Abstract
Context: Polycystic ovary syndrome (PCOS) is a complex disorder having both genetic and environmental components. A number of association studies based on candidate genes have reported significant association, but few have been replicated. D19S884, a polymorphic marker in fibrillin 3 (FBN3), is one of the few association findings that has been replicated in independent sets of families.
Objective: The aims of the study are: 1) to genotype single nucleotide polymorphisms (SNPs) in the region of D19S884; and 2) to follow up with an independent data set, published results reporting evidence for PCOS candidate gene associations.
Design: The transmission disequilibrium test (TDT) was used to analyze linkage and association between PCOS and SNPs in candidate genes previously reported by us and by others as significantly associated with PCOS.
Setting: The study was conducted at academic medical centers.
Patients or Other Participants: A total of 453 families having a proband with PCOS participated in the study. Sisters with PCOS were also included. There was a total of 502 probands and sisters with PCOS.
Intervention(s): There were no interventions.
Main Outcome Measure(s): The outcome measure was transmission frequency of SNP alleles.
Results: We identified a six-SNP haplotype block spanning a 6.7-kb region on chromosome 19p13.2 that includes D19S884. SNP haplotype allele-C alone and in combination with D19S884-allele 8 is significantly associated with PCOS: haplotype-C TDT χ2 = 10.0 (P = 0.0016) and haplotype-C/A8 TDT χ2 = 7.6 (P = 0.006). SNPs in four of the other 26 putative candidate genes that were tested using the TDT were nominally significant (ACVR2A, POMC, FEM1B, and SGTA). One SNP in POMC (rs12473543, χ2 = 9.1; Pcorrected = 0.042) is significant after correction for multiple testing.
Conclusions: A polymorphic variant, D19S884, in FBN3 is associated with risk of PCOS. POMC is also a candidate gene of interest.
Family-based transmission disequilibrium test analysis provides evidence for genetic association between PCOS and polymorphic variants in two genes, FBN3 and POMC.
Polycystic ovary syndrome (PCOS) is a common endocrine disorder with a prevalence between 5 and 10% of reproductive-age women (1). In addition to the hallmark features of hyperandrogenemia, chronic anovulation, and infertility, PCOS women are at increased risk for obesity, insulin resistance, type 2 diabetes, and cardiovascular disease (2). Thus, PCOS represents a disorder with broader implications to a woman’s health than reproduction.
Legro et al. (3) described evidence for familial aggregation of hyperandrogenemia in PCOS that is consistent with a genetic contribution to the disease. This observation has been confirmed by others (4,5), providing a strong foundation for research into the genetic contributions to PCOS risk. However, to date, there is no consensus in the literature regarding any proposed PCOS candidate gene, despite the fact that numerous association studies have been published. Most nominally significant positive findings have not been replicated or have not been confirmed in follow-up studies (5,6).
We identified a PCOS candidate region surrounding D19S884, a microsatellite marker in intron 55 of fibrillin 3 (FBN3), located on chromosome 19p13.2 about 800 kb centromeric to the insulin receptor (INSR) gene (7). Previously, using the transmission disequilibrium test (TDT), we replicated significant association and linkage with this marker and PCOS in three independent sets of samples (7,8,9,10). D19S884 was also shown to be associated with PCOS in two independent association studies (11,12), but others have failed to find association in smaller study populations (13,14). The FBN3 gene encodes an extracellular matrix protein, and although the role of FBN3 in PCOS remains unclear, FBN3 expression can be demonstrated in the human ovary (14,15). Furthermore, other members of the fibrillin family of proteins are known to bind members of the TGFβ family (16), which play significant roles in controlling ovarian follicular development (17), as well as muscle and adipocyte development and differentiation (18,19,20), all processes that can be impaired in PCOS.
To further investigate the role of variation in the region of D19S884, we extended our earlier TDT observations of D19S884 by identifying a six-single nucleotide polymorphism (SNP) haplotype allele that is significantly associated with PCOS. We also performed family-based analysis using the TDT to assess linkage and association between PCOS and other candidate genes that arose from our earlier work (7) or published studies from other laboratories (21,22,23,24,25,26,27,28,29,30). We followed up 14 genes that showed either increased allele sharing among siblings or nominally significant TDT results in Urbanek et al. (7): ACVR2A, CYP11A, CYP17, CYP19, HSD17B2, IGF1R, INHBA, INHBB, INSL3, INSR, SMAD4, LEP, POMC, and SHBG. We also followed up SNPs and SNP haplotypes in 12 genes shown to have a significant association with PCOS when analyzed in case-control studies in Caucasian women. These include ADIPOQ (25), CAPN5 (21), ENPP1/PC-1 (26), EPHX1 (29), FEM1A and FEM1B (22), H6PD (30), HSD17B6 (27), IL1A (28), SGTA (24), SRD5A1, and SRD5A2 (23). Among these genes, ACVR2A, POMC, FEM1B, and SGTA were nominally significant, but only POMC is significant after correction for multiple testing.
