Abstract
Background.
Short-term adherence to physical activity (PA) in older adults improves psychomotor processing abilities and is associated with greater brain activation. It is not known whether these associations are also significant for longer-term adherence to moderate-intensity activities.
Methods.
We measured the cross-sectional association of regular walking with brain activation while performing the digit symbol substitution test (DSST). Participants of the lifestyle interventions and independence for elders—pilot study were examined 2 years after completing a 1-year treatment, consisting of either PA or education in successful aging (SA). Data were obtained from 20 PA participants who reported having remained active for 2 years after the end of the treatment and from 10 SA participants who reported having remained sedentary during the same period (mean age: 81.5 and 80.8 years). Complete brain activation and behavioral data were available for 17 PA and 10 SA participants.
Results.
Two years after the formal intervention had ended, the PA group engaged in more minutes of moderate activity and had significantly greater DSST score and higher brain activation within regions important for processing speed (left dorsolateral prefrontal, posterior parietal, and anterior cingulate cortices). Associations were independent of self-reported health, blood pressure, cognition, medication records, gray matter atrophy, and white matter hyperintensities.
Conclusions.
Persistent engagement in PA may have beneficial effects on psychomotor processing speed and brain activation, even for moderate levels and even when started late in life. Future studies are warranted to assess whether these beneficial effects are explained by delayed neuronal degeneration and/or new neurogenesis.
Keywords: Physical activity, Sedentary, fMRI, Executive control function, Older adults
DESCRIPTIVE studies have shown that older adults with greater physical activity (PA) levels are less likely to develop dementia or other cognitive functional impairments compared with those who do not exercise (1,2). Gains in the cognitive domain seem to be strongest for executive control function (ECF), psychomotor processing speed, and attention (3,4). Intervention studies to increase PA in previously sedentary older adults have also shown beneficial short-term effects in cognition (1,3,5), as well as structural changes in ECF-related brain regions in frontoparietal lobes (6) and overall greater brain functional activation (7,8). It has been proposed that PA is associated with brain changes through overall cardiovascular conditioning and specifically improved cerebrovascular blood flow and neuronal oxygenation. Animal studies have also shown that increased PA is associated with the development of new neurons (4,9). These events may in turn delay age-related degenerative processes or promote new neurogenesis and thus delay brain structural and functional declines. These findings suggest that older adults maintain brain plasticity and that the brain can respond to behavioral changes even late in life. However, previous studies have not measured such associations in very old and sedentary adults who begin and remain engaged in moderate to low levels of PA, such as regular walking, beyond 1 year of time. It is also not clear whether specific regions within the ECF frontoparietal circuits are selectively affected by PA or whether there is a generalized positive effect of PA on the brain.
It has been recently shown that the participants of the 1-year randomized controlled trial lifestyle interventions and independence for elders—pilot (LIFE-p) (10), who had been assigned to the PA arm, maintained greater physical function and higher score on the digit symbol substitution test (DSST) 2 years after the end of the treatment compared with those in the control group (11,12). The purpose of this study is to measure the spatial distribution and intensity of brain functional activation in relationship with moderate PA levels in a subsampleof this cohort, 2 years after formal PA intervention has terminated.
We hypothesize that the PA LIFE-p participants who have maintained active would have greater functional magnetic resonance imaging (fMRI) activation while performing the DSST compared with the successful aging (SA) participants who had remained sedentary. Specifically, we hypothesize that group differences would be localized within the ECF regions of the dorsolateral prefrontal, posterior parietal, and anterior cingulate cortices.
METHODS
The LIFE-p was a 1-year single-blinded, multicenter randomized controlled trial conducted in sedentary older adults and consisted of a PA intervention arm compared with a health education in SA intervention arm (10,13). The study took place at four field centers (Cooper Institute, Stanford University, University of Pittsburgh, and Wake Forest University) in adherence with the principles of the Declaration of Helsinki and is registered at www.ClinicalTrials.gov (registration number NCT00116194). For this study, participants were selected from the Pittsburgh site, 2 years after the end of the 1-year intervention trial. Inclusion into this study is described in the Participants section.
Overview of LIFE-p Trial
The design of the LIFE-p trial has been described in detail elsewhere (10,13). Briefly, inclusion criteria were having an age of 70–89 years, having a sedentary lifestyle (<20 minutes/week spent in structured PA during the past month), being able to walk 400 m within 15 minutes without sitting and without use of any assistive device, having a Short Physical Performance Battery score 9 (on a scale of 0–12), having completed behavioral tests related to logging health behavior, giving informed consent, living in the study area, and not planning to move for at least 9 months.
