Abstract
The pregnancy-associated plasma protein-A knockout (PAPP-A KO) mouse is a model of reduced local insulin-like growth factor (IGF)-I activity with normal circulating IGF-I levels. In this study, PAPP-A KO mice had significantly increased mean (27%), median (27%), and maximum (35%) life span compared with wild-type (WT) littermates. End-of-life pathology indicated that the incidence of neoplastic disease was not significantly different in the two groups of mice; however, it occurred in older aged PAPP-A KO compared with WT mice. Furthermore, PAPP-A KO mice were less likely to show degenerative changes of age. Scheduled pathologies at 78, 104, and 130 weeks of age indicated that WT mice, in general, had more degenerative changes and tumors earlier than PAPP-A KO mice. This was particularly true for abnormalities in heart, testes, brain, kidney, spleen, and thymus. In summary, the major contributors to the extended life span of PAPP-A KO mice are delayed occurrence of fatal neoplasias and decreased incidence of age-related degenerative changes.
Keywords: Longevity, Pathology, Pregnancy-associated plasma protein-A, Insulin-like growth factor I, Mouse model
PREGNANCY-ASSOCIATED plasma protein-A (PAPP-A) is a zinc metalloproteinase that enhances insulin-like growth factor (IGF) bioactivity through degradation of inhibitory IGF-binding proteins in the pericellular microenvironment in vitro and in vivo (1,2). Conversely, inhibition or loss of PAPP-A suppresses IGF-I receptor-mediated action without altering IGF or IGF-I receptor expression (3–5). Characterization of the PAPP-A knockout (KO) mouse indicated its value as a model of local suppression of IGF activity (5,6). We recently reported that PAPP-A KO mice have life-span extension of ∼30% compared with WT littermates, with no reduction in food intake, secondary endocrine abnormalities, or altered metabolism (7,8). By gross inspection at end-of-life necropsy, these animals had an apparent reduction in incidence and burden of spontaneous tumors (7). So what do PAPP-A KO mice die of? The studies herein were designed to answer this important question and thereby shed light on the role of autocrine and paracrine distinct from endocrine regulation of aging by IGF-I.
MATERIALS AND METHODS
Mice
Mice with the targeted deletion of the Pappa gene were generated through homologous recombination in embryonic stem cells; these Pappa−/− (PAPP-A KO) mice are on a mixed C57BL/6 and 129Sv/E background (5). WT and PAPP-A KO littermates from heterozygous breedings were used in these studies. Genotyping was performed by polymerase chain reaction as described previously (6). WT and PAPP-A KO mice were divided into two groups: a Longevity group to determine the effect of PAPP-A gene deletion on mortality parameters and end-of-life pathology and a Scheduled Sacrifice group for direct comparison of WT and PAPP-A KO pathology at specific chronological ages (78, 104, and 130 weeks). Genotypes were confirmed with tail DNA collected at the end of the experiment. Mice in the Longevity group were housed (up to 5 per cage, separated by sex but not by genotype) in a specific pathogen-free (SPF) barrier facility. Before entering, personnel don sterile gowns, caps, booties, and chemically sanitized gloves. Mice for the Scheduled Sacrifice pathology were housed in a general clean room (up to 5 per cage, separated by sex but not by genotype) and only handled by gowned and gloved personnel in a laminar flow hood. Caging in both the barrier and the general housing rooms was Allentown 67 square inch polysulfone static microisolator “shoebox” caging (Allentown Caging Equipment Co., Inc., Allentown, NJ) with aspen wood chip bedding (Sani-Chips; P.J. Murphy Forest Products, Montville, NJ). In the barrier, caging, bedding, and water bottles were autoclaved after being sanitized by washing at 180°F for 3 minutes. In the general housing rooms, the caging and water bottles were sanitized by washing at 180°F for 3 minutes but not autoclaved. In the barrier, sterilized potable tap water was provided in autoclaved polysulfone water bottles with shoulder holes. Mouse rooms at both sites had an automated 12-h light/dark cycle. Nesting material (Nestlets; AnCare Corp., Bellmore, NY) was added as environmental enrichment to the cages. Mice were fed ad libitum irradiated diet (PicoLab diet 20 [5053]; PMI Nutrition International, Richmond, IN) containing 5% fat and 20% protein. Sentinel mice in both barrier and general housing rooms were present on each side of each rack and evaluated via serology for Sendai virus, pneumonia virus of mice, mouse hepatitis virus, minute virus of mice, Theiler murine encephalomyelitis virus, reovirus 3, Mycoplasma pulmonis, lymphocytic choriomeningitis virus, Ectromelia, K virus, polyoma virus, mouse adenovirus type 1, mouse adenovirus type 2, enzootic diarrhea of infant mice, mouse cytomegalovirus, hantavirus, Encephalitozoon cuniculi, cilia-associated respiratory bacillus, mouse parovirus, and mouse lymphotropic virus. Sentinel evaluation also included quarterly testing for ectoparasites by pelage examination and for endoparasites (pinworms) by anal tape test, fecal flotation, and cecal content examination. All such tests were negative throughout the study. All procedures were approved by the Institutional Animal Care and Use Committee of Mayo Clinic.
Necropsy Methods
Mice were examined daily, including weekends and holidays. For the Longevity group, mice were considered to be at end of life and euthanized by carbon dioxide inhalation according to American Veterinary Medical Association guidelines when they were moribund and demonstrated one or more clinical signs suggesting imminent death: nonresponsive to being touched, labored breathing, failure to eat or drink. Mice found dead in cage were also submitted for necropsy. Liver, kidney, spleen, thymus (if detectable), colon, heart, lung plus any visible abnormalities were preserved in 10% neutral phosphate-buffered formalin. Fixed tissues were embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin–eosin at the Small Animal Histology Core at Mayo Clinic, Scottsdale, Arizona.
Whole-body formalin fixation was used for mice in the Scheduled Sacrifice group. After euthanasia by carbon dioxide inhalation, brain, thoracic, and abdominal cavities were exposed and lung, stomach, and small and large intestines were flushed with 10% formalin and then placed in a container of formalin. Bodies were fixed for several weeks prior to dissection and processing by standard operating procedures at Charles River Laboratories, Frederick, Maryland.