Subjects and Methods
PCOS families
SNP markers were genotyped in 453 families with PCOS: 406 simplex families (one affected daughter and both parents) and 47 multiplex families (two or more affected daughters and both parents). The total number of offspring with PCOS was 502, including 49 affected sisters in the 44 multiplex families. The self-identified ethnicities of probands in the families were: 87% white, 4% Hispanic, 1% black, and 7% other or unknown. A total of 373 families are in common with those reported in Stewart et al. (8). Ninety-two families from the collection reported in Stewart et al. (8) were not included in the current study due to consistent Mendelian exclusions (n = 2), depleted DNA stock (n = 25), parental DNA that was not obtainable (n = 62), or changes of proband phenotype (n = 3). Since the Stewart et al. (8) publication, an additional 80 families have been added to the collection.
Diagnostic criteria have been described in detail elsewhere (3,7). Probands and sisters were considered affected if they had six or fewer menses per year and elevated total testosterone (greater than 58 ng/dl) or elevated non-SHBG-bound testosterone (greater than 15 ng/dl); these thresholds are 2 sd greater than the mean of our normal control individuals (3,7). To ensure a more homogeneous collection of PCOS women, we included only those sisters who met the same diagnostic criteria as the proband. This represents a change from the previous assignment of affected status for sisters, which was based on elevated total testosterone or non-SHBG-bound testosterone only, without a requirement of six or fewer menses per year.
This study was approved by the institutional review boards of the University of Pennsylvania, Penn State University College of Medicine, Brigham and Women’s Hospital, and Northwestern University. Written informed consent was obtained from all adult subjects and from a parent or guardian for minor subjects.
SNP genotyping
We genotyped 104 SNPs in 27 candidate genes. SNPs in 12 of these genes have been found to be associated with PCOS disease risk by others in case-control studies. To increase our likelihood of replicating results from previous PCOS association studies, we restricted our follow-up studies to those that were carried out in Caucasian women having at least 100 PCOS cases. Whenever possible, we genotyped the same SNP as reported in the literature. In three cases (rs1640262 in SGTA, rs3797179 in SRD5A1, and rs2754530 in SDR5A2), the assay for the reported SNP failed, and a nearby SNP in strong linkage disequilibrium (LD) was substituted. For replication of linkage and association results with microsatellite markers located near candidate genes reported in Urbanek et al. (7), we chose SNPs with minor allele frequency greater than 0.2 spaced approximately every 10 kb across the gene. SNPs were genotyped with Applied Biosystems TaqMan SNP Genotyping Assays (Applied Biosystems, Foster City, CA). Allelic PCR products were analyzed using the Applied Biosystems 7900HT Sequence Detection System and SDS 2.2 software. Genotypes were auto-called by SDS 2.2 software with the quality value set at 0.95. Three Centre d’Etude du Polymorphisme Humain individuals were genotyped on each of 16 96-well plates; no discrepancies were observed for any of the SNPs. All SNPs were in Hardy-Weinberg equilibrium. (Details of the location and size of the genes studied, SNP location, and SNP genotyping assays can be found in Supplemental Tables 1 and 2, published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org.)
Statistical analysis
Error-checking of genotypes in the family material was performed with Merlin software (31), and families with one or more Mendelian discrepancies for a marker were excluded in the analysis of that marker. Association between SNPs and PCOS was tested with the TDT (32), which tests for the combined presence of linkage and association with the marker.
Because markers within a gene tend to be correlated to varying degrees, correction for multiple testing with Bonferroni methods using the total number of markers tested is likely to be too stringent. We corrected for the number of genes tested separately in each of the two categories of follow-up studies: 1) 14 genes with suggestive evidence for linkage or association with PCOS in Urbanek et al. (7); and 2) 12 genes shown to be associated with PCOS in studies by other laboratories (21,22,23,24,25,26,27,28,29,30). The corrected P value in each case is 0.0036 and 0.0042, respectively, corresponding to a nominal P of 0.05.
The haplotype structure for SNPs in FBN3 and D19S884 was determined using HAPLORE v242 (http://www.soph.hab.edu/stratgenetics/People/KZhang/HAPLORE) (33) which allows input of multiple D19S884 alleles. In all other cases, haplotypes were constructed and analyzed with Haploview v4.1 (http://www.broadinstitute.org/mpq/haploview) (34).
Evolutionary conservation
We compared an approximately 2.2-kb interval in FBN3 containing D19S884 (chr 19: 8,054,042–8,056,288; March 2006 human genome assembly version hg18) against multiple other species using the Multiz Alignment and Conservation (44 species), Basewise Conservation by PhyloP for 28-Species (Placental Mammal and Vertebrate), PhastCons Placental Mammal Conserved Elements (28-way Multiz Alignment), PhastCons Vertebrate Conserved Elements (28-way Multiz Alignment), and PhastCons Vertebrate Conserved Elements (17-way Vertebrate Multiz Alignment) tracks in the University of California Santa Cruz Genome Browser (http://genome.usc.edu). To more quantitatively determine the evolutionary conservation of the intron harboring D19S884 (between exons 55 and 56 in FBN3), we compared the distribution of its 28-way PhyloP scores to the distribution from 1000 random introns using the Wilcoxon rank sum test.