The PA intervention consisted of a combination of aerobic, strength, balance, and flexibility exercises, divided into adoption (Weeks 1–8), transition (Weeks 9–24), and maintenance (Week 25 to the end of the trial) phase, and walking was the primary mode of exercise. The goal was walking for at least 150 minutes over the course of the week. The SA group served as active comparison group and met in small groups weekly for the first 26 weeks and then monthly. Sessions included health topics relevant to older adults such as nutrition, medications, foot care, and recommended preventive services at different ages.
Participants
At the Pittsburgh site, 104 participants completed the 1-year trial (average: 14.5 months, standard deviation [SD]: 2 months). Two years after the end of the study (average: 20 ± 1.7 months), participants were screened for interest in participating in this brain fMRI study. Those interested were included if they reported to have remained adherent to their original group assignment since the closeout of the study and if they were eligible for a brain MRI. Adherence to the original group assignment was ascertained separately for the two arms. The PA group was asked: “Since the LIFE Study ended, have you completely stopped your regular physical activity?” and participants were included if they responded “No.” The SA group was asked the question: “Since the LIFE-p Study ended, have you spent at least 20 minutes a week getting regular exercise? Regular exercise includes activities like: brisk walking, jogging, weight lifting, cycling, aerobics, or dancing” and they were included if they responded “No.” To corroborate the self-report adherence ascertained through this initial question, PA levels were also measured through detailed interview using the Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire (14), described later in the Other Measurements section.
MRI eligibility was assessed through interview and review of medical records. The study was approved by the University of Pittsburgh Institutional Review Board, and all participants gave written informed consent.
A total of 30 participants (n = 20 from the PA group and n = 10 from the SA group) who indicated adherence to their original group assignment were selected for this study (Supplementary Figure 1) and underwent a brain MRI and a clinic exam. The average time interval between study closeout and the time of the brain MRI was similar in the two groups (p = .7). The average interval of time between the phone follow-up and the brain MRI was less than 2 months (M: 1.9 months, SD: 1.8 months).
Masking to Intervention Group Assignment
Masking was maintained for the other data collection including mobility measures, cognitive measures, and brain MRI and for data entry and brain fMRI preprocessing. The recruitment for this fMRI study was not blinded to intervention assignment because the goal of the recruitment was to obtain participants from both groups who had remained adherent to their original assignment. During all evaluations, the participant was reminded again not to mention his or her group assignment to the staff or investigators. In addition to the measurements previously obtained at study entry (baseline) and at the end of the trial (closeout), functional brain MRI task–related activation scores on the brain volume and white matter hyperintensities volume were obtained at follow-up.
Digit Symbol Substitution Test
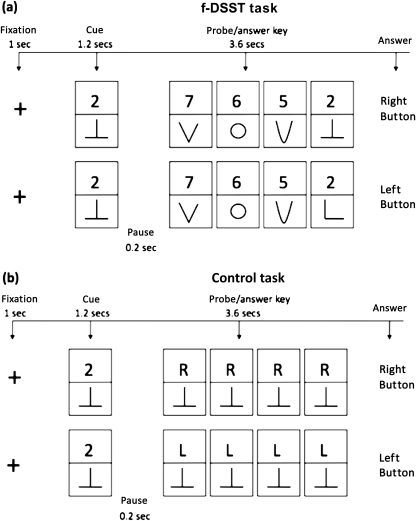
DSST is a test of psychomotor performance, and it requires incidental memory, perceptual organization, visuomotor coordination, and selective attention to filter out irrelevant information (eg, symbols that may look alike). The test has high test–retest reliability. For this study, participants performed the pencil and paper version of the DSST, previously employed in epidemiological studies of older adults (15). A computerized version adapted for the fMRI protocol (fDSST) (16) was developed for this study (see Figure 1). Each participant was instructed on the task outside the scanner just before the fMRI experiment begun and practiced with 12 trials (6 trials for the control condition and 6 trials for the fDSST).
Figure 1.