Pathology
Longevity group.—
Tissues were examined microscopically and assessed by an American College of Veterinary Pathologists (ACVP) board-certified veterinary pathologist (R.J.M.) as to probable cause of morbidity/mortality. The determination was based on the pathology in the tissue sections, degree of tissue involvement, severity of the changes, and whether the effects would be expected to contribute or lead to the death of the animal. Comorbidity was recorded if more than one histopathologic change could be a possible contributory cause of death.
Scheduled sacrifice group.—
The following tissues were examined histologically by an ACVP board-certified veterinary pathologist (K.P.K): adrenals, aorta, femur, sternum, brain, cecum, colon, duodenum, esophagus, eyes, heart, ileum, jejunum, kidneys, liver, lungs, ovary, pancreas, pituitary, prostate, rectum, salivary gland, seminal vesicles, skeletal muscle, skin, spinal cord, spleen, stomach, testes, thymus, thyroid, trachea, and urinary bladder. The histopathology definitions for the major lesions and tumors observed in this study were based on standard descriptions and terminology currently as described in standard texts and guidelines. Most of the nontumor lesions were given one of four subjective grades of minimal, mild, moderate, and marked where appropriate. The minimal grade represents a very small amount of the given organ affected by the change and the marked grade indicates a very large amount of a given organ affected. For hyperplastic lesions, grades are based on the extent of the organ involvement and/or the number of foci observed depending on the morphological change. For tumors, the organ-specific diagnosis is accompanied by modifiers that may be descriptive and then an indication if the tumor is benign or malignant and, if malignant, whether it is a primary or secondary (metastasis) tumor. All microscopic pathology findings for the Scheduled Sacrifice group were directly entered into a validated pathology computer program Provantis NT 2000, Data Management System.
Serum IGF-I
Blood was collected by cardiac puncture at the times of scheduled pathology (78, 104, and 130 weeks) for measurement of serum IGF-I levels using a rat/mouse IGF-I two-site immunoenzymatic assay kit from Immunodiagnostic Systems (Fountain Hills, AZ). Intra- and interassay variation is 8%. All samples for a particular age were run in duplicate within the same assay.
Statistical Analyses
Kaplan–Meier survival curves were compared using a log-rank test. Fisher’s exact text was used to compare proportions of mice between groups. Student’s t test was used to compare serum IGF-I levels in PAPP-A KO and WT mice. The p value <.05 was considered statistically significant.
RESULTS
Longevity
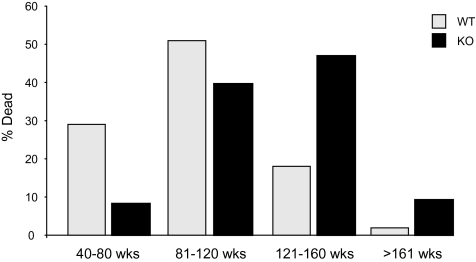
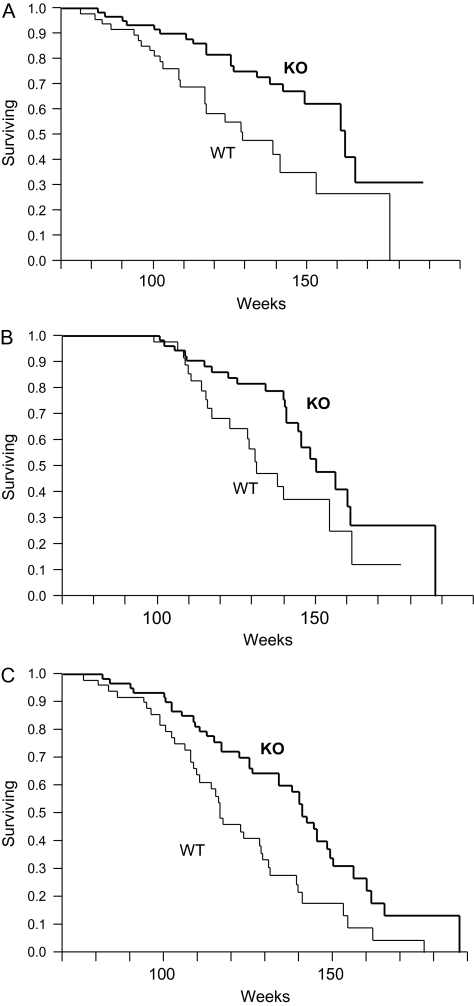
Survival data were collected on 78 PAPP-A KO mice (38 females) and 95 WT mice (50 females) that were housed in an SPF barrier facility throughout their life. Longevity was significantly increased in PAPP-A KO mice (p < .0001; Figure 1A) even when analyzed separately for females (p < .0001; Figure 1B) and males (p = .0042; Figure 1C). Mean, median, and maximum life spans are summarized in Table 1. Mean and median life spans were increased 27% in PAPP-A KO mice (p < .0001). Maximum life span was increased 35% in PAPP-A KO mice (p = .0002). There was no significant difference in longevity (by log-rank test) between females and males, either WT or PAPP-A KO mice, and subsequent data were pooled for analyses. Mortality rates of life-span quartiles indicate that deaths of PAPP-A KO mice were lower than WT at young adult ages and higher than WT at older ages. This distribution shift (Figure 2) was highly significant (p < .0001).
Figure 1.
Survival distribution of PAPP-A KO (thick lines) and WT (thin lines) mice. (A) All mice, (B) females only, and (C) males only. Data are from 78 PAPP-A KO mice (38 females) and 95 WT mice (50 females). Notes: PAPP-A KO = pregnancy-associated plasma protein-A knockout; WT = wild type.
Table 1.
Longevity Analyses
| Weeks |
|||
| Life Span | WT | KO | % Increase |
| All mice | |||
| Mean | 96 | 122 | 27 |
| Median | 97 | 123 | 27 |
| Maximum | 101 | 136 | 35 |
| Female mice | |||
| Mean | 96 | 124 | 29 |
| Median | 96 | 126 | 33 |
| Maximum | 102 | 141 | 38 |
| Male mice | |||
| Mean | 97 | 121 | 25 |
| Median | 97 | 116 | 20 |
| Maximum | 110 | 134 | 22 |
Note: These analyses were calculated from the survival data in Figure 1 on 95 WT mice (50 females) and 78 PAPP-A KO mice (38 females). KO = knockout; WT = wild type.