DNA topography
The three-dimensional molecular structure of DNA is affected by nucleotide changes, which can cause differences in protein binding and affinity. We measured the level of change in DNA shape that arises from noncoding SNPs (rs3745393, rs2303169, rs17160147, rs17202517, rs73503752, and rs28525575) near D19S884 (35). We compared these levels of change across haplotype alleles by ranking the structure-change-value as determined by predicted hydroxyl radical cleavage patterns (36) for the six SNPs in the haplotype. All the structure change comparisons are relative to the reference allele. We used the Wilcoxon rank sum test to compare the distribution of changes associated with each haplotype to determine significance.
Results
SNPs in the D19S884 region of FNB3
The region around D19S884 continues to have the most convincing evidence for association with PCOS, having been replicated in three independent data sets by us (7,8,9) and others (11,12). To further investigate this region, we genotyped 17 SNPs spanning a 77-kb region of FBN3 that included D19S884 in our full collection of 453 families (Table 1). The strongest evidence for association with PCOS based on our existing collection of families is allele 8 (A8) of D19S884 (χ2 = 13.64; P = 0.0002). Three SNPs located within a 2-kb region of introns 55 and 56 telomeric to D19S884 were also significant: rs17160147 (χ2 = 4.06; P = 0.044), rs17202517 (χ2 = 10.51; P = 0.0012), and rs73503752 [χ2 = 10.84; P = 0.001; identified as FBN3-6197 in Stewart et al. (8)]. SNPs rs17202517 and rs73503752 are significant after correction for testing 18 markers in FBN3.
Table 1.
TDT results for the microsatellite marker D19S884 and 17 SNPs in FBN3
| Marker ID | Position | Allelesa | MAF | Overtransmitted allele | T | Not T | Total TDT | Transmission frequency | TDT χ2 | P value (nominal) |
|---|---|---|---|---|---|---|---|---|---|---|
| rs2287937 | 8036419 | C/G | 0.18 | C | 114 | 96 | 210 | 0.543 | 1.54 | >0.05 |
| rs3745393 | 8049164 | A/G | 0.437 | A | 245 | 211 | 456 | 0.537 | 2.53 | >0.05 |
| rs2303169 | 8052113 | G/A | 0.441 | G | 242 | 215 | 457 | 0.530 | 1.60 | >0.05 |
| rs17160147 | 8054041 | C/G | 0.317 | C | 217 | 177 | 394 | 0.551 | 4.06 | 0.044 |
| rs17202517 | 8054300 | C/T | 0.231 | T | 195 | 136 | 331 | 0.589 | 10.51 | 0.0012b |
| rs73503752c | 8055015 | G/A | 0.238 | A | 190 | 131 | 321 | 0.592 | 10.84 | 9.9E-04b |
| rs28525575 | 8055474 | A/C | 0.487 | C | 238 | 212 | 450 | 0.529 | 1.50 | >0.05 |
| D19S884 | 8056105-8056141 | 7 | 106 | 101 | 207 | 0.512 | 0.12 | >0.05 | ||
| 8 | 177 | 114 | 291 | 0.608 | 13.64 | 2.2E-04b | ||||
| 9 | 96 | 109 | 205 | 0.468 | 0.82 | >0.05 | ||||
| 11 | 82 | 73 | 155 | 0.529 | 0.52 | >0.05 | ||||
| 13 | 153 | 168 | 321 | 0.477 | 0.70 | >0.05 | ||||
| rs7260399 | 8056661 | C/T | 0.478 | T | 196 | 166 | 362 | 0.541 | 2.49 | >0.05 |
| rs8103000 | 8057238 | C/G | 0.282 | C | 135 | 128 | 263 | 0.513 | 0.19 | >0.05 |
| rs12460643 | 8058724 | C/T | 0.266 | T | 139 | 133 | 272 | 0.511 | 0.13 | >0.05 |
| rs12981294 | 8062220 | A/G | 0.227 | G | 142 | 121 | 263 | 0.540 | 1.68 | >0.05 |
| rs7245552 | 8065408 | T/G | 0.38 | T | 190 | 175 | 365 | 0.520 | 0.62 | >0.05 |
| rs3813780 | 8067609 | C/T | 0.228 | T | 117 | 104 | 221 | 0.529 | 0.76 | >0.05 |
| rs2086149 | 8083619 | C/T | 0.278 | C | 136 | 136 | 272 | 0.500 | 0 | >0.05 |
| rs4804064 | 8094934 | G/A | 0.268 | A | 122 | 119 | 241 | 0.506 | 0.04 | >0.05 |
| rs12974280 | 8102507 | G/C | 0.379 | C | 150 | 149 | 299 | 0.502 | 0.003 | >0.05 |
| rs12162237 | 8113069 | G/A | 0.455 | A | 182 | 175 | 357 | 0.510 | 0.14 | >0.05 |
MAF, Minor allele frequency; T, number of transmissions in TDT analysis.