Digit symbol substitution test (DSST) and control condition tasks. Schematic representation of the DSST and control conditions tasks administered during the brain functional magnetic resonance imaging (fMRI) scanning session. fDSST = DSST modified for the fMRI protocol. Two examples are shown for the fDSST (matching: push right button; not matching : push left button) and for the control condition ( ‘R’: push right button; ‘L’: push left button). The participant sees on a computer screen one number–symbol matching pair (cue). After the cue disappears, an answer key (probe) appears containing a grid of four number–symbol matching pairs. The participant is instructed to push the right index finger button if the probe contains one number–symbol that matches the cue and to push the left index finger button if the probe does not contain any number–symbol that matches the cue. Instructions are to respond “as fast as you possibly can.” In the control condition, the probe is a grid of four “R-symbol” pairs or “L-symbol” pairs to which the participant is instructed to respond by pushing the right or the left button, respectively, using the index finger. Eye movements to visually scan the screen in the control condition and in the fDSST condition are estimated to account for non–task-specific brain activation (frontal eye field and visual cortex for eye movements and motor cortex for the index finger movements). In all trials, cues and a probe flashed on a black screen that had a 0.1-cm bright white fixation cross at the center, according to specific timing (cue: 1.0 seconds; pause: 0.2 seconds; probe: 3.6 seconds; pause: 1.2 seconds). The fDSST was presented using the block design, in which one block (eight trials per block) alternated with one block (eight trials per block) of a control condition. Each block lasted for 48 seconds, and the participants were reminded the instruction for 8 seconds at the start of each block. There were five fDSST blocks alternated with five control blocks for a total of 9 minutes and 20 seconds. No cue was repeated within the same block. The two task conditions (matching vs nonmatching) were randomized 1:1 across participants and block.
Each participant was then asked whether he or she wanted additional practice trial before starting the scan. None of the participants asked for additional practice time.
Performance on the fDSST was measured as accuracy (number of right responses/total number of probes) and response time (time between appearance of the probe and response time). Responses occurring after the probe disappeared (after 3.6 seconds) entered the analyses as “wrong” with a response time = 3.6 seconds. Analyses were also repeated after excluding these responses.
MRI Protocol and Processing
MR images were acquired in a 3-T Siemens Trio MR-scanner using a Siemens 12-channel head coil. Functional time series consisted of whole-brain gradient-echo echoplanar images (EPIs) (repetition time [TR]: 2 seconds; echo time [TE]: 32 ms; resolution: 128 × 128; slice thickness: 3.0 mm; 29 axial slices; GRAPPA with acceleration factor of 2). A three-dimensional magnetization prepared rapid gradient echo high-resolution T1-weighted image (TR: 2.3 seconds; TE: 3.43 ms; slice thickness: 1 mm; 176 slices) was acquired first for anatomical detail, and fluid attenuated inversion recovery T2-weighted image (TR: 9 seconds; TE: 102 ms; longitudinal relaxation time: 2.5 seconds; slice thickness: 3 mm; 49 slices) for the white matter hyperintensities. Statistical parametric mapping 5 (SPM5) (17) was implemented in MatLab (MathWorks, Natick, MA). EPI volumes were realigned to the first volume of the first time series, and a mean image of the realigned volumes was created. The realigned images were coregistered to the anatomical T1-weighted image. The anatomical image and the EPIs were normalized to a standard SPM reference. The derived normalization parameters of the anatomical image were applied to the EPIs, which were subsampled to a voxel size of 2 × 2 × 3 mm and smoothed with a Gaussian kernel of 8 mm full width at half maximum.
Brain volume of gray matter and white matter hyperintensities was measured because they could be potential confounders of the main association between adherence to PA and brain fMRI and DSST score. A fully deformable automatic algorithm previously described was used (18–23).
Other Measurements
A self-reported PA questionnaire, the CHAMPS (14), was used to measure participation (yes or no) and frequency (days per week and hours per day) for the following activities: walk leisurely, walk for errands, ride a bicycle, use aerobic machines, exercise (both strength and water exercise), stretching, and general conditioning. Measures that indicate overall functioning and health-related status were obtained concurrently with the fMRI because they could be confounders of the association between group assignment and brain fMRI signal and performance. These included in-person interview, anthropometric measures, physical exam, blood pressure, prevalence of clinical conditions (assessed using self-reported physician-diagnosed disease information), and medication use. Cognitive function was measured using the Mini-Mental State Examination (MMSE) test at baseline, at closeout, and at the time of follow-up concurrent with the brain MRI. Total MMSE score and scores of individual domains were obtained, including orientation (state the time, season, month, city, etc.), immediate and delayed words recall, calculation (subtract 7 starting from 100), naming (object and body parts), words repeating, three-stage movement (take the paper in the right hand, fold it in half, put it on the floor), and copying (octagonal drawing).
Analysis
Wilcoxon signed-rank test was used to test significance of within-group differences in PA obtained at the beginning, at the end, and at 2 years after the end of the formal intervention program. Mann–Whitney test and Fisher’s exact tests were used to compare between-group differences in means and proportion for continuous and categorical variables, respectively. Based on the power calculations of our previous work (18) and on sample size computations for fMRI blood oxygen level dependent (BOLD) signal (24), this study had a power of 80% and alpha of .05 to detect a signal change mean difference between groups of ≤0.18%. All analyses were done using SPSS 15.0 (SPSS, Inc., Chicago, IL).
