that project diffusely throughout the brain and spinal cord (Gaspar et al. 2003).
In contrast to aminergic neurons, most projection neurons establish some precisely patterned projections upon entering their target domain. Although activity-dependent mechanisms control the final refinement of these projections, their targeting to specific layers and neurons, as well as their subcellular localization, are primarily instructed by extrinsic cues.
In the developing hippocampus, axons from the entorhinal cortex synapse onto the distal part of granule cell dendrites in the dentate gyrus, whereas the proximal region of the dendrite is targeted by commissural/associational axons from the contralateral hippocampus (Super and Soriano 1994; Forster et al. 2006). Prior to contacting granule cells, entorhinal axons also project onto a transient neuronal population, the Cajal-Retzius cells. Preventing this contact severely perturbs their final targeting (Del Rio et al. 1997) and Reelin, secreted by the Cajal-Retzius cells, may be involved.
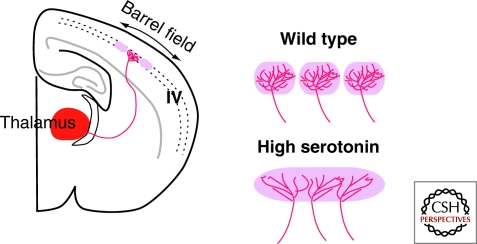
Other examples illustrating the role of axon guidance molecules in projection map formation are presented in O'Leary (2010). Interestingly, mounting evidence suggests that the development of point-to-point projections is influenced by aminergic systems, in particular serotonin. Serotonin is expressed early in embryonic development and growing axons can release it before synaptogenesis. The segregation of thalamocortical axons in the barrel field of the somatosensory cortex is blocked in several lines of genetically modified mice with increased levels of serotonin (Gaspar et al. 2003) (Fig. 6). This also affects the segregation of ipsilateral/contralateral eye inputs in the lateral geniculate nucleus. At earlier ages, serotonin can also influence the guidance of thalamocortical axons by converting Netrin1 activity from attraction to repulsion (Bonnin et al. 2007). The action of serotonin on developing axons appears mediated to a large extent by Gi/o-coupled 5-HT1B receptors, which induce a decrease of cAMP level.
Figure 6.
Serotonin influences axonal arborization during development. In layer IV of the somatosensory cortex, thalamic axons conveying sensory information from the same whisker cluster and arborize in the same domain called a “barrel.” When the level of serotonin is increased during development, such as occurs in MaoA and SERT knockout mice, the barrel field does not form and thalamic axon terminals corresponding to distinct whiskers overlap. Adapted from Gaspar et al., 2003.
A similar role for other biogenic amines has not yet been demonstrated, but acetylcholine produced by some amacrine cells is essential for the establishment of visual projection maps (Cang et al. 2008; Huberman et al. 2008). Acetylcholine attracts spinal cord axons in the Xenopus turning assay (Zheng et al. 1994), suggesting that this neurotransmitter could influence axon guidance in many brain areas. Interestingly, other circulating proteins such as endocannabinoids (Berghuis et al. 2007) and leptin (Bouret et al. 2004) can also influence axon guidance.
CURRENT DIRECTIONS IN MAMMALIAN BRAIN WIRING
The work described here has demonstrated some overarching mechanistic principles in mammalian brain wiring. Pioneer axons first set the stage for the general axonal map of the brain. Second, neuronal populations are generated and specified by the expression of transcription factors, with these neurons sending out axons that grow towards their final targets through the use of intermediate targets such as glial and corridor cells along the pathway. Target and synapse specificity in the region of the final target is coordinated both through molecular and activity-dependent mechanisms. These sequential mechanisms, working together in neurons forming different, but vital, functions in the brain underlie the functional circuitry of the adult brain. Future directions for this field will involve exciting technical advances in observing single neurons and neuronal circuits with fluorescent labels or via brain imaging technologies (e.g., see lines available from the Gensat consortium) (Gong et al. 2003 and Brainbow technology; Livet et al. 2007) (Fig. 7). A coordinated effort is also now underway to map neuronal connectivity in the brains of multiple organisms (Bohland et al. 2009), particularly mouse and macaque, and to develop the tools for mapping human neuronal connectivity on an unprecedented scale. This research will lead to another leap in our understanding of brain structure and function as we will be able to dissect neuronal circuits at both the microscopic and systems levels in ways that were previously impossible.
Figure 7.