Figure 2.
Age-specific mortality. Life-span quartiles of WT (light gray bars) and PAPP-A KO (black bars) mice (see Figure 1). Notes: PAPP-A KO = pregnancy-associated plasma protein-A knockout; WT = wild type.
Although life-span data on these larger group sizes allowed analysis of survival curves in more detail, information on pathology was necessary to determine likely cause of death and progression of specific pathological lesions in PAPP-A KO compared with WT mice.
End-of-Life Pathology
Despite daily checks for moribund animals, a large proportion of mice were found dead in cage with tissues too autolyzed to permit satisfactory pathological analyses. Thus, the terminal pathology reports were on 47 WT mice (25 females) and 60 PAPP-A KO mice (29 females).
Summary of probable cause of death based on histopathology is presented in Table 2. When no disease was considered severe enough to terminate the animal’s life, the probable cause of death was categorized as “undetermined.” Approximately 30% of PAPP-A KO mice and 6% of WT mice died without such histological evidence of lethal pathological changes (p = .014). Hematopoietic (HP) tumors, including lymphomas, leukemia, and histiocytic sarcomas, were a major cause of death regardless of genotype. Presumably fatal non-HP neoplastic diseases (hemangioma/hemangiosarcoma of the liver and spleen, hepatocellular carcinoma, and bronchioalveolar carcinoma of the lung) were also similar in PAPP-A KO and WT mice. Other contributing non-HP neoplasias (mammary gland adenocarcinoma, ovarian granulosa cell tumor, and endometrial stromal tumor) were identified in WT mice but not in PAPP-A KO mice (p = .042). Although most animals in this study had neoplasia at end of life, PAPP-A KO mice had significantly delayed occurrence of presumably fatal neoplastic disease compared with WT siblings. As shown in Figure 3, this delay was evident when assessed for HP (p = .004), non-HP (p = .026), and all neoplasias (p = .002). Thus, changes in incidence of fatal disease did not seem to be a major contributor to extended life span. Rather, fatal neoplastic disease occurred in older aged PAPP-A KO mice compared with WT littermates.
Table 2.
Probable Cause-of-Death Histopathology
| WT | KO | p | |
| Undetermined* | 3 (6) | 18 (30) | .014 |
| HP neoplastic disease† | 23 (49) | 20 (33) | .366 |
| Non-HP neoplasic disease | |||
| Hemangioma/hemangiosarcoma | 4 (9) | 2 (3) | .407 |
| Hepatocellular carcinoma | 4 (9) | 10 (17) | .390 |
| Bronchioalveolar carcinoma | 8 (17) | 14 (23) | .638 |
| Other‡ | 4 (9) | 0 | .042 |
| Degenerative disease | |||
| Atrial thrombosis | 5 (11) | 0 | .019 |
| Nephropathy | 3 (6) | 0 | .091 |
| Other§ | 9 (13) | 1 (2) | .007 |
| Comorbidities‖ | 15 (32) | 6 (10) | .030 |
Notes: Tissues from mice in the Longevity group ([n = 47 WT mice [25 females] and 60 PAPP-A KO mice [29 females]) were examined histologically and assessed by R.J.M. for probable cause of death. Data are presented as number of mice affected (% given in parentheses). HP = hematopoietic; KO = knockout; WT = wild type.
No disease found to be severe enough to cause death.
HP tumors including lymphomas, leukemia, and histiocytic sarcoma.
Mammary gland adenocarcinoma, ovarian granulosa cell tumor, and endometrial stroma tumor.
Ectasia, heart, lung and ovarian cyst hemorrhage, spleen and liver thrombi, liver necrosis, hydronephrosis, and amyloidosis.
Co-occurring contributing lesions per mouse.
Figure 3.
Age distribution of presumably fatal neoplastic disease in PAPP-A KO (thick lines) and WT (thin lines) mice. (A) HP neoplasias, (B) non-HP neoplasias, and (C) all neoplasias. Notes: PAPP-A KO = pregnancy-associated plasma protein-A knockout; WT = wild type; HP= hematopoietic; KO = knockout.
As also indicated in Table 2, PAPP-A KO mice were less likely to show degenerative changes of age even though the average age at death was higher. The major presumptive fatal degenerative diseases in WT mice were thrombosis in heart and chronic nephropathy in kidney. In contrast, these degenerative changes were not found as contributory lesions in any of the PAPP-A KO mice. As a group, other degenerative changes associated with aging (ectasia, heart, lung and ovarian cyst hemorrhage, spleen and liver thrombi, liver necrosis, hydronephrosis, and amyloid) were significantly higher in WT than in PAPP-A KO mice (p = .007). Comorbidities, that is, co-occurring contributory lesions per mouse, were significantly reduced in PAPP-A KO mice (p = .030).
Scheduled Sacrifice Pathology
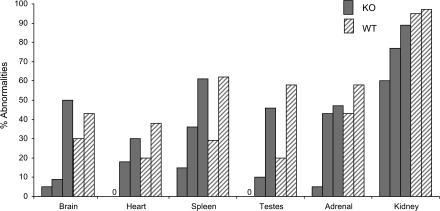
Pathological analyses of WT and PAPP-A KO mice were performed at 78, 104, and 130 weeks of age. Table 3 provides a simplified overview of the tissue-specific findings presented as noted abnormalities in each tissue expressed as percentage of the number of mice evaluated in each group. In general, the WT mice appeared to have more degenerative changes and tumors at the 78- and 104-week time points than the PAPP-A KO mice. At 130 weeks, only four male and three female WT mice were available for evaluation, so the data are provided but not included in further analyses. The lesions and tumors seen in the more numerous PAPP-A KO mice at 130 weeks probably reflect their greater survival and delayed onset of aging changes rather than a real increase in lesion or tumor incidence. In particular, heart, testis, brain, and spleen showed markedly reduced abnormalities in PAPP-A KO compared with WT mice at 78 and 104 weeks. Abnormalities in kidney, pituitary gland, stomach, adrenal gland, and bladder also appeared reduced in PAPP-A KO versus WT mice when compared at 78 weeks. Liver, lung, salivary gland, ovary, uterus, and bone had high percentage of abnormalities noted in both groups of mice. Esophagus, eye, intestine, skeletal muscle, pancreas, prostate gland, seminal vesicles, skin, spinal cord, thyroid, aorta, trachea, and mammary gland had low and/or variable incidence of abnormalities, especially at 78 and 104 weeks. Figure 4 illustrates delayed progression of disease burden (% abnormalities) in brain, heart, spleen, testes, adrenal, and kidney of PAPP-A KO mice compared with WT mice. For WT mice, only 78 and 104 weeks are used. Brain and testes of PAPP-A KO mice exhibited the most pronounced delay, with disease burden at 104 weeks remaining markedly below WT at 78 weeks; disease burden at 130 weeks was equivalent to WT at 104 weeks. A similar pattern was seen with spleen and kidney. Disease burden of PAPP-A KO heart and adrenal was less than WT at 78 weeks, but at 104 weeks, it was similar to WT at 78 weeks.