Within-group differences in mean group fMRI activation maps were estimated between the two conditions (fDSST vs control) in the context of the general linear model (17). Each condition was modeled using a delayed boxcar function convolved with the SPM5 hemodynamic response function. Additionally, movement parameters derived from realignment were added as covariates of no interest to correct for confounding effects induced by head movement. Contrasts of interest (fDSST > control and control > fDSST) were first estimated for each participant individually (averaging activation across runs) and then subjected to a second-level random-effects analysis.
The association of fMRI mean signal with PA was tested using a one-sample two-tailed t test. Within-group and between-group differences in fMRI signal were estimated in both directions (fDSST > control and control > fDSST) using a two-sample two-tailed t test. The two-sample t test addresses sample size differences by comparing the groups in the same model and fits the fMRI BOLD design of comparing the primary contrast obtained for each participant (fDSST − control condition). In all analyses, a voxel-wise approach was used with t maps thresholded at a minimal voxel entry value of t > 1.71 (p < .05 uncorrected) and corrected for multiple comparison using AlphaSim (gray matter mask, 1000 Monte Carlo simulations, and smoothness were obtained using full-width at half maximum in X dimension) (25). WFU_Pickatlas (26) was used to accept and identify the clusters with probability (alpha) of .001 (extent threshold: 433 voxels) and .01 (extent threshold: 270 voxels) for the one-sample and the two-sample t test analyses, respectively. Between-group analyses were also adjusted for accuracy and response time.
RESULTS
Participants’ Characteristics
The participants included in this study (n = 30) had similar baseline characteristics (p > .14 for all comparisons) compared with those excluded (n = 74), with the exception of race (blacks: 10% vs 37% in the fMRI vs not fMRI group, Fisher’s exact test: p = .004). Inclusion of the four participants who completed the 1-year trial and died by the time of the second follow-up (n = 2 in the PA and n = 2 in the SA group) did not change these results. Among those included in this fMRI study (Table 1), adults in this PA group were less likely to be black compared with those in the SA group (0% vs 30%, Fisher’s exact test: p = .03). An imbalance in ethnicity distribution between groups was also observed in the total sample of n = 104 (blacks: 24% vs 34% in the PA vs SA group, Fisher’s exact test: p = .2). The other baseline characteristics, including MMSE score (total and by domains), did not significantly differ between groups (p ≥ .2 for all comparisons). Differences in neuroradiological markers and self-reported health were also not significant. The similarities between groups indicate that randomized design was maintained in this substudy. There was a within-group increase (p = .025) in the three-stage task in the PA group but not in the SA group (see Supplementary Figure 3).
Table 1.
Participants’ Characteristics Measured at the Follow-up Visit at Time of fMRI (n = 30)
| Successful Aging Group (n = 10) | Physical Activity Group (n = 20) | p Value of Group Comparison | |
| Age, y, M (SD) | 81.45 (2.77) | 80.8 (3.95) | .5 |
| Male, n (%) | 3 (30.0) | 5 (25.0) | .4 |
| Education, completed high school or equivalent, n (%) | 4 (40.0) | 9 (45.0) | .5 |
| Mini-mental score, M (SD) | 27.5 (2.2) | 27.9 (1.8) | .8 |
| Self-reported rating of very good health, n (%) | 4 (40) | 8 (40) | .9 |
| Brain volume, mm3, M (SD)* | 1296.5 (167.5) | 1350.7 (94.7) | .1 |
| Atrophy index (gray matter/CSF volume), M (SD)* | 1.4 (0.1) | 1.4 (0.1) | .9 |
| White matter hyperintensities, % of total white matter volume, M (SD)* | 0.35 (0.3) | 0.33 (0.2) | .8 |
| DSST during the fMRI task, accuracy, M (SD)† | 66 (0.27) | 82 (0.12) | .02 |
| DSST during the fMRI task, response time, ms, M (SD)‡ | 3086.5 (364.8) | 2680.2 (534.7) | .01 |
Notes: CSF = cerebro-spinal fluid; DSST = digit symbol substitution test; fMRI = functional magnetic resonance imaging.
Obtained for n = 18 of 20 in the physical activity intervention group.
Number of correct answer/total number of trial × 100.
Time between stimulus presentation and time of response recording.