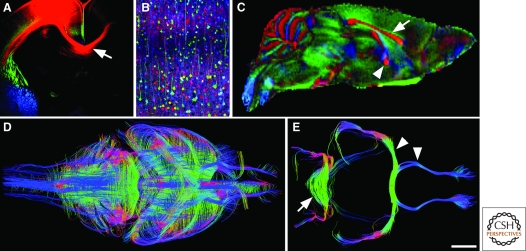
Advances in techniques for labeling axon tracts and circuits. (A) Carbocyanine dye labeling in the developing brain. DiI-labeled callosal axons are shown in red (arrow in A). Labeling was performed on fixed embryonic day 17 mouse brain. (image courtesy of Dr Celine Plachez, University of Maryland). (B) Labeling of neurons in the brain of a “brainbow” mouse. Different neurons are visualized with different hues of color generated by Cre/loxP recombination in transgenic mice (image courtesy of Dr Jeff Lichtman and Dr Tamily Weissman, Harvard University). (C) Diffusion-weighted (30 directions) magnetic resonance image acquired at 16.4 Tesla—colormap demonstrating commissural tracts in a midsagittal view. Based on their orientation, commissural fibers have been color-coded in red, including the corpus callosum (arrow in C) and anterior commissure (arrowhead in C). (D and E) are tractography images of high angular resolution imaging (HARDI/q-ball). In D, regions of interest (ROI) were selected across the brain, with axon tracts shown that pass through the midline. E demonstrates a more selective placement of ROI’s, one at the midline within the anterior commissure (arrowheads in E depict both the anterior and posterior arms of the anterior commissure that pass through the ROI at the midline), and one in the hindbrain at the midline within the middle cerebellar peduncle and pontine transverse fibers (arrowhead in E). Images in C–E courtesy of Dr Nyoman Kurniawan and Dr Randal Moldrich (The University of Queensland). Scale bar in E = 400 µm in A, 80 µm in B, 2 mm in C and E and 1.35 mm in D.
Imaging the entire brain to understand systems level questions is also progressing at a rapid rate. Magnetic resonance imaging has been used to examine the gross anatomy of the living human brain. Recent advances in this field have led to developments in non-invasive axonal tractography and tract tracing. Diffusion tensor, and diffusion weighted, imaging allow the color-coding of axonal tracts in the brain depending on the fiber orientation (Behrens et al. 2003) (Fig. 7). Newer methods known as high angular resolution diffusion imaging (HARDI) and q-Ball tractography allow even greater accuracy, particularly in regions of crossing fibers. Techniques such as these can be used on both fixed tissue and living brains and can provide incredible three-dimensional reconstruction of axonal pathways and their relationship to other axonal tracts in the brain (Fig. 7). Recent use of this technique in patients with callosal hypogenesis has demonstrated a remarkable array of differences in brain wiring between patients with similar gross-anatomical features (Wahl et al. 2009) and has even highlighted the formation of ectopic connections in these patients (Tovar-Moll et al. 2007). This work has set the stage for an unprecedented understanding and measurement of human brain plasticity during development and following injury or disease, as well as the possibility to relate actual brain connectivity with human behavior.
ACKNOWLEDGMENTS
Alain Chédotal is supported by the Institut National de la Santé et de la Recherche Médicale, the Agence Nationale pour la recherche (programmes Blanc and MNP) and the Fondation pour la Recherche Médicale (programme équipe FRM). Linda Richards is supported by a Senior Research Fellowship form the National Health and Medical Research Council of Australia. We thank John Baisden and Ian Glidden for graphics assistance and Rowan Tweedale for critical reading of the text.
Footnotes
Editors: Marc Tessier-Lavigne and Alex L. Kolodkin
Additional Perspectives on Neuronal Guidance available at www.cshperspectives.org
REFERENCES
- Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Farinas I, Grosschedl R, McConnell SK 2008. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron 57:364–377 [DOI] [PubMed] [Google Scholar]
- Andrews GL, Mastick GS 2003. R-cadherin is a Pax6-regulated, growth-promoting cue for pioneer axons. J Neurosci 23:9873–9880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ 2004. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell 119:257–272 [DOI] [PubMed] [Google Scholar]
- Ango F, Wu C, Van der Want JJ, Wu P, Schachner M, Huang ZJ 2008. Bergmann glia and the recognition molecule CHL1 organize GABAergic axons and direct innervation of Purkinje cell dendrites. PLoS Biol 6:e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux BJ, Jabaudon D, Yoshida Y, Macklis JD 2008. Ctip2 controls the differentiation of medium spiny neurons and the establishment of the cellular architecture of the striatum. J Neurosci 28:622–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, et al. 2008. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci 9:557–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub AE, Kostovic I 2009. New horizons for the subplate zone and its pioneering neurons. Cereb Cortex 19:1705–1707 [DOI] [PubMed] [Google Scholar]
- Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M 2002. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron 33:233–248 [DOI] [PubMed] [Google Scholar]
- Bak M, Fraser SE 2003. Axon fasciculation and differences in midline kinetics between pioneer and follower axons within commissural fascicles. Development 130:4999–5008 [DOI] [PubMed] [Google Scholar]
- Barreiro-Iglesias A, Villar-Cheda B, Abalo XM, Anadon R, Rodicio MC 2008. The early scaffold of axon tracts in the brain of a primitive vertebrate, the sea lamprey. Brain Res Bull 75:42–52 [DOI] [PubMed] [Google Scholar]
- Barry G, Piper M, Lindwall C, Moldrich R, Mason S, Little E, Sarkar A, Tole S, Gronostajski RM, Richards LJ 2008. Specific glial populations regulate hippocampal morphogenesis. J Neurosci 28:12328–12340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Fishell G 2009. The developmental integration of cortical interneurons into a functional network. Curr Top Dev Biol 87:81–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, et al. 2003. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6:750–757 [DOI] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, et al. 2007. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science 316:1212–1216 [DOI] [PubMed] [Google Scholar]
- Bohland JW, Wu C, Barbas H, Bokil H, Bota M, Breiter HC, Cline HT, Doyle JC, Freed PJ, Greenspan RJ, et al. 2009. A proposal for a coordinated effort for the determination of brainwide neuroanatomical connectivity in model organisms at a mesoscopic scale. PLoS Comput Biol 5:e1000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A, Torii M, Wang L, Rakic P, Levitt P 2007. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci 10:588–597 [DOI] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB 2004. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304:108–110 [DOI] [PubMed] [Google Scholar]
- Braga-de-Souza S, Lent R 2004. Temporal and spatial regulation of chondroitin sulfate, radial glial cells, growing commissural axons, and other hippocampal efferents in developing hamsters. J Comp Neurol 468:217–232 [DOI] [PubMed] [Google Scholar]
- Braisted JE, Catalano SM, Stimac R, Kennedy TE, Tessier-Lavigne M, Shatz CJ, O’Leary DD 2000. Netrin-1 promotes thalamic axon growth and is required for proper development of the thalamocortical projection. J Neurosci 20:5792–5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britanova O, de Juan Romero C, Cheung A, Kwan KY, Schwark M, Gyorgy A, Vogel T, Akopov S, Mitkovski M, Agoston D, et al. 2008. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron 57:378–392 [DOI] [PubMed] [Google Scholar]
- Brunet I, Di Nardo AA, Sonnier L, Beurdeley M, Prochiantz A 2007. The topological role of homeoproteins in the developing central nervous system. Trends Neurosci 30:260–267 [DOI] [PubMed] [Google Scholar]
- Brunet I, Weinl C, Piper M, Trembleau A, Volovitch M, Harris W, Prochiantz A, Holt C 2005. The transcription factor Engrailed-2 guides retinal axons. Nature 438:94–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, Niell CM, Liu X, Pfeiffenberger C, Feldheim DA, Stryker MP 2008. Selective disruption of one Cartesian axis of cortical maps and receptive fields by deficiency in ephrin-As and structured activity. Neuron 57:511–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canty AJ, Murphy M 2008. Molecular mechanisms of axon guidance in the developing corticospinal tract. Prog Neurobiol 85:214–235 [DOI] [PubMed] [Google Scholar]
- Chédotal A, Rijli FM 2009. Transcriptional regulation of tangential neuronal migration in the developing forebrain. Curr Opin Neurobiol 19:139–145 [DOI] [PubMed] [Google Scholar]
- Chédotal A, Pourquie O, Sotelo C 1995. Initial tract formation in the brain of the chick embryo: selective expression of the BEN/SC1/DM-GRASP cell adhesion molecule. Eur J Neurosci 7:198–212 [DOI] [PubMed] [Google Scholar]
- Chen Y, Mohammadi M, Flanagan JG 2009. Graded levels of FGF protein span the midbrain and can instruct graded induction and repression of neural mapping labels. Neuron 62:773–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Wang SS, Hattox AM, Rayburn H, Nelson SB, McConnell SK 2008. The Fezf2-Ctip2 genetic pathway regulates the fate choice of subcortical projection neurons in the developing cerebral cortex. Proc Natl Acad Sci U S A 105:11382–11387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis AB, Kuwada JY 1991. Elimination of a brain tract increases errors in pathfinding by follower growth cones in the zebrafish embryo. Neuron 7:277–285 [DOI] [PubMed] [Google Scholar]
- Chitnis AB, Patel CK, Kim S, Kuwada JY 1992. A specific brain tract guides follower growth cones in two regions of the zebrafish brain. J Neurobiol 23:845–854 [DOI] [PubMed] [Google Scholar]