Table 3.
Overview of Scheduled Sacrifice Pathology
| Abnormalities* |
||||||
| 78 wk |
104 wk |
130 wk |
||||
| WT | KO | WT | KO | WT | KO | |
| Apparent differences at 78 and 104 wk | ||||||
| Heart | 20 | 0 | 38 | 18 | 43 | 30 |
| Testis | 20 | 0 | 58 | 10 | 50 | 46 |
| Brain | 30 | 5 | 41 | 9 | 43 | 50 |
| Spleen | 29 | 15 | 62 | 36 | 71 | 61 |
| Apparent differences at 78 wk | ||||||
| Kidney | 95 | 60 | 97 | 77 | 100 | 89 |
| Pituitary gland | 11 | 5 | 14 | 9 | 20 | 5 |
| Stomach | 15 | 5 | 14 | 14 | 57 | 25 |
| Adrenal | 43 | 5 | 58 | 43 | 50 | 47 |
| Bladder | 30 | 21 | 38 | 32 | 86 | 50 |
| High % abnormalities | ||||||
| Liver | 62 | 65 | 79 | 77 | 100 | 96 |
| Lung | 71 | 50 | 69 | 63 | 100 | 86 |
| Salivary gland | 45 | 46 | 55 | 41 | 57 | 54 |
| Ovary | 90 | 90 | 100 | 100 | 100 | 100 |
| Uterus | 80 | 80 | 100 | 100 | 100 | 100 |
| Sternum | 35 | 37 | 38 | 14 | 57 | 54 |
| Femur | 65 | 63 | 45 | 18 | 43 | 67 |
| Bone marrow | —† | — | 66 | 50 | 100 | 100 |
| Low or variable incidence abnormalities | ||||||
| Esophagus | 0 | 0 | 3 | 0 | 0 | 0 |
| Eye | 40 | 16 | 7 | 9 | 0 | 33 |
| Intestine | 0 | 0 | 14 | 5 | 14 | 4 |
| Skeletal muscle | 0 | 5 | 0 | 5 | 0 | 0 |
| Pancreas | 0 | 0 | 5 | 0 | 61 | 14 |
| Prostate gland | 0 | 12 | 0 | 12 | 50 | 100 |
| Seminal vesicles | 0 | 0 | 0 | 12 | 25 | 8 |
| Skin | 15 | 5 | 10 | 5 | 14 | 7 |
| Spinal cord | 0 | 0 | 10 | 13 | 14 | 0 |
| Thyroid | 5 | 11 | 31 | 0 | 40 | 12 |
| Aorta | 5 | 0 | 11 | 0 | 0 | 14 |
| Trachea | 0 | 0 | 3 | 4 | 0 | 0 |
| Mammary gland | 14 | 0 | 3 | 0 | — | 0 |
Notes: Tissues from mice in the Scheduled Sacrifice group were examined histologically by K.P.K. KO = knockout; WT = wild type.
The data are presented as a summary of all abnormalities noted in individual tissues/organs, expressed as percentage of both sexes of mice evaluated in each group.
Insufficient number available for analysis.
Figure 4.
Lesion progression. PAPP-A KO (solid gray bars) and WT (hatched gray bars) mice in the following order: PAPP-A KO 78, 104, and 130 weeks and WT 78 and 104 weeks (see Table 3). Notes: PAPP-A KO = pregnancy-associated plasma protein-A knockout; WT = wild type; 0 = no abnormalities.
Table 4 presents more detailed information on tissues of particular interest at 78 and 104 weeks. In the hearts of the WT mice, a higher incidence of cardiomyopathy (myocyte degeneration, mixed cellular infiltrates, and interstitial fibrosis; Supplementary Figure 1) was noted, suggesting an earlier onset of age-related cardiovascular disease. In the kidneys of the WT mice, there was a higher incidence and severity of chronic nephropathy (multifocal areas of glomerulosclerosis, tubular dilation and basophilia, interstitial infiltrates and fibrosis, and basement membrane thickening; Supplementary Figure 2). In the lungs of both WT and PAPP-A KO mice, an unusually high incidence of chronic bronchioalveolar inflammation, foamy alveolar macrophages, and eosinophilic crystals was noted and was frequently associated with abundant eosinophilic alveolar fluid. These lesions were not associated with severe cardiovascular changes and did not appear to be age related. In the testes of the WT mice, an increased incidence of seminiferous tubule atrophy (age-related degeneration; Supplementary Figure 3) was noted compared with PAPP-A KO mice at similar age. The female WT and PAPP-A KO mice had ovarian atrophy and cystic endometrial hyperplasia that appeared to be more severe in WT mice at 78 and 104 weeks, suggesting an earlier onset of reproductive senescence in the WT females. In the endocrine organs, the WT mice had occasional thyroid follicular cell hyperplasias, pancreatic islet cell hyperplasia, pituitary adenomas and focal hyperplasia of the pars distalis, and a higher incidence of adrenal subcapsular cell hyperplasias relative to PAPP-A KO mice. However, these hyperplasias were of overall low incidence in small groups of WT mice. In the thymus of WT mice at 78 weeks, an increased incidence and severity of thymic involution (atrophy) was observed. This involution resulting in progressive loss of thymocytes and collapse of thymic structure has been reported to be delayed in the PAPP-A KO mice of this stock (9). The observations at 104 weeks in the present study were limited because of the large number of missing thymi in the slides submitted for evaluation, consistent with massive involution in both genotypes at late age. Observations in the spleen were generally secondary responses to chronic blood loss or anemia in other organs (ie, increased hematopoiesis and hemosiderin) or immune stimulation (lymphoid hyperplasia). Additional age-related lesions and tumors in this study were of a similar incidence between WT and PAPP-A KO groups and typical of those seen in the background strains of these stocks. (Further details are available upon request from the corresponding author.)