Minor allele in bold.
Remained significant after correction for multiple testing.
SNP rs73503752 was identified as FBN3-6197 in Stewart et al. (8).
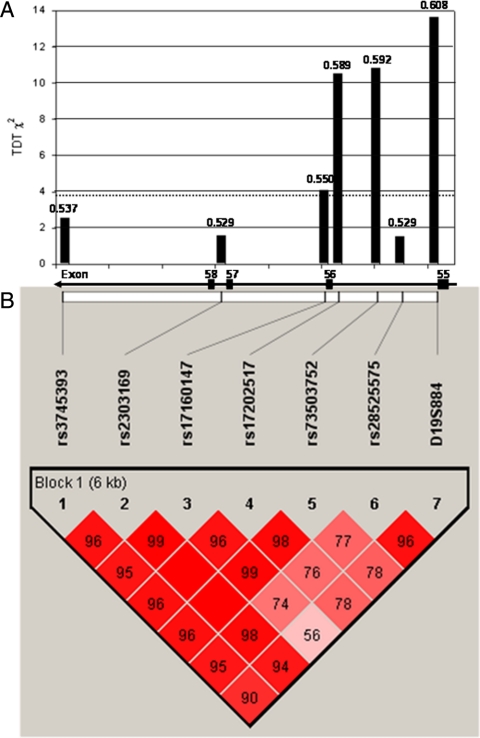
These three SNPs plus rs3745393, rs2303169, rs28525575, and D19S884 constitute a haplotype block (Fig. 1). LD among these seven markers in our study population is strong and ranged between 0.77 and 0.99 (average D′ = 0.94; median = 0.96). (The range of r2 was 0.13 to 0.94; average r2 = 0.46.) We first carried out TDT analysis with haplotypes defined by the SNPs alone, without including alleles of D19S884; the third most common haplotype, designated allele-C, is significantly overtransmitted to offspring with PCOS (χ2 = 10.0; P = 0.0016) (Table 2). Although not significant, haplotypes A and B are both undertransmitted. When D19S884 alleles were included in the haplotype, A8 was found on the same haplotype as allele-C 80.1% of the time. Together, haplotype allele-C and A8 are overtransmitted in the TDT analysis (χ2 = 7.6; P = 0.0059) (Table 2).
Figure 1.
TDT and haplotype analysis of SNPs in the region of D19S884. A, Individual TDT results are shown for six SNPs and D19S884 located between exons 55 and 59 of FBN3 (number above each bar represents transmission frequency, and dashed line represents nominally significant χ2 = 3.84). Also shown are the positions of the markers relative to the exons. B, Pairwise LD plot showing D′ values (percent) in the PCOS families. For construction of haplotypes in Haploview, D19S884 was coded as a two-allele system: allele 8 or not-8. (The x-axis displays location of chromosome 19: 8049164-8056141.)
Table 2.
TDT analysis for the most common FBN3 SNP haplotypes and the SNP haplotypes combined with D19S884 alleles
| Allele frequency | T | Not T | Total | Transmission frequency | TDT χ2 | P value | |
|---|---|---|---|---|---|---|---|
| SNP haloptype allelea | |||||||
| A. GACCGA | 0.454 | 117 | 141 | 258 | 0.453 | 2.23 | >0.05 |
| B. AGGCGC | 0.278 | 94 | 118 | 212 | 0.443 | 2.72 | >0.05 |
| C. AGCTAC | 0.184 | 100 | 60 | 160 | 0.625 | 10.00 | 0.0016 |
| D. AGGCGA | 0.030 | 21 | 17 | 38 | 0.553 | 0.42 | >0.05 |
| E. AGCTAA | 0.021 | 13 | 10 | 23 | 0.565 | 0.39 | >0.05 |
| All others | 0.032 | ||||||
| Haloptype allele/D19S884 allele | |||||||
| A/11 | 0.067 | 33 | 41 | 74 | 0.446 | 0.86 | >0.05 |
| A/13 | 0.192 | 86 | 81 | 167 | 0.515 | 0.15 | >0.05 |
| B/7 | 0.107 | 54 | 54 | 108 | 0.500 | 0 | >0.05 |
| B/8 | 0.027 | 16 | 19 | 35 | 0.457 | 0.26 | >0.05 |
| B/9 | 0.092 | 41 | 53 | 94 | 0.436 | 1.53 | >0.05 |
| C/8 | 0.138 | 79 | 48 | 127 | 0.622 | 7.57 | 0.0059 |
| C/9 | 0.025 | 17 | 11 | 28 | 0.607 | 1.29 | >0.05 |
T, Number of transmissions in TDT analysis.