- de Castro F, Hu L, Drabkin H, Sotelo C, Chédotal A 1999. Chemoattraction and chemorepulsion of olfactory bulb axons by different secreted semaphorins. J Neurosci 19:4428–4436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier-Lavigne M, Sretavan DW 1997. Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron 19:575–589 [DOI] [PubMed] [Google Scholar]
- Del Rio JA, Heimrich B, Borrell V, Forster E, Drakew A, Alcantara S, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, et al. 1997. A role for Cajal-Retzius cells and reelin in the development of hippocampal connections. Nature 385:70–74 [DOI] [PubMed] [Google Scholar]
- Devine CA, Key B 2008. Robo-Slit interactions regulate longitudinal axon pathfinding in the embryonic vertebrate brain. Dev Biol 313:371–383 [DOI] [PubMed] [Google Scholar]
- Dufour A, Seibt J, Passante L, Depaepe V, Ciossek T, Frisen J, Kullander K, Flanagan JG, Polleux F, Vanderhaeghen P 2003. Area specificity and topography of thalamocortical projections are controlled by ephrin/Eph genes. Neuron 39:453–465 [DOI] [PubMed] [Google Scholar]
- Easter SS Jr, Burrill J, Marcus RC, Ross LS, Taylor JS, Wilson SW 1994. Initial tract formation in the vertebrate brain. Prog Brain Res 102:79–93 [DOI] [PubMed] [Google Scholar]
- Easter SS Jr, Ross LS, Frankfurter A 1993. Initial tract formation in the mouse brain. J Neurosci 13:285–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine L, Williams SE, Brose K, Kidd T, Rachel RA, Goodman CS, Tessier-Lavigne M, Mason CA 2000. Retinal ganglion cell axon guidance in the mouse optic chiasm: expression and function of robos and slits. J Neurosci 20:4975–4982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk J, Bechara A, Fiore R, Nawabi H, Zhou H, Hoyo-Becerra C, Bozon M, Rougon G, Grumet M, Puschel AW, et al. 2005. Dual functional activity of semaphorin 3B is required for positioning the anterior commissure. Neuron 48:63–75 [DOI] [PubMed] [Google Scholar]
- Farmer WT, Altick AL, Nural HF, Dugan JP, Kidd T, Charron F, Mastick GS 2008. Pioneer longitudinal axons navigate using floorplate and Slit/Robo signals. Development 135:3643–3653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Rayburn H, et al. 1997. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature 386:796–804 [DOI] [PubMed] [Google Scholar]
- Figdor MC, Stern CD 1993. Segmental organization of embryonic diencephalon. Nature 363:630–634 [DOI] [PubMed] [Google Scholar]
- Forster E, Zhao S, Frotscher M 2006. Laminating the hippocampus. Nat Rev Neurosci 7:259–267 [DOI] [PubMed] [Google Scholar]
- Fouquet C, Di Meglio T, Ma L, Kawasaki T, Long H, Hirata T, Tessier-Lavigne M, Chédotal A, Nguyen-Ba-Charvet KT 2007. Robo1 and robo2 control the development of the lateral olfactory tract. J Neurosci 27:3037–3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L 2003. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci 4:1002–1012 [DOI] [PubMed] [Google Scholar]
- Geisen MJ, Di Meglio T, Pasqualetti M, Ducret S, Brunet JF, Chédotal A, Rijli FM 2008. Hox paralog group 2 genes control the migration of mouse pontine neurons through slit-robo signaling. PLoS Biol 6:e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Shatz CJ 1992. Involvement of subplate neurons in the formation of ocular dominance columns. Science 255:1441–1443 [DOI] [PubMed] [Google Scholar]
- Ghosh A, Antonini A, McConnell SK, Shatz CJ 1990. Requirement for subplate neurons in the formation of thalamocortical connections. Nature 347:179–181 [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, et al. 2003. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425:917–925 [DOI] [PubMed] [Google Scholar]
- Goodhill GJ, Richards LJ 1999. Retinotectal maps: molecules, models and misplaced data. Trends Neurosci 22:529–534 [DOI] [PubMed] [Google Scholar]
- Goodman CS, Shatz CJ 1993. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell 72:77–98 [DOI] [PubMed] [Google Scholar]
- Gould E, Woolf NJ, Butcher LL 1991. Postnatal development of cholinergic neurons in the rat: I. Forebrain. Brain Res Bull 27:767–789 [DOI] [PubMed] [Google Scholar]
- Hatanaka Y, Matsumoto T, Yanagawa Y, Fujisawa H, Murakami F, Masu M 2009. Distinct roles of neuropilin 1 signaling for radial and tangential extension of callosal axons. J Comp Neurol 514:215–225 [DOI] [PubMed] [Google Scholar]
- Hernandez-Montiel HL, Tamariz E, Sandoval-Minero MT, Varela-Echavarria A 2008. Semaphorins 3A, 3C, and 3F in mesencephalic dopaminergic axon pathfinding. J Comp Neurol 506:387–397 [DOI] [PubMed] [Google Scholar]
- Hjorth JT, Key B 2001. Are pioneer axons guided by regulatory gene expression domains in the zebrafish forebrain? High-resolution analysis of the patterning of the zebrafish brain during axon tract formation. Dev Biol 229:271–286 [DOI] [PubMed] [Google Scholar]
- Hjorth J, Key B 2002. Development of axon pathways in the zebrafish central nervous system. Int J Dev Biol 46:609–619 [PubMed] [Google Scholar]