Table 4.
Scheduled Sacrifice Pathology
| 78 wk |
104 wk |
|||
| WT | KO | WT | KO | |
| Heart | ||||
| Number of examined | 20 | 19 | 29 | 22 |
| WNL | 16 (80) | 19 (100) | 18 (62) | 18 (82) |
| Cardiomyopathy | 3 (15) | 0 | 9 (31) | 3 (14) |
| Inflammation, chronic active, coronary artery | 1 (5) | 0 | 3 (10) | 0 |
| Moderate | 1 | 0 | 2 | 0 |
| Marked | 0 | 0 | 1 | 0 |
| Mineralization | 0 | 0 | 1 (3) | 0 |
| HP malignancy, metastasis | 0 | 0 | 1 | 0 |
| Kidney | ||||
| Number of examined | 20 | 19 | 29 | 22 |
| WNL | 1 (5) | 7 (37) | 1 (3) | 5 (23) |
| Nephropathy, chronic | 15 (75) | 9 (47) | 28 (97) | 6 (27) |
| Minimal | 11 | 7 | 10 | 4 |
| Mild | 4 | 2 | 17 | 2 |
| Moderate | 0 | 0 | 1 | 0 |
| Hydronephrosis | 2 (10) | 2 (10) | 3 (10) | 4 (18) |
| Inflammation, chronic active, artery | 0 | 0 | 2 (7) | 1 |
| Mineralization | 5 (24) | 0 | 13 (45) | 1 (5) |
| Cyst | 0 | 0 | 2 (7) | 0 |
| Vacuolation, tubular | 6 (30) | 2 (11) | 9 (31) | 0 |
| Minimal | 4 | 2 | 4 | 0 |
| Mild | 2 | 0 | 4 | 0 |
| Moderate | 0 | 0 | 1 | 0 |
| Hyaline droplets, tubular | 1 (5) | 0 | 0 | 0 |
| Infiltration, mononuclear cell | 1 (5) | 0 | 0 | 6 (27) |
| HP malignancy, metastasis | 2 (10) | 0 | 6 (21) | 3 (14) |
| Lungs | ||||
| Number of examined | 20 | 19 | 29 | 22 |
| WNL | 5 (25) | 9 (47) | 9 (31) | 8 (36) |
| Infiltration | 12 (57) | 10 (50) | 20 (69) | 11 (50) |
| Inflammation | 13 (62) | 5 (25) | 8 (28) | 8 (36) |
| Crystals, eosinophilic | 10 (50) | 4 (21) | 10 (34) | 8 (36) |
| Hemorrhage | 0 | 1 (5) | 1 (3) | 0 |
| Hyperplasia | 0 | 0 | 2 (7) | 1 (5) |
| Mineralization | 1 (5) | 0 | 0 | 0 |
| Bronchio-alveolar adenoma | 4 (19) | 2 (10) | 3 (10) | 1 (5) |
| Bronchioalverolar adenocarcinoma | 1 (5) | 1 (5) | 1 (3) | 1 (5) |
| HP-malignancy, metastasis | 1 (5) | 0 | 6 (21) | 2 (9) |
| Testes | ||||
| Number of examined | 10 | 9 | 14 | 10 |
| WNL | 8 (80) | 9 (100) | 6 (43) | 9 (90) |
| Atrophy, seminiferous tubule | 2 (20) | 0 | 7 (50) | 1 (10) |
| Minimal | 2 | 0 | 5 | 1 |
| Mild | 0 | 0 | 1 | 0 |
| Moderate | 0 | 0 | 1 | 0 |
| Mineralization, seminiferous tubule | 0 | 0 | 2 (14) | 1 (10) |
| Hyperplasia, interstitial cell | 1 (10) | 0 | 0 | 0 |
| Sperm granuloma | 0 | 0 | 1 (7) | 0 |
| Ovary | ||||
| Number of examined | 10 | 10 | 15 | 11 |
| WNL | 1 (10) | 1 (10) | 0 | 0 |
| Atrophy | 9 (90) | 9 (90) | 14 (93) | 11 (100) |
| Mild | 1 | 5 | 0 | 0 |
| Moderate | 7 | 2 | 8 | 6 |
| Marked | 1 | 2 | 6 | 5 |
| Cyst, bursa | 1 (10) | 0 | 1 (10) | 1 (9) |
| Cyst, epithelial | 3 (30) | 1 (10) | 5 (33) | 1 (9) |
| Mild | 1 | 1 | 5 | 1 |
| Moderate | 1 | 0 | 0 | 0 |
| Marked | 1 | 0 | 0 | 0 |
| Angiectasis | 0 | 0 | 1 (7) | 0 |
| HP malignancy, metastasis | 0 | 0 | 8 (33) | 1 (9) |
| Granulosa cell tumor | 0 | 0 | 1 (7) | 0 |
| Uterus | ||||
| Number of examined | 10 | 10 | 15 | 12 |
| WNL | 2 (20) | 2 (20) | 0 | 0 |
| Hyperplasia, cystic, endometrial | 7 (70) | 5 (50) | 13 (87) | 10 (83) |
| Minimal | 2 | 4 | 1 | 2 |
| Mild | 5 | 1 | 5 | 3 |
| Moderate | 0 | 0 | 3 | 5 |
| Marked | 0 | 0 | 4 | 0 |
| Atrophy | 1 (10) | 3 (30) | 0 | 2 (17) |
| HP malignancy, metastasis | 0 | 0 | 3 (20) | 0 |
| Thyroid | ||||
| Number of examined | 20 | 18 | 26 | 21 |
| WNL | 19 (95) | 16 (88) | 20 (77) | 21 (100) |
| Hyperplasia, follicular cell | 1 (5) | 0 | 2 (8) | 0 |
| Inflammation | 0 | 0 | 3 (12) | 0 |
| Cysts | 0 | 0 | 1 (4) | 0 |
| Pancreas | ||||
| Number of examined | 19 | 18 | 18 | 22 |
| WNL | 18 (100) | 18 (100) | 19 (70) | 18 (82) |
| Atrophy, acinar cell | 0 | 0 | 1 (4) | 0 |
| Hyperplasia | 0 | 0 | 2 (7) | 0 |
| HP malignancy, metastasis | 0 | 0 | 3 (11) | 3 (14) |
| Pituitary | ||||
| Number of examined | 18 | 14 | 18 | 9 |
| WNL | 16 (89) | 13 (93) | 15 (83) | 7 (78) |
| Hyperplasia, pars distalis | 1 (6) | 0 | 1 (6) | 0 |
| Adenoma | 1 (6) | 0 | 1 (6) | 0 |
| Adrenal | ||||
| Number of examined | 20 | 19 | 29 | 21 |
| WNL | 11 (55) | 18 (95) | 11 (42) | 12 (57) |
| Hyperplasia, subcapsular cells | 8 (40) | 1 (5) | 17 (65) | 7 (33) |
| Minimal | 6 | 1 | 13 | 5 |
| Mild | 2 | 0 | 4 | 2 |
| Angiectasis | 0 | 0 | 1 (3) | 0 |
| HP malignancy, metastasis | 0 | 0 | 3 (10) | 0 |
| Thymus | ||||
| Number of examined | 20 | 19 | 29 | 22 |
| Not present on slide | 3 (15) | 2 (11) | 13 (45) | 11 (50) |
| WNL | 5 (29) | 9 (53) | 3 (19) | 0 |