Order of SNPs in haplotype: rs3745393, rs2303169, rs17160147, rs17202517, rs73503752, and rs28525575.
Evolutionary conservation and DNA topography in the D19S884 region of FNB3
We investigated the degree of evolutionary conservation in a 2.2-kb interval surrounding D19S884. Aside from the expected conservation of the exons, neither the PhastCons tracks nor the PhyloP algorithm shows any region of the intron as conserved. The intron harboring D19S884 had significantly less conservation compared with 1000 random introns (P < 2.2 × 10−6; see Supplemental Figs. 1 and 2).
We also investigated whether SNPs near D19S884 might alter the three-dimensional molecular structure of the DNA, which could potentially influence protein binding and affinity properties. Three of the six SNPs had DNA structural change values greater than 0.8, suggestive of potentially functional consequences (Table 3). However, the magnitude of this structure-change-value did not correlate well with association with PCOS, as determined by TDT (Table 2). Comparison of haplotypes A–E showed that the low-frequency haplotype E had the most drastic DNA structural changes, followed by haplotype C (see Supplemental Fig. 3). However, there was no statistically significant difference (P > 0.05) in predicted DNA structure changes associated with haplotype C relative to haplotype A or B.
Table 3.
Predicted changes in three-dimensional molecular structure of DNA at noncoding SNPs near D19S884
| SNP | Position | TDT χ2 | P value (nominal) | DNA structural change |
|---|---|---|---|---|
| rs3745393 | 8049164 | 2.53 | >0.05 | 0.511 |
| rs2303169 | 8052113 | 1.60 | >0.05 | 0.262 |
| rs17160147 | 8054041 | 4.06 | 0.044 | 0.839 |
| rs17202517 | 8054300 | 10.51 | 0.0012 | 0.895 |
| rs73503752 | 8055015 | 10.84 | 9.9E-04 | 0.440 |
| rs28525575 | 8055474 | 1.50 | >0.05 | 1.395 |
DNA structural change value greater than 0.8 is suggestive of potentially functional consequences. (See Ref. 35.)
Family-based analysis of other PCOS candidate genes
Eighty-seven SNPs in 26 PCOS candidate genes were genotyped and analyzed using the TDT. Fourteen of these genes (ACVR2A, CYP11A, CYP17, CYP19, HSD17B2, IGF1R, INHBA, INHBB, INSL3, INSR, SMAD4, LEP, POMC, and SHBG) were follow-up studies of genes originally analyzed by Urbanek et al. (7). In that study, microsatellite markers that were, in some cases, quite far from the gene were used. To further investigate these candidate genes, we genotyped SNPs located approximately every 12.5 kb in each gene. We also followed up 12 genes studied in case-control studies by others: ADIPOQ (25), CAPN5 (21), ENPP1/PC-1 (26), EPHX1 (29), FEM1A and FEM1B (22), H6PD (30), HSD17B6 (27), IL1A (28), SGTA (24), SRD5A1 and SRD5A2 (23). SNPs in four of these genes had a nominally significant TDT result: ACVR2A and POMC (7), FEM1B (22), and SGTA (24) (Table 4). (See Supplemental Table 3 for a complete list of TDT results of all SNPs that were genotyped.)
Table 4.
PCOS candidate genes with one or more significant SNP (P ≤ 0.05)
| Gene (Ref.) | SNP ID | Position | Overtransmitted allele | T | Not T | Total T | Transmission frequency | TDT χ2 | P value (nominal) |
|---|---|---|---|---|---|---|---|---|---|
| ACVR2A (7) | rs1364658 | chr2:148324277 | C | 123 | 118 | 241 | 0.510 | 0.10 | >0.05 |
| rs1895694 | chr2:148336215 | T | 227 | 193 | 420 | 0.541 | 2.75 | >0.05 | |
| rs1424941 | chr2:148359587 | T | 156 | 113 | 269 | 0.580 | 6.87 | 0.009 | |
| rs12987286 | chr2:148380037 | T | 120 | 110 | 230 | 0.522 | 0.44 | >0.05 | |
| rs3768688 | chr2:148387570 | G | 189 | 146 | 335 | 0.564 | 5.52 | 0.019 | |
| rs2303392 | chr2:148396896 | C | 119 | 106 | 225 | 0.529 | 0.75 | >0.05 | |
| rs3768686 | chr2:148409245 | T | 229 | 182 | 411 | 0.557 | 5.38 | 0.020 | |
| POMC (7) | rs1042571 | chr2:25237390 | G | 113 | 107 | 220 | 0.514 | 0.16 | >0.05 |
| rs12473543 | chr2:25240684 | T | 181 | 128 | 309 | 0.586 | 9.09 | 0.003 | |
| rs934778 | chr2:25242727 | G | 96 | 88 | 184 | 0.522 | 0.35 | >0.05 | |
| rs1009388 | chr2:25244604 | C | 123 | 119 | 242 | 0.508 | 0.07 | >0.05 | |
| FEM1B (22) | rs10152450 | chr15:66358532 | G | 191 | 239 | 430 | 0.447 | 5.36 | 0.021 |
| rs6494730 | chr15:66371610 | T | 175 | 215 | 390 | 0.449 | 4.10 | 0.043 | |
| Haplotype | G-T | 197 | 238 | 435 | 0.453 | 3.86 | 0.049 | ||
| SGTA (24) | rs2238614 | chr19:2706338 | G | 175 | 134 | 309 | 0.567 | 5.44 | 0.019 |
| rs741103 | chr19:2722640 | T | 140 | 118 | 258 | 0.543 | 1.88 | >0.05 | |
| rs1005556 | chr19:2729542 | G | 181 | 144 | 325 | 0.557 | 4.21 | 0.040 | |
| Haplotype | G-T-G | 216 | 165 | 381 | 0.646 | 7.08 | 0.008 |
T, Number of transmissions in TDT analysis.