- Huang ZJ, Di Cristo G, Ango F 2007. Development of GABA innervation in the cerebral and cerebellar cortices. Nature Reviews Neuroscience 8:673–686 [DOI] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B 2008. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci 31:479–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving C, Malhas A, Guthrie S, Mason I 2002. Establishing the trochlear motor axon trajectory: role of the isthmic organiser and Fgf8. Development 129:5389–5398 [DOI] [PubMed] [Google Scholar]
- Islam SM, Shinmyo Y, Okafuji T, Su Y, Naser IB, Ahmed G, Zhang S, Chen S, Ohta K, Kiyonari H, et al. 2009. Draxin, a repulsive guidance protein for spinal cord and forebrain commissures. Science 323:388–393 [DOI] [PubMed] [Google Scholar]
- Itasaki N, Nakamura H 1996. A role for gradient en expression in positional specification on the optic tectum. Neuron 16:55–62 [DOI] [PubMed] [Google Scholar]
- Ito K, Kawasaki T, Takashima S, Matsuda I, Aiba A, Hirata T 2008. Semaphorin 3F confines ventral tangential migration of lateral olfactory tract neurons onto the telencephalon surface. J Neurosci 28:4414–4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MB, Kawasawa YI, Mason CE, Krsnik Z, Coppola G, Bogdanovic D, Geschwind DH, Mane SM, State MW, Sestan N 2009. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron 62:494–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Kara P, Reid RC, Shatz CJ 2003. Role of subplate neurons in functional maturation of visual cortical columns. Science 301:521–525 [DOI] [PubMed] [Google Scholar]
- Kastenhuber E, Kern U, Bonkowsky JL, Chien CB, Driever W, Schweitzer J 2009. Netrin-DCC, Robo-Slit, and heparan sulfate proteoglycans coordinate lateral positioning of longitudinal dopaminergic diencephalospinal axons. J Neurosci 29:8914–8926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Ito K, Hirata T 2006. Netrin 1 regulates ventral tangential migration of guidepost neurons in the lateral olfactory tract. Development 133:845–853 [DOI] [PubMed] [Google Scholar]
- Keeble TR, Cooper HM 2006. Ryk: a novel Wnt receptor regulating axon pathfinding. Int J Biochem Cell Biol 38:2011–2017 [DOI] [PubMed] [Google Scholar]
- Kiecker C, Lumsden A 2005. Compartments and their boundaries in vertebrate brain development. Nat Rev Neurosci 6:553–564 [DOI] [PubMed] [Google Scholar]
- Koester SE, O’Leary DD 1994. Axons of early generated neurons in cingulate cortex pioneer the corpus callosum. J Neurosci 14:6608–6620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolk SM, Gunput RA, Tran TS, van den Heuvel DM, Prasad AA, Hellemons AJ, Adolfs Y, Ginty DD, Kolodkin AL, Burbach JP, et al. 2009. Semaphorin 3F is a bifunctional guidance cue for dopaminergic axons and controls their fasciculation, channeling, rostral growth, and intracortical targeting. J Neurosci 29:12542–12557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Johansen T, Korzh V, Fjose A 1991. Expression pattern of zebrafish pax genes suggests a role in early brain regionalization. Nature 353:267–270 [DOI] [PubMed] [Google Scholar]
- Kulkarni RP, Bak-Maier M, Fraser SE 2007. Differences in protein mobility between pioneer versus follower growth cones. Proc Natl Acad Sci U S A 104:1207–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwada JY 1986. Cell recognition by neuronal growth cones in a simple vertebrate embryo. Science 233:740–746 [DOI] [PubMed] [Google Scholar]
- Kwan KY, Lam MM, Krsnik Z, Kawasawa YI, Lefebvre V, Sestan N 2008. SOX5 postmitotically regulates migration, postmigratory differentiation, and projections of subplate and deep-layer neocortical neurons. Proc Natl Acad Sci U S A 105:16021–16026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai T, Jabaudon D, Molyneaux BJ, Azim E, Arlotta P, Menezes JR, Macklis JD 2008. SOX5 controls the sequential generation of distinct corticofugal neuron subtypes. Neuron 57:232–247 [DOI] [PubMed] [Google Scholar]
- Lent R, Uziel D, Baudrimont M, Fallet C 2005. Cellular and molecular tunnels surrounding the forebrain commissures of human fetuses. J Comp Neurol 483:375–382 [DOI] [PubMed] [Google Scholar]
- Leone DP, Srinivasan K, Chen B, Alcamo E, McConnell SK 2008. The determination of projection neuron identity in the developing cerebral cortex. Curr Opin Neurobiol 18:28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillesaar C, Stigloher C, Tannhauser B, Wullimann MF, Bally-Cuif L 2009. Axonal projections originating from raphe serotonergic neurons in the developing and adult zebrafish, Danio rerio, using transgenics to visualize raphe-specific pet1 expression. J Comp Neurol 512:158–182 [DOI] [PubMed] [Google Scholar]
- Lin L, Rao Y, Isacson O 2005. Netrin-1 and slit-2 regulate and direct neurite growth of ventral midbrain dopaminergic neurons. Mol Cell Neurosci 28:547–555 [DOI] [PubMed] [Google Scholar]