| Involution | 11 (65) | 4 (24) | 4 (25) | 5 (62) |
| Minimal | 4 | 2 | 0 | 0 |
| Mild | 4 | 1 | 1 | 2 |
| Moderate | 3 | 1 | 3 | 3 |
| Spleen | ||||
| Number of examined | 20 | 19 | 29 | 22 |
| WNL | 14 (70) | 16 (84) | 11 (38) | 14 (64) |
| Extramedullary hematopoiesis, increased | 4 (20) | 3 (16) | 6 (21) | 3 (14) |
| Pigmentation, hemosiderin | 3 (15) | 0 | 2 (7) | 3 (14) |
| Hyperplasia, lymphoid | 0 | 0 | 4 (14) | 1 (5) |
| Angiectasis | 0 | 0 | 1 (3) | 0 |
| Thrombosis | 0 | 0 | 1 (3) | 0 |
| HP malignancy, metastasis | 1 (5) | 0 | 5 (17) | 3 (14) |
| Systemic HP neoplasia | ||||
| Number of examined | 20 | 19 | 29 | 22 |
| WNL | 19 (95) | 19 (100) | 20 (69) | 19 (86) |
| Lymphoma, all types | 0 | 0 | 9 (31) | 3 (14) |
| Histiocytic sarcoma | 1 (5) | 0 | 0 | 0 |
Notes: Tissues from mice in the Scheduled Sacrifice group were examined histologically by K.P.K. (see Table 3). Results are presented as number of mice with observed pathology in each group with percent of mice in parentheses. Individual mice may have more than one type of pathology. Severity was graded as minimal, mild, moderate, and marked (see Methods section). KO = knockout; WNL = within normal limits; WT = wild type; HP = hematopoietic.
Blood was collected from mice at the time of sacrifice (78, 104, and 130 weeks) for measurement of serum IGF-I. As shown in Table 5, serum IGF-I levels did not differ significantly between WT and PAPP-A KO mice at any of the ages tested.
Table 5.
Serum IGF-I Levels
| IGF-I (ng/mL) |
|||
| 78 wk | 104 wk | 130 wk | |
| Male | |||
| WT | 239 ± 9 | 420 ± 29 | 460 ± 22 |
| KO | 237 ± 19 | 358 ± 42 | 427 ± 16 |
| Female | |||
| WT | 298 ± 17 | 508 ± 30 | 368 ± 36 |
| KO | 300 ± 8 | 422 ± 31 | 433 ± 28 |
Note: Results are mean ± SEM, n = 6–10 per group. KO = knockout; WT = wild type.
DISCUSSION
The extensive pathology performed for this study clearly indicates that the increased life span of PAPP-A KO mice is associated with reduced incidence and/or delayed occurrence of various age-related diseases in multiple tissues. These findings are important because they shed light on mechanisms of autocrine and paracrine separate from endocrine regulation of aging by IGF-I and suggest that PAPP-A might serve as a broad-spectrum preventative target for tumors and diseases of aging.
Longevity
PAPP-A KO mice had highly significant increases in life span compared with WT mice, confirming a previous report on a relatively small number of mice (7). The larger group sizes in this study allowed analysis of survival curves in more detail. When analyzed separately, life span was significantly extended in both female and male PAPP-A KO mice. Median life span was increased 32% in female mice and 20% in male mice. Maximum life span was increased 38% in female mice and 21% in male mice. There was no significant difference in life span between female and male mice in our study. In comparison, a significant longevity phenotype in females compared with males has been reported for growth hormone (GH)-deficient Ames and Snell mice and heterozygous IGF-I receptor mutant mice (10–12). The increase in maximum life span and shift in age-related mortality indicate significantly enhanced longevity in PAPP-A KO mice. Although the median life span (96 weeks) of WT mice on a mixed C57BL/6 and 129Sv/E background in this study is shorter than that reported for WT mice on the C57BL/6 background, it is consistent with previous studies. Moreover, the C57BL/6 background is considered one of the longer lived strains, whereas the 129/Sv background is a much shorter living strain (13).
Reduced serum IGF-I levels have been implicated as a major contributor to the life span increase in GH- and GH receptor/binding protein (GHRBP)–deficient mice as well as in calorically restricted mice (14–18). However, the longevity phenotype of PAPP-A KO mice is associated with normal serum levels of IGF-I. Thus, control of tissue availability of IGF-I, independent from systemic levels, can influence the aging process. Furthermore, a moderate reduction in IGF-I receptor activation through local sequestration of ligand rather than complete inhibition of IGF-I signaling may be important for extended life span considering the perinatal lethality of IGF-I receptor KO mice (19).