ACVR2A and POMC
Seven SNPs that span ACVR2A and four SNPs in POMC were genotyped (Table 4). Two SNPs were nominally significant in ACVR2A: rs1424941 (χ2 = 6.87; P = 0.009) and rs3768688 (χ2 = 5.52; P = 0.019), but neither was significant after correction for multiple testing for 14 genes. One of the four SNPs in POMC, rs12473543, remained significant after correction (χ2 = 9.09; P = 0.0026; Pcorrected=0.036). This SNP is located 329 bp 3′ of exon 2 in the 7.8-kb POMC gene. None of the ACVR2A or POMC SNP haplotype blocks were significantly associated with PCOS in the TDT analysis.
FEM1B and SGTA
Our results for two SNPs in FEM1B and three SNPs in SGTA confirm the findings of Goodarzi et al. (22,24) (Table 4). In our results and those of Goodarzi et al. (22), the same (minor) alleles of rs10152450 and rs6494730 in FEM1B had a reduced frequency with PCOS or were undertransmitted in the TDT analysis. Goodarzi et al. (22) also reported a five-SNP haplotype with reduced frequency with PCOS: G-G-A-A-T, P = 0.004. We genotyped only the first and last SNPs that tagged their significant haplotype; the allele carrying the minor alleles of both SNPs, G and T, was also undertransmitted in the TDT analysis (χ2 =3.86; P = 0.049).
Goodarzi et al. (24) analyzed three SNPs (rs2238614, rs741103, and rs1640262) in SGTA, a gene on chromosome 19p13, 5.3 MB telomeric to D19S884. Only rs1640262 was significant, with the minor allele CC genotype being protective against PCOS (P = 0.009). We genotyped both rs2238614 and rs741103 but replaced rs1640262 with rs1005556. [The replacement SNP rs1005556 is located in 590 bp 5′ of rs1640262, and the two SNPs are in complete LD (D′ = 1 in HapMap_CEU).] In our analysis, rs1005556 was also significant; the minor T allele was undertransmitted, in agreement with Goodarzi et al. (24) (TDT χ2 = 4.2; P = 0.040). The most common haplotype containing the major alleles at all three SNPs was significant in both the Goodarzi et al. study (rs2238614-rs741103-rs1640262: G-A-T; P = 0.015) and our analysis (rs2238614-rs741103-rs1005556: G-T-G; TDT χ2 =7.08; P = 0.008). However, none of the results for these four SNPs were significant after correcting for the 12 genes tested in this follow-up study.
None of the other SNPs genotyped in this family-based replication of previously reported case-control studies showed significant evidence for linkage or association in our TDT analysis (Table 5). This includes SNPs and SNP haplotypes in ADIPOQ (25), CAPN5 (21), ENPP1/PC-1 (26), EPHX1 (29), FEM1A (22), H6PD (30), HSD17B6 (27), IL1A (28), SRD5A1 and SRD5A2 (23).
Table 5.