PA Measures
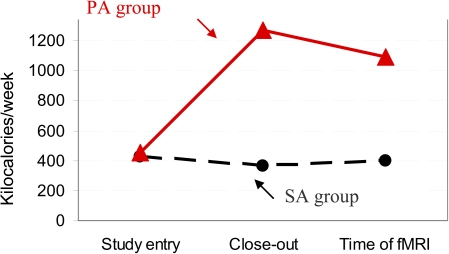
The level of PA was greater in the PA compared with SA group recruited for this study (Figure 2) at the study closeout and 2 years after the end of the treatment (Mann–Whitney test: p = .008 and .01, respectively) but not at baseline (p = .98). The PA group continued to remain engaged in more minutes of PA at closeout and at the 2-year follow-up compared with baseline (within-group comparison, Wilcoxon signed-rank test: p < .007 for both). The SA group of this study did not increase its level of PA from baseline through the closeout of the formal intervention or through follow-up (within-group comparison, Wilcoxon signed-rank test: p > .5 for both comparisons). Results were similar for those not included in the fMRI study (not shown). The type of PA was primarily walking and stretching (Supplementary Figure 2).
Figure 2.
Kilocalories expenditures were computed using the Community Healthy Activities Model Program for Seniors for participants who were in the physical activity (PA) arm and in the successful aging (SA) group. Mean kilocalories expenditures per week were measured at study entry, at closeout, and at the follow-up clinic exam at the time of the functional magnetic resonance imaging (fMRI) for the SA (dashed line, filled circles) and for the PA (solid line, triangles). Kilocalories expenditures were computed to perform the following activities: walk leisurely, walk for errands, ride a bicycle, use aerobic machines, do water exercise, stretching, heavy strength exercise, light strength exercise, and general conditioning. See Supplementary Figure 2 for percentage distribution.
PA, fDSST Performance, and Spatial Distribution of Functional Brain MRI Signal
Complete behavioral data were obtained for 29 participants (19 persistent exercisers and 10 from persistent sedentary group). Complete fMRI analysis was performed on 27 participants, 17 participants of the persistent new exercisers group and 10 participants of the persistent sedentary group. Alignment errors (n = 2) and motion artifacts (n = 1) prevented fMRI analyses of three PA participants.
The PA group performed the fDSST with shorter response times (Mann–Whitney test: p = .01) and greater accuracy (Mann–Whitney test: p = .02) compared with the SA group (Table 1). Greater PA as ascertained through CHAMPS score at time of fMRI was associated with faster fDSST responses in all groups (gender-adjusted correlation coefficient: r = −.33, p = .04). Associations with accuracy followed similar trends (gender-adjusted correlation coefficient: r = .29, p = .066).
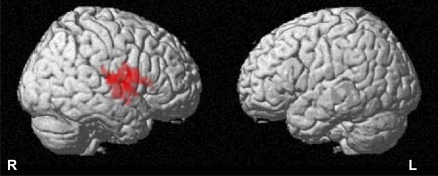
Overall, greater PA was also associated with greater fMRI activation (Figure 3) within the left inferior frontal gyrus, with activation extending caudally toward the temporal lobe cortex and deeply to include the ipsilateral insula (cluster size = 551 voxels, peak Z score = 3.45, Talairach coordinates [x, y, z]: 55.4, 4.7, 16.3).
Figure 3.
Functional brain magnetic resonance imaging (MRI) activation in relationship with physical activity. Highlighted regions indicate regions with functional MRI signal positively correlated with physical activity (kilocalories). t maps of analysis of brain MRI activation in relationship with physical activity (kilocalories). See text for spatial coordinates of the regions. R = right, L = left.
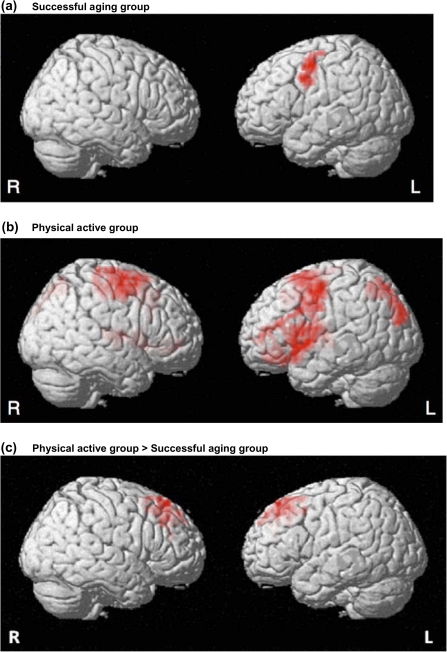
Both groups showed increased brain activation while performing the fDSST test compared with the control condition (Figure 4a and b). However, in the PA group the main effect for fDSST versus control condition was significant in a more widely distributed network that included ECF regions within the dorsolateral prefrontal, posterior parietal, and anterior cingulate cortices compared with the regions active in the SA group (Table 2). Between-group comparisons of fMRI activation (Figure 4c; Table 2) showed that the fMRI signal was significantly greater in the PA compared with the SA group in selected cortical regions bilaterally, including ECF-related regions within the dorsolateral prefrontal and anterior cingulate cortices. Analyses repeated using a voxel-wise approach showed that the fMRI signal was not significantly greater in the SA versus the PA group.