- Little GE, Lopez-Bendito G, Runker AE, Garcia N, Pinon MC, Chédotal A, Molnar Z, Mitchell KJ 2009. Specificity and plasticity of thalamocortical connections in Sema6A mutant mice. PLoS Biol 7:e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Shi J, Lu CC, Wang ZB, Lyuksyutova AI, Song XJ, Zou Y 2005. Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat Neurosci 8:1151–1159 [DOI] [PubMed] [Google Scholar]
- Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW 2007. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450:56–62 [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Cautinat A, Sanchez JA, Bielle F, Flames N, Garratt AN, Talmage DA, Role LW, Charnay P, Marin O, et al. 2006. Tangential neuronal migration controls axon guidance: A role for neuregulin-1 in thalamocortical axon navigation. Cell 125:127–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden A, Keynes R 1989. Segmental patterns of neuronal development in the chick hindbrain. Nature 337:424–428 [DOI] [PubMed] [Google Scholar]
- Macdonald R, Xu Q, Barth KA, Mikkola I, Holder N, Fjose A, Krauss S, Wilson SW 1994. Regulatory gene expression boundaries demarcate sites of neuronal differentiation in the embryonic zebrafish forebrain. Neuron 13:1039–1053 [DOI] [PubMed] [Google Scholar]
- Marcus RC, Mason CA 1995. The first retinal axon growth in the mouse optic chiasm: axon patterning and the cellular environment. J Neurosci 15:6389–6402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus RC, Blazeski R, Godement P, Mason CA 1995. Retinal axon divergence in the optic chiasm: uncrossed axons diverge from crossed axons within a midline glial specialization. J Neurosci 15:3716–3729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marillat V, Cases O, Nguyen-Ba-Charvet KT, Tessier-Lavigne M, Sotelo C, Chédotal A 2002. Spatiotemporal expression patterns of slit and robo genes in the rat brain. J Comp Neurol 442:130–155 [DOI] [PubMed] [Google Scholar]
- Mastick GS, Davis NM, Andrew GL, Easter SS Jr 1997. Pax-6 functions in boundary formation and axon guidance in the embryonic mouse forebrain. Development 124:1985–1997 [DOI] [PubMed] [Google Scholar]
- McConnell SK, Ghosh A, Shatz CJ 1989. Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science 245:978–982 [DOI] [PubMed] [Google Scholar]
- McLean DL, Fetcho JR 2004. Ontogeny and innervation patterns of dopaminergic, noradrenergic, and serotonergic neurons in larval zebrafish. J Comp Neurol 480:38–56 [DOI] [PubMed] [Google Scholar]
- Mendes SW, Henkemeyer M, Liebl DJ 2006. Multiple Eph receptors and B-class ephrins regulate midline crossing of corpus callosum fibers in the developing mouse forebrain. J Neurosci 26:882–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metin C, Deleglise D, Serafini T, Kennedy TE, Tessier-Lavigne M 1997. A role for netrin-1 in the guidance of cortical efferents. Development 124:5063–5074 [DOI] [PubMed] [Google Scholar]
- Molle KD, Chédotal A, Rao Y, Lumsden A, Wizenmann A 2004. Local inhibition guides the trajectory of early longitudinal tracts in the developing chick brain. Mech Dev 121:143–156 [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD 2005. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron 47:817–831 [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD 2007. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci 8:427–437 [DOI] [PubMed] [Google Scholar]
- Mortimer D, Feldner J, Vaughan T, Vetter I, Pujic Z, Rosoff WJ, Burrage K, Dayan P, Richards LJ, Goodhill GJ 2009. Bayesian model predicts the response of axons to molecular gradients. Proc Natl Acad Sci U S A 106:10296–10301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Ito Y, Shirasaki R, Murakami F 2000. Local directional cues control growth polarity of dopaminergic axons along the rostrocaudal axis. J Neurosci 20:4112–4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naser IB, Su Y, Islam SM, Shinmyo Y, Zhang S, Ahmed G, Chen S, Tanaka H 2009. Analysis of a repulsive axon guidance molecule, draxin, on ventrally directed axon projection in chick early embryonic midbrain. Dev Biol 332:351–359 [DOI] [PubMed] [Google Scholar]
- Nguyen Ba-Charvet KT, Brose K, Marillat V, Kidd T, Goodman CS, Tessier-Lavigne M, Sotelo C, Chédotal A 1999. Slit2-Mediated chemorepulsion and collapse of developing forebrain axons. Neuron 22:463–473 [DOI] [PubMed] [Google Scholar]
- Nguyen-Ba-Charvet KT, Plump AS, Tessier-Lavigne M, Chédotal A 2002. Slit1 and slit2 proteins control the development of the lateral olfactory tract. J Neurosci 22:5473–5480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Ba-Charvet KT, von Boxberg Y, Guazzi S, Boncinelli E, Godement P 1998. A potential role for the OTX2 homeoprotein in creating early ‘highways’ for axon extension in the rostral brain. Development 125:4273–4282 [DOI] [PubMed] [Google Scholar]