End-of-Life Pathology
Previous gross inspection at necropsy indicated reduced tumor incidence and burden in PAPP-A KO mice (7). However, the histopathology in the present study modified these conclusions somewhat because HP neoplasias were of the highest incidence and the probable cause of death in both PAPP-A KO and WT mice. These HP tumors were not likely detected at a gross level. Abnormal masses noted in liver and lung were confirmed as carcinomas by histology and also were of similar incidence in PAPP-A KO and WT mice. On the other hand, tumors besides liver and lung that were identified in end-of-life pathology were significantly decreased in PAPP-A KO compared with WT mice. Several of these, that is, mammary gland, ovarian, and endometrial, appear to be IGF-dependent tumors (20), and thus, their growth may be particularly repressed in PAPP-A KO mice. Although the absolute incidence of neoplasia was not reduced in PAPP-A KO mice at end of life, there was a significant delay in occurrence. This was true whether analyzed for HP, non-HP, or total neoplasias. Thus, retardation in cancer occurrence likely accounts, in part, for the increased life span of PAPP-A KO mice.
Reduction in age-related degenerative diseases also appeared to contribute to the extended life span in these PAPP-A KO mice. Degenerative disease was a highly significant cause of death in WT but was essentially absent in PAPP-A KO mice. Atrial thrombosis in WT mice was of particular interest because of implications for PAPP-A in cardiovascular disease (21,22).
Overall disease burden was significantly higher in WT than in PAPP-A KO mice. Thus, extension of longevity was not through an effect on specific lethal diseases but through a more general retardation of senescent and neoplastic processes. Importantly, no disease severe enough to cause death was noted for 30% of PAPP-A KO mice and 6% of WT mice. This may skew the data further in terms of a longer apparently disease-free life span in PAPP-A KO mice, although not all tissues were examined in the Longevity group, and there may be illnesses not easily identified by histopathologic examination alone.
End-of-life necropsies have been performed on long-lived Ames and Snell dwarf, GHRBP KO, and calorically restricted mice (23–28). In common with the PAPP-A KO mice, these mice on different genetic backgrounds [ie, C57BL/6 (23,25,27), mixed 129Ola and BalbC (26), and heterogeneous (24)] all showed delayed occurrence of fatal neoplastic disease, reduced contributing degenerative lesions, and lower overall disease burden. Decreased cardiac and renal pathology was also seen in GHRBP KO and calorically restricted mice at time of death (26,27). However, Ames dwarf mice had decreased and calorically restricted mice had increased incidence of adenocarcinoma of the lung (24,27). This could be due to the decrease in GH and/or other pituitary hormones in the Ames dwarf mice (13,14) and the increase in vitamin A given to calorically restricted mice (27). PAPP-A KO and WT mice with normal GH (7) and fed similar diets were not significantly different in terms of presumably fatal lung cancer.
Scheduled Pathology
It was important to determine whether this extension of life span in PAPP-A KO mice was accompanied by improvement in health during aging. To accomplish this, we performed comprehensive pathology on WT and PAPP-A KO mice at 78, 104, and 130 weeks of age. In general, there were lower levels of pathology in PAPP-A KO mice compared with WT mice at each age. The WT mice appeared to have more age-related degenerative lesions and tumors, and an earlier onset of these changes, than the PAPP-A KO mice. Specifically, cardiomyopathy, nephropathy, neurogenerative lesions, and testicular, and ovarian and thymic atrophy were more evident in WT than PAPP-A KO mice at the time points studied. Hyperplasias (thyroids, pancreatic islets, adrenals, and uterus) and pituitary adenomas and lymphomas were more evident in WT at earlier time points than in PAPP-A KO mice. There was a trend for delayed occurrence of lesions, especially noted for testes, brain, and kidney, where at 104 weeks, disorders were still less than WT at 78 weeks. In heart, spleen, and adrenals, incidence at 104 weeks was similar to WT at 78 weeks. These results are consistent with a general delay in age-related degenerative disease in PAPP-A KO mice, which likely enabled them to maintain a better health status compared with WT mice. This type of scheduled comprehensive pathology has also been performed in calorically restricted mice (23,27). The findings of delayed onset of lymphoma and decreased age-related cardiac, renal, brain, and endocrine system pathologies are very similar to those in PAPP-A KO mice.
The pathological findings in the thymus warrant further discussion. Recently, we reported that PAPP-A KO mice were resistant to age-dependent thymic atrophy (9). At approximately 78 weeks of age, the thymi of WT mice were very small in size with extensive fatty tissue infiltrates and few thymocytes. In contrast, PAPP-A KO mice maintained thymic structure and normal histology and cellularity. The pathology on 78 week-old-mice in this study confirmed these findings. However, at 104 weeks, obvious thymic tissue was not present in most of the PAPP-A KO mice, suggesting delay rather than absolute resistance to thymic involution later in life. We speculate that this delayed loss of thymic structure and accompanying immune homeostasis may contribute to the delayed occurrence of neoplasia, especially lymphomas, in PAPP-A KO mice.
Overall, survival curves and pathology indicate an extended life span of PAPP-A KO mice accompanied by lower age-related accumulation of various pathological changes. Although these data provide clues to mechanisms contributing to the multifactorial processes of aging and longevity, they raise many additional questions, such as What role does PAPP-A play in development of cardiomyopathies and tumor growth? Does the delay in brain lesions contribute to improved co-ordination/cognition in PAPP-A KO mice? Is intact thymus an important tumor suppressor during aging? Determining the answers to these and other questions could lead to novel approaches using PAPP-A as a target for healthy aging.
FUNDING
This work was supported by the National Institute on Aging (R01AG028141) and The Ellison Medical Foundation.
SUPPLEMENTARY MATERIAL
Supplementary material can be found at: http://biomed.gerontologyjournals.org/
Supplementary Material
Acknowledgments
The authors thank Jacquelyn Grell, Stephanie Thomas, and Kelly Thompson for their excellent technical assistance.