Figure 4.
Functional brain magnetic resonance imaging activation during fDSST performance. t maps of analysis of task-related activity from (a) the successful aging (SA) group and (b) the physical activity (PA) group and (c) between comparisons of PA > SA. Results for SA group > PA group were nonsignificant. R = right; L = Left. See Table 2 for spatial coordinates of the corresponding regions.
Table 2.
Spatial Distribution of Functional Brain MRI Activation During fDSST Performance as Illustrated in Figure 4
| Regions and Corresponding BA Classification | Cluster Size (voxels) | Peak Z Score | Talairach Coordinates of Peak Z Score | Corrected Alpha for the Cluster (α)* |
| SA group | ||||
| Left dorsolateral prefrontal cortex (BA 9) | 283 | 2.97 | −35.6, −3.6, 44.4† | <.01 |
| PA group | ||||
| Left dorsolateral prefrontal cortex (BA 9, 11, 44, 45, 46, and 47) | 3708 | 4.55 | −17.8, 8.4, 52.1‡ | <.001 |
| Left superior posterior parietal cortex (BA 7) | 722 | 3.20 | −9.9, −61.0, 61.1§ | <.001 |
| Anterior cingulate cortex (BA 24 and 32) | 757 | 2.84 | 1.9, −5.7, 3.1‖ | <.001 |
| Right middle frontal gyrus | 1454 | 3.76 | 27.7, 1.1, 60.7¶ | <.001 |
| PA group > SA group | ||||
| Left dorsolateral prefrontal cortex (BA 9) | 407 | 2.80 | −4.0, 31.4, 45.4# | .002 |
| Right dorsolateral prefrontal cortex (BA 9) | 298 | 2.92 | 13.9, 37.4, 47.9** | .01 |
Notes: Analyses were adjusted for accuracy and reaction time. Results are for participants in the SA group and the PA group and for the between-group comparison of functional brain MRI activation in the PA > SA. Results for SA > PA comparisons were nonsignificant. BA = Brodmann area; DSST = digit symbol substitution test; MRI = magnetic resonance imaging; PA = physical activity; SA = successful aging.
Probability of a false positive detection based on the combination of individual voxel probability thresholding and minimum cluster size thresholding.
The cluster had its peak in the left middle frontal gyrus, and it included the left BA 9.
This large cluster had its peak in the left superior frontal gyrus, and it extended dorsolaterally toward the dorsolateral prefrontal cortex as well as deeply to include the left insula.
This cluster had its peak in the left superior posterior parietal cortex (BA 7), and it also included the left parastriatal visual cortex (BA 19).
This cluster had its peak in the right thalamus, and it extended laterally to include the right striatum and rostrally toward the anterior cingulate cortex and right orbitofrontal gyrus (BA 10).
This cluster had its peak in the right middle frontal gyrus, and it extended toward the right post-central gyrus (BA 4).
The cluster had its peak in the left superior frontal gyrus, and it extended dorsolaterally toward the left BA 9 and 6.
This cluster had its peak in the right superior frontal gyrus, and it extended dorsolaterally toward the left BA 9 and ventrally toward the anterior cingulate cortex.
DISCUSSION
In this group of previously sedentary older adults, those who began and remained adherent to a moderate PA program had distinct patterns of brain activation and higher performance on a test of psychomotor speed compared with those who remained sedentary over the same period of time. Specifically, differences in brain activation during task performance were localized within regions important for ECF. Greater PA levels were also directly associated with greater fMRI signal restricted to the inferior frontal gyrus, 2 years after the intervention ended.
Our results are consistent with the previous studies in older adults showing a relationship between PA and greater executive performance (see (3) for a review). Previous fMRI studies, including ours (18,27,28), have also shown activation in dorsolateral prefrontal, posterior parietal, and anterior cingulate cortices during the execution of ECF tasks. However, this is the first study to examine a relationship between PA, ECF performance, and ECF-related brain activation in very old participants with long follow-up after the end of the intervention.