- Niquille M, Garel S, Mann F, Hornung JP, Otsmane B, Chevalley S, Parras C, Guillemot F, Gaspar P, Yanagawa Y, et al. 2009. Transient neuronal populations are required to guide callosal axons: a role for semaphorin 3C. PLoS Biol 7:e1000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary DDM 2010. Topographic mapping: the visual system. Cold Spring Harb Perspect Biol 2:a001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros TJ, Rebsam A, Mason CA 2008. Retinal axon growth at the optic chiasm: to cross or not to cross. Annu Rev Neurosci 31:295–315 [DOI] [PubMed] [Google Scholar]
- Pierani A, Wassef M 2009. Cerebral cortex development: From progenitors patterning to neocortical size during evolution. Dev Growth Differ 51:325–342 [DOI] [PubMed] [Google Scholar]
- Pike SH, Melancon EF, Eisen JS 1992. Pathfinding by zebrafish motoneurons in the absence of normal pioneer axons. Development 114:825–831 [DOI] [PubMed] [Google Scholar]
- Piper M, Plachez C, Zalucki O, Fothergill T, Goudreau G, Erzurumlu R, Gu C, Richards LJ 2009. Neuropilin 1-Sema signaling regulates crossing of cingulate pioneering axons during development of the corpus callosum. Cereb Cortex 19:i11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires-Neto MA, Braga-De-Souza S, Lent R 1998. Molecular tunnels and boundaries for growing axons in the anterior commissure of hamster embryos. J Comp Neurol 399:176–188 [DOI] [PubMed] [Google Scholar]
- Plump AS, Erskine L, Sabatier C, Brose K, Epstein CJ, Goodman CS, Mason CA, Tessier-Lavigne M 2002. Slit1 and Slit2 cooperate to prevent premature midline crossing of retinal axons in the mouse visual system. Neuron 33:219–232 [DOI] [PubMed] [Google Scholar]
- Polleux F, Ince-Dunn G, Ghosh A 2007. Transcriptional regulation of vertebrate axon guidance and synapse formation. Nat Rev Neurosci 8:331–340 [DOI] [PubMed] [Google Scholar]
- Powell AW, Sassa T, Wu Y, Tessier-Lavigne M, Polleux F 2008. Topography of thalamic projections requires attractive and repulsive functions of Netrin-1 in the ventral telencephalon. PLoS Biol 6:e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochiantz A, Joliot A 2003. Can transcription factors function as cell-cell signalling molecules? Nat Rev Mol Cell Biol 4:814–819 [DOI] [PubMed] [Google Scholar]
- Puelles L, Rubenstein JL 2003. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci 26:469–476 [DOI] [PubMed] [Google Scholar]
- Pujic Z, Mortimer D, Feldner J, Goodhill GJ 2009. Assays for eukaryotic cell chemotaxis. Comb Chem High Throughput Screen 12:580–588 [DOI] [PubMed] [Google Scholar]
- Rash BG, Richards LJ 2001. A role for cingulate pioneering axons in the development of the corpus callosum. J Comp Neurol 434:147–157 [DOI] [PubMed] [Google Scholar]
- Richards LJ, Koester SE, Tuttle R, O’Leary DD 1997. Directed growth of early cortical axons is influenced by a chemoattractant released from an intermediate target. J Neurosci 17:2445–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer BM, Berbel P, Innocenti GM 2004. Neurons in the corpus callosum of the cat during postnatal development. Eur J Neurosci 19:2039–2046 [DOI] [PubMed] [Google Scholar]
- Sechrist J, Bronner-Fraser M 1991. Birth and differentiation of reticular neurons in the chick hindbrain: ontogeny of the first neuronal population. Neuron 7:947–963 [DOI] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M 1996. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87:1001–1014 [DOI] [PubMed] [Google Scholar]
- Shanmugalingam S, Houart C, Picker A, Reifers F, Macdonald R, Barth A, Griffin K, Brand M, Wilson SW 2000. Ace/Fgf8 is required for forebrain commissure formation and patterning of the telencephalon. Development 127:2549–2561 [DOI] [PubMed] [Google Scholar]
- Shu T, Richards LJ 2001. Cortical axon guidance by the glial wedge during the development of the corpus callosum. J Neurosci 21:2749–2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Butz KG, Plachez C, Gronostajski RM, Richards LJ 2003a. Abnormal development of forebrain midline glia and commissural projections in Nfia knock-out mice. J Neurosci 23:203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Li Y, Keller A, Richards LJ 2003b. The glial sling is a migratory population of developing neurons. Development 130:2929–2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Sundaresan V, McCarthy MM, Richards LJ 2003c. Slit2 guides both precrossing and postcrossing callosal axons at the midline in vivo. J Neurosci 23:8176–8184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Edwards MA, Levitt P 1993. Immunocytochemical demonstration of early appearing astroglial structures that form boundaries and pathways along axon tracts in the fetal brain. J Comp Neurol 328:415–436 [DOI] [PubMed] [Google Scholar]