TDT analysis for SNPs and haplotypes in candidate genes previously reported in the literature as being associated with PCOS
| Gene tested (Ref.) | SNP tested | Major/ minor alleles (+ strand) | Literature studies
|
TDT results
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of cases: controls | Population | Reported association: P value | Allele | T | Not T | Total | Transmission frequency | TDT χ2 | P value | |||
| ADIPOQ (25) | rs2241766 | T/G | 143:245 | Finland | ns | T | 46 | 44 | 90 | 0.511 | 0.04 | 0.841 |
| rs1501299 | G/T | T reduced frequency: 0.047 | T | 193 | 160 | 353 | 0.547 | 3.09 | 0.079 | |||
| Haplotype | ns | TT | 114.8 | 95.9 | 210.7 | 0.545 | 1.70 | 0.192 | ||||
| CAPN5 (21) | rs948976 | A/G | 148:606 | Spain | ns | G | 184 | 169 | 353 | 0.521 | 0.64 | 0.425 |
| rs4945140 | G/A | ns | G | 219 | 212 | 431 | 0.508 | 0.11 | 0.736 | |||
| rs2233546 | C/T | ns | T | 44 | 39 | 83 | 0.530 | 0.30 | 0.583 | |||
| rs2233549 | G/A | ns | A | 159 | 152 | 311 | 0.511 | 0.16 | 0.691 | |||
| Haplotype | GGCA: 0.009 | GGCA | 49.3 | 44.5 | 93.8 | 0.526 | 0.24 | 0.623 | ||||
| Haplotype | GGTG: 0.001 | GGTG | 25.3 | 20.5 | 45.8 | 0.552 | 0.50 | 0.478 | ||||
| ENPP1/ PC1 (26) | rs1044498 | A/C | 143:115 | Finland | AC or CC: <0.01 | A | 131 | 104 | 235 | 0.557 | 3.10 | 0.078 |
| EPHX1 (29) | rs1051740 | T/C | 112:115 | Finland | ns | C | 196 | 179 | 375 | 0.523 | 0.77 | 0.380 |
| rs2234922 | A/G | ns | G | 86 | 72 | 158 | 0.544 | 1.24 | 0.265 | |||
| Haplotype | CG: 0.026 | CG | 35.2 | 48.3 | 83.5 | 0.422 | 0.92 | 0.338 | ||||
| FEM1A (22) | rs1044386 | G/A | 287:187 | United States (Alabama) | 0.013 | A | 158 | 146 | 304 | 0.520 | 0.474 | 0.491 |
| rs8111933 | C/G | 0.001 | G | 217 | 215 | 432 | 0.502 | 0.01 | 0.924 | |||
| Haplotype | AC: 0.013 | AC | 162.8 | 154.7 | 317.5 | 0.513 | 0.21 | 0.651 | ||||
| Haplotype | GG: 0.001 | GG | 226.3 | 229.1 | 455.4 | 0.497 | 0.02 | 0.897 | ||||
| FEM1B (22) | rs10152450 | T/G | 287:187 | United States (Alabama) | G reduced frequency: 0.005 | T | 239 | 191 | 430 | 0.556 | 5.36 | 0.021 |
| rs6494730 | G/T | ns | G | 215 | 175 | 390 | 0.551 | 4.10 | 0.043 | |||
| Haplotype | G(GAA)T: 0.004 | GT | 197 | 238 | 435 | 0.453 | 3.86 | 0.049 | ||||
| H6PD (30) | rs6688832 | G/A | 116:76 | Spain | Genotype frequency: 0.011 | G | 178 | 166 | 344 | 0.517 | 0.42 | 0.517 |
| HSD17B6 (27) | rs898611 | T/C | 173:107 | Australia | T reduced frequency: 0.03 | T | 218 | 208 | 426 | 0.512 | 0.24 | 0.628 |
| IL1A (28) | rs1800587 | G/A | 105:102 | Austria | Genotype frequency: 0.04 | A | 190 | 189 | 379 | 0.501 | 0.00 | 0.956 |
| SGTA (24) | rs2238614 | G/A | 287:187 | United States (Alabama) | ns | G | 175 | 134 | 309 | 0.566 | 5.44 | 0.020 |
| rs741103 | T/C | ns | T | 140 | 118 | 258 | 0.543 | 1.88 | 0.171 | |||
| rs1640262 | T/C | CC protective: 0.009 | Did not genotype | |||||||||
| rs1005556a | G/T | G | 181 | 144 | 325 | 0.557 | 4.21 | 0.040 | ||||
| Haplotype | GAT: 0.015 | GTG | 216.4 | 164.5 | 380.9 | 0.568 | 7.08 | 0.008 | ||||
| SRD5A1 (23) | rs3797179 | G/A | 287:187 | United States (Alabama) | ns | Did not genotype | ||||||
| rs6872996b | C/T | C | 193 | 172 | 365 | 0.529 | 1.21 | 0.272 | ||||
| rs39848 | T/C | ns | T | 231 | 205 | 436 | 0.530 | 1.55 | 0.213 | |||
| Haplotype | TA: 0.03 | TC | 182.6 | 200.1 | 382.7 | 0.477 | 0.80 | 0.371 | ||||
| (Continued) | ||||||||||||
Table 5A.