The spatial distribution of brain changes associated with increased PA has been examined mostly in animal studies (4,29) and in few human studies. Colcombe and colleagues have shown that a 6-month PA program is associated with ECF frontoparietal fMRI signal changes similar to those observed here (30) and with fronto-temporo-parietal structural gray matter changes (7). If PA affects the brain through increased cardiovascular conditioning, then it is likely that the watershed region, such as the dorsolateral prefrontal cortex, would be the most sensitive to cerebrovascular and oxygenation levels’ changes after PA. If PA has a stronger effect on these regions than on others, then this could explain why the effect of PA on cognitive function is stronger for the ECF domain.
Although the fDSST task mainly taps ECF function, our fMRI analyses were not limited to the ECF regions of interest alone and considered all the brain tissue captured in the field of view. Importantly, our analyses examined group differences using two-tailed tests. In addition to tests for greater activation in the persistent exercisers versus persistent sedentary, we also tested for greater activation in the sedentary versus exercisers and found that none of the regions were significantly more active in the sedentary versus exercisers group. Although this study cannot conclude that ECF is the only cognitive domain affected by PA, scores on tests of general cognition and of individual cognitive domains did not differ between groups at baseline or at follow-up (Supplementary Figure 3). The within-group increase in the “three-stage movement” in the PA group may indicate a gain in short-term memory and praxis in relationship with PA. However, we are very cautious in interpreting these results as a lack of effect on other domains and emphasize that this facet of the study needs further investigation. Specifically, intervention studies should include careful and repeated neuropsychological assessment and neuroimaging measures to provide conclusive evidence that PA is associated with improved versus preserved function over time and what domains are affected the most.
Strengths of this study include the comprehensive clinical characterization and the thorough measures of PA for up to 3 years prior to the time of the fMRI. Specifically, PA was directly monitored during the 1-year intervention trial and had been measured for a follow-up of 2 years until the time of fMRI both in this study sample and in the larger group (11,12). In this fMRI study of aging, we used standard registration methods and smoothing parameters, which is a potential limitation as some have suggested that structural variability in older adults limits the registration and can confound the interpretation of the fMRI results. One would expect this confound to lead to decreased activation in participants with greater atrophy. In this study, we found no difference in activation that correlated atrophy index, thus we suspect that our results are not confounded by differences in brain registration. Although this study was cross-sectional, retrospective thorough information on PA was available from 3 years prior (1-year intervention trial and 2 years of follow-up). This study did not directly investigate the reasons why some but not all the participants of the original PA arm remained active during follow-up.
Individuals in the PA group may have been selective in their decision, or need, to remain active, and this motivation may partially account for results obtained, especially in light of the role of prefrontal cortices in motivational behavior. Other possible reasons may include overall better health, greater physical fitness, or greater ECF at baseline. However, neither health status, clinical characteristics, nor cognitive tests of global function significantly differed between groups at baseline or at the time of fMRI. It is also possible that differences were not significant due to the relatively small sample size. Future intervention studies in larger groups with detailed assessments of mood, cognition, and brain neuroimaging are warranted to examine potential explanatory factors of the association between long-term adherence to PA and greater brain activation and also to examine whether long-term adherence to PA is related to distinct patterns of brain activation compared with short-term adherence.
Funding
This work was supported in part by the Intramural Research program and the National Institute on Aging, National Institutes of Health. Dr. Rosano is a Pepper Scholar (1P30 AGO24827-01) and Beeson Scholar (K23AG028966-01) and is supported by the National Institute on Aging (grant numbers RO3 AG025076-02 and R01 AG029232).
Supplementary Material
Supplementary material can be found at: http://biomed.gerontologyjournals.org/
Acknowledgments
The authors thank Pamela Vincent, Piera Kost, and Susan Urda for assistance in obtaining the data necessary for this analysis. There are no actual or potential conflicts of interest.