References
- 1.Lawrence JB, Oxvig C, Overgaard MT, et al. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy associated plasma protein-A. Proc Natl Acad Sci USA. 1999;96:3149–3153. doi: 10.1073/pnas.96.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boldt HB, Conover CA. Pregnancy-associated plasma protein-A (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Horm IGF Res. 2007;17:10–18. doi: 10.1016/j.ghir.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Byun D, Mohan S, Yoo M, Sexton C, Baylink DJ, Qin X. Pregnancy-associated plasma protein-A accounts for the insulin-like growth factor (IGF)-binding protein-4 (IGFBP-4) proteolytic activity in human pregnancy serum and enhances the mitogenic activity of IGF by degrading IGFBP-4 in vitro. J Clin Endocrinol Metab. 2001;86:847–854. doi: 10.1210/jcem.86.2.7223. [DOI] [PubMed] [Google Scholar]
- 4.Ortiz C, Chen B-K, Bale L, Overgaard M, Oxvig C, Conover CA. Transforming growth factor-β regulation of the insulin-like growth factor binding protein-4 protease system in cultured human osteoblasts. J Bone Miner Res. 2003;18:1066–1072. doi: 10.1359/jbmr.2003.18.6.1066. [DOI] [PubMed] [Google Scholar]
- 5.Conover CA, Bale LK, Overgaard MT, et al. Metalloproteinase pregnancy-associated plasma protein-A is a critical growth regulatory factor during fetal development. Development. 2004;131:1187–1194. doi: 10.1242/dev.00997. [DOI] [PubMed] [Google Scholar]
- 6.Bale LK, Conover CA. Disruption of insulin-like growth factor-II imprinting during embryonic development rescues the dwarf phenotype of mice null for pregnancy-associated plasma protein-A. J Endocrinol. 2005;186:325–331. doi: 10.1677/joe.1.06259. [DOI] [PubMed] [Google Scholar]
- 7.Conover CA, Bale LK. Loss of pregnancy-associated plasma protein A extends lifespan in mice. Aging Cell. 2007;6:727–729. doi: 10.1111/j.1474-9726.2007.00328.x. [DOI] [PubMed] [Google Scholar]
- 8.Conover CA, Mason MA, Levine JA, Novak CM. Metabolic consequences of pregnancy-associated plasma protein-A deficiency in mice: exploring possible relationship to the longevity phenotype. J Endocrinol. 2008;198:599–605. doi: 10.1677/JOE-08-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallejo AN, Michel JJ, Bale LK, Lemster BH, Borghesi L, Conover CA. Resistance to age-dependent thymic atrophy in long-lived mice that are deficient in pregnancy-associated plasma protein A. Proc Natl Acad Sci USA. 2009;106:11252–11257. doi: 10.1073/pnas.0807025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the aging process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 11.Flurkey K, Papaconstantinou J, Harrison DE. The Snell dwarf mutation Pit 1dw can increase life span in mice. Mech Ageing Dev. 2002;123:121–130. doi: 10.1016/s0047-6374(01)00339-6. [DOI] [PubMed] [Google Scholar]
- 12.Holzenberger M, Dupont J, Ducos B, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 13.Brown-Borg HM. Hormonal control of aging in rodents: the somatotropic axis. Mol Cell Endocrinol. 2009;299:64–71. doi: 10.1016/j.mce.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartke A. Impact of reduced insulin-like growth factor-1/insulin signaling on aging in mammals: novel findings. Aging Cell. 2008;7:285–290. doi: 10.1111/j.1474-9726.2008.00387.x. [DOI] [PubMed] [Google Scholar]
- 15.Berryman DE, Christiansen JS, Johannsson G, Thorner MO, Kopchick JJ. Role of the GH/IGF-1 axis in lifespan and healthspan: lessons from animal models. Growth Horm IGF Res. 2008;18:455–471. doi: 10.1016/j.ghir.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandrashekar V, Bartke A. Induction of endogenous insulin-like growth factor-I secretion alters the hypothalamic-pituitary-testicular function in growth hormone-deficient adult dwarf mice. Biol Reprod. 1993;48:544–551. doi: 10.1095/biolreprod48.3.544. [DOI] [PubMed] [Google Scholar]
- 17.Breese CR, Ingram RL, Sonntag WE. Influence of age and long-term dietary restriction on plasma insulin-like growth factor-I (IGF-I), IGF-I gene expression, and IGF-I binding proteins. J Gerontol. 1991;46:B180–B187. doi: 10.1093/geronj/46.5.b180. [DOI] [PubMed] [Google Scholar]
- 18.Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- 19.Liu J-P, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 20.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 21.Bayes-Genis A, Conover CA, Overgaard MT, et al. Pregnancy-associated plasma protein A as a marker of acute coronary syndromes. N Engl J Med. 2001;345:1022–1029. doi: 10.1056/NEJMoa003147. [DOI] [PubMed] [Google Scholar]
- 22.Harrington SC, Simari RD, Conover CA. Genetic deletion of pregnancy-associated plasma protein-A is associated with resistance to atherosclerotic lesion development in apolipoprotein E-deficient mice challenged with a high-fat diet. Circ Res. 2007;100:1696–1702. doi: 10.1161/CIRCRESAHA.106.146183. [DOI] [PubMed] [Google Scholar]
- 23.Blackwell B-N, Bucci TJ, Hart RW, Turturro A. Longevity, body weight, and neoplasia in ad libitum-fed and diet-restricted C57BL6 mice fed NIH-31 open formula diet. Toxicol Pathol. 1995;23:570–582. doi: 10.1177/019262339502300503. [DOI] [PubMed] [Google Scholar]
- 24.Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in Ames dwarf mice: correlation to extend longevity. J Gerontol Biol Sci. 2003;58A:291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- 25.Ikeno Y, Hubbard GB, Lee S, et al. Housing density does not influence the longevity effect of calorie restriction. J Gerontol Biol Sci. 2005;50A:1510–1517. doi: 10.1093/gerona/60.12.1510. [DOI] [PubMed] [Google Scholar]
- 26.Ikeno Y, Hubbard GB, Lee S, et al. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol Biol Sci. 2009;64A:522–529. doi: 10.1093/gerona/glp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turturro A, Duffy P, Haas B, Kodell R, Hart R. Survival characteristics and age-adjusted disease incidences in C57BL/6 mice fed a commonly used cereal-based diet modulated by dietary restriction. J Gerontol Biol Sci. 2002;57A:B379–B389. doi: 10.1093/gerona/57.11.b379. [DOI] [PubMed] [Google Scholar]
- 28.Vergara M, Smith-Wheelock M, Harper JM, Sigler R, Miller RA. Hormone-treated Snell dwarf mice regain fertility but remain long lived and disease resistant. J Gerontol. 2004;59A:1244–1250. doi: 10.1093/gerona/59.12.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.