- Silver J, Lorenz SE, Wahlsten D, Coughlin J 1982. Axonal guidance during development of the great cerebral commissures: descriptive and experimental studies, in vivo, on the role of preformed glial pathways. J Comp Neurol 210:10–29 [DOI] [PubMed] [Google Scholar]
- Smith KM, Ohkubo Y, Maragnoli ME, Rasin MR, Schwartz ML, Sestan N, Vaccarino FM 2006. Midline radial glia translocation and corpus callosum formation require FGF signaling. Nat Neurosci 9:787–797 [DOI] [PubMed] [Google Scholar]
- Sotelo C 2008. Development of “Pinceaux” formations and dendritic translocation of climbing fibers during the acquisition of the balance between glutamatergic and gamma-aminobutyric acidergic inputs in developing Purkinje cells. J Comp Neurol 506:240–262 [DOI] [PubMed] [Google Scholar]
- Sretavan DW, Pure E, Siegel MW, Reichardt LF 1995. Disruption of retinal axon ingrowth by ablation of embryonic mouse optic chiasm neurons. Science 269:98–101 [DOI] [PubMed] [Google Scholar]
- Steele-Perkins G, Plachez C, Butz KG, Yang G, Bachurski CJ, Kinsman SL, Litwack ED, Richards LJ, Gronostajski RM 2005. The transcription factor gene Nfib is essential for both lung maturation and brain development. Mol Cell Biol 25:685–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, Hensch TK 2008. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell 134:508–520 [DOI] [PubMed] [Google Scholar]
- Super H, Soriano E 1994. The organization of the embryonic and early postnatal murine hippocampus. II. Development of entorhinal, commissural, and septal connections studied with the lipophilic tracer DiI. J Comp Neurol 344:101–120 [DOI] [PubMed] [Google Scholar]
- Sur M, Rubenstein JL 2005. Patterning and plasticity of the cerebral cortex. Science 310:805–810 [DOI] [PubMed] [Google Scholar]
- Tomioka N, Osumi N, Sato Y, Inoue T, Nakamura S, Fujisawa H, Hirata T 2000. Neocortical origin and tangential migration of guidepost neurons in the lateral olfactory tract. J Neurosci 20:5802–5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Moll F, Moll J, de Oliveira-Souza R, Bramati I, Andreiuolo PA, Lent R 2007. Neuroplasticity in human callosal dysgenesis: a diffusion tensor imaging study. Cereb Cortex 17:531–541 [DOI] [PubMed] [Google Scholar]
- Uziel T, Zindy F, Xie S, Lee Y, Forget A, Magdaleno S, Rehg JE, Calabrese C, Solecki D, Eberhart CG, et al. 2005. The tumor suppressors Ink4c and p53 collaborate independently with Patched to suppress medulloblastoma formation. Genes Dev 19:2656–2667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel DM, Pasterkamp RJ 2008. Getting connected in the dopamine system. Prog Neurobiol 85:75–93 [DOI] [PubMed] [Google Scholar]
- Wahl M, Strominger Z, Jeremy RJ, Barkovich AJ, Wakahiro M, Sherr EH, Mukherjee P 2009. Variability of homotopic and heterotopic callosal connectivity in partial agenesis of the corpus callosum: a 3T diffusion tensor imaging and Q-ball tractography study. AJNR Am J Neuroradiol 30:282–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LC, Rachel RA, Marcus RC, Mason CA 1996. Chemosuppression of retinal axon growth by the mouse optic chiasm. Neuron 17:849–862 [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Toyoda R, Nakamura H 2004. Navigation of trochlear motor axons along the midbrain-hindbrain boundary by neuropilin 2. Development 131:681–692 [DOI] [PubMed] [Google Scholar]
- Williams SE, Mann F, Erskine L, Sakurai T, Wei S, Rossi DJ, Gale NW, Holt CE, Mason CA, Henkemeyer M 2003. Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron 39:919–935 [DOI] [PubMed] [Google Scholar]
- Wilson NH, Key B 2006. Neogenin interacts with RGMa and Netrin-1 to guide axons within the embryonic vertebrate forebrain. Dev Biol 296:485–498 [DOI] [PubMed] [Google Scholar]
- Wilson SW, Brennan C, Macdonald R, Brand M, Holder N 1997. Analysis of axon tract formation in the zebrafish brain: the role of territories of gene expression and their boundaries. Cell Tissue Res 290:189–196 [DOI] [PubMed] [Google Scholar]
- Wolman MA, Liu Y, Tawarayama H, Shoji W, Halloran MC 2004. Repulsion and attraction of axons by semaphorin3D are mediated by different neuropilins in vivo. J Neurosci 24:8428–8435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolman MA, Regnery AM, Becker T, Becker CG, Halloran MC 2007. Semaphorin3D regulates axon axon interactions by modulating levels of L1 cell adhesion molecule. J Neurosci 27:9653–9663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR 2008. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature 451:465–469 [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Mizushima S, Tamada A, Yamamoto N, Takashima S, Murakami F 2009. FGF8 signaling regulates growth of midbrain dopaminergic axons by inducing semaphorin 3F. J Neurosci 29:4044–4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JQ, Felder M, Connor JA, Poo MM 1994. Turning of nerve growth cones induced by neurotransmitters. Nature 368:140–144 [DOI] [PubMed] [Google Scholar]