References
- 1.Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11(8):342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Taylor AH, Cable NT, Faulkner G, Hillsdon M, Narici M, Van Der Bij AK. Physical activity and older adults: a review of health benefits and the effectiveness of interventions. J Sports Sci. 2004;22(8):703–725. doi: 10.1080/02640410410001712421. [DOI] [PubMed] [Google Scholar]
- 3.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 4.Dishman RK, Berthoud HR, Booth FW. Neurobiology of exercise. Obesity (Silver Spring) 2006;14(3):345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- 5.Small GW, Silverman DH, Siddarth P, Ercoli LM, Miller KJ, Lavretsky H, Wright BC. Effects of a 14-day healthy longevity lifestyle program on cognition and brain function. Am J Geriatr Psychiatry. 2006;14(6):538–545. doi: 10.1097/01.JGP.0000219279.72210.ca. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 7.Colcombe SJ, Erickson KI, Raz N. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58(2):176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 8.Colcombe SJ, Erickson KI, Scalf PE. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 9.Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22(3):612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pahor M, Blair S, Espeland M. Effects of a physical activity intervention on measures of physical performance: results of the lifestyle interventions and independence for elders pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61:1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 11.Williamson JD, Espeland M, Kritchevsky SB, Newman AB, King AC, Pahor M, Guralnik JM, et al. Changes in cognitive function in a randomized trial of physical activity: results of the lifestyle interventions and independence for elders pilot study. J Gerontol A Biol Sci Med Sci. 2009;64(6):688–694. doi: 10.1093/gerona/glp014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rejeski WJ, Marsh AP, Chmelo E, Prescott AJ, Dobrosielski M, Walkup MP, Espeland M, et al. The lifestyle interventions and independence for elders pilot (LIFE-P): 2-year follow-up. J Gerontol A Biol Sci Med Sci. 2009;64(4):462–467. doi: 10.1093/gerona/gln041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katula JA, Kritchevsky SB, Guralnik JM, Glynn NW, Pruitt L, Wallace K, Walkup MP, et al. Lifestyle interventions and independence for elders pilot study: recruitment and baseline characteristics. J Am Geriatr Soc. 2007;55(5):674–683. doi: 10.1111/j.1532-5415.2007.01136.x. [DOI] [PubMed] [Google Scholar]
- 14.Stewart AL, Verboncoeur CJ, McLellan BY. Physical activity outcomes of CHAMPS II: a physical activity promotion programs for older adults. J Gerontol A Biol Sci Med Sci. 2001;56A:M465–M470. doi: 10.1093/gerona/56.8.m465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosano C, Newman AB, Katz R, Hirsch CH, Kuller LH. Association between lower digit symbol substitution test score and slower gait and greater risk of mortality and of developing incident disability in well-functioning older adults. J Am Geriatr Soc. 2008;56(9):1618–1625. doi: 10.1111/j.1532-5415.2008.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salthouse TA. The role of memory in the age decline in digit-symbol substitution performance. J Gerontol. 1978;33:232–238. doi: 10.1093/geronj/33.2.232. [DOI] [PubMed] [Google Scholar]
- 17.Friston K, Holmes A, Worsley K. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 18.Rosano C, Aizenstein HJ, Cochran JL, Saxton JA, De Kosky ST, Newman AB, Kuller C, et al. Event-related functional magnetic resonance imaging investigation of executive control in very old individuals with mild cognitive impairment. Biol Psychiatry. 2005;57(7):761–767. doi: 10.1016/j.biopsych.2004.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Brady M, Smith SM. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 21.Wu M, Carmichael O, Lopez-Garcia P, Carter CS, Aizenstein HJ. Quantitative comparison of AIR, SPM, and the fully deformable model for atlas-based segmentation of functional and structural MR images. Hum Brain Mapp. 2006;27(9):747–754. doi: 10.1002/hbm.20216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thirion JP. Image matching as a diffusion process: an analogy with Maxwell's demons. Med Image Anal. 1998;2(3):243–260. doi: 10.1016/s1361-8415(98)80022-4. [DOI] [PubMed] [Google Scholar]
- 23.Udupa JK, Samarasekera S. Fuzzy connectedness and object definition: theory, algorithms, and applications in image segmentation. Graph Models Image Process. 1996;58:246–261. [Google Scholar]
- 24.Zandbelt BB, Gladwin TE, Raemaekers M, van Buuren M, Neggers SF, Kahn RS, Ramsey NF, et al. Within-subject variation in BOLD-fMRI signal changes across repeated measurements: quantification and implications for sample size. Neuroimage. 2008;42(1):196–206. doi: 10.1016/j.neuroimage.2008.04.183. [DOI] [PubMed] [Google Scholar]
- 25.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 26.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 27.Rosano C, Becker J, Lopez O, Lopez-Garcia P, Carter CS, Newman A, Kuller L, et al. Morphometric analysis of gray matter volume in demented older adults: exploratory analysis of the cardiovascular health study brain MRI database. Neuroepidemiology. 2005;24(4):221–229. doi: 10.1159/000085140. [DOI] [PubMed] [Google Scholar]
- 28.Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 29.McCloskey DP, Adamo DS, Anderson BJ. Exercise increases metabolic capacity in the motor cortex and striatum, but not in the hippocampus. Brain Resolut. 2001;891(1–2):168–175. doi: 10.1016/s0006-8993(00)03200-5. [DOI] [PubMed] [Google Scholar]
- 30.Colcombe SJ, Kramer AF, Erickson KI. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101(9):3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]