Continued
| Gene tested (Ref.) | SNP tested | Major/ minor alleles (+ strand) | Literature studies
|
TDT results
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of cases: controls | Population | Reported association: P value | Allele | T | Not T | Total | Transmission frequency | TDT χ2 | P value | |||
| SRD5A2 (23) | rs11889731 | T/G | 287:187 | United States (Alabama) | Data not shown | T | 107 | 104 | 211 | 0.507 | 0.04 | 0.836 |
| rs7571644 | A/G | Data not shown | Did not genotype | |||||||||
| rs12470143 | C/T | Data not shown | C | 235 | 227 | 462 | 0.509 | 0.14 | 0.709 | |||
| rs12467911 | C/T | Data not shown | T | 200 | 192 | 392 | 0.510 | 0.16 | 0.686 | |||
| rs2300697 | T/C | Data not shown | Did not genotype | |||||||||
| Haplotype A | T(A)CC(C): 0.003 | TCC | 89.6 | 94.5 | 184.1 | 0.487 | 0.13 | 0.718 | ||||
| rs11675297 | G/A | Data not shown | G | 43 | 32 | 75 | 0.573 | 1.61 | 0.204 | |||
| rs2754530 | C/T | Data not shown | Did not genotype | |||||||||
| rs765138c | G/T | G | 208 | 203 | 411 | 0.506 | 0.06 | 0.805 | ||||
| Haplotype B | GC: 0.019 | GG | 241.1 | 231.8 | 472.9 | 0.510 | 0.18 | 0.669 | ||||
| GT: 0.021 | GT | 229.8 | 227.8 | 457.6 | 0.503 | 0.01 | 0.925 | |||||
| rs523349 | C/G | C:0.015 | C | 183 | 175 | 358 | 0.511 | 0.18 | 0.672 | |||
Minor allele in bold. ns, Not significant.
SGTA rs1640262 genotyping failed and was replaced with rs1005556, which is also in intron 1, 590 bp 3′ of rs1640262; rs1005556: G/T.
SRD5A1 rs3797179 genotyping assay failed and was replaced with rs6872996, which is also in intron 4 180 bp 3′ of rs3797179; rs6872996: C/T.
SRD5A2 rs2754530 genotyping assay failed and was replaced with rs765138, which is also in intron 1 536 bp 3′ of rs2754530; rs765138: G/T.
Gene expression of FBN3, ACVR2, POMC, FEM1B, and SGTA was analyzed in cultured human theca cells by PCR, and all five genes found to be associated with PCOS by TDT analysis were also found to be expressed in these cells (Supplemental Fig. 4).
Discussion
Previous studies from our laboratory and others have presented strong evidence that D19S884 itself, or a nearby determinant, acts as a susceptibility locus for PCOS (7,8,9,10,11,12). In this present study using TDT analysis, we identified a six-SNP haplotype, designated allele-C, which is in LD with D19S884 and is significantly overtransmitted to PCOS offspring. When the SNP-haplotype alleles are combined with D19S884 alleles, the frequency of A8 association with haplotype allele-C is 0.81. Together, allele-C and A8 are also significantly overtransmitted (frequency = 0.622; χ2 = 7.57; P = 0.006).
Investigation of evolutionary conservation in the region of D19S884 or changes in molecular DNA structure due to the SNPs in the haplotype associated with PCOS failed to provide any information on the role this region plays in PCOS. We were not able to correlate predicted changes in DNA molecule shape with either SNPs or haplotypes known to be associated with PCOS. (The analysis considered only changes in DNA topography that are induced by SNPs, not the effects of different alleles of D19S884.) Furthermore, the 2.2-kb interval that includes D19S884 and the intron between exons 55 and 56 is significantly less evolutionarily conserved relative to 1000 other random human introns. This lack of intronic evolutionary conservation and DNA structural changes from SNPs suggests that these variants may not be functionally relevant. Instead, there may be a functional role for specific alleles of D19S884, rather than nearby (but unknown) regulatory variants.
Two recent studies by Prodoehl et al. (14) and Urbanek et al. (10) examined a large number of SNPs in FBN3 for association with PCOS. The case-control study in Australians reported in Prodoehl failed to find an association with D19S884-A8 (Pcorrected = 1.0). They also genotyped 30 SNPs in FBN3, but only rs2303169 and rs17160147 were in the region of our PCOS-associated haplotype. Our study included both of these SNPs. Although rs2303169 was part of the haplotype block, by itself it was not associated with PCOS, and whereas rs17160147 was nominally significant in the TDT, it was not significant after correction for multiple testing. One explanation for the discrepancy in the D19S884 results between these two studies is differences in study design: case-control compared with a family-based method. In addition, there are differences in diagnostic criteria that are commented on below.
Urbanek et al. (10) also reported TDT results for a large number of SNPs in FBN3 in a collection of families that largely overlaps the families genotyped in the present study. Four of the FBN3 SNPs genotyped in the current study were in common with Urbanek et al. (10): rs17202517 (designated F9 in Urbanek et al.) was significant in both studies; rs17160147 (Fin55a) was nominally significant in the current study and not significant in Urbanek et al., whereas rs28525575 (Fin55e) and rs2303169 (F8) were not significant in either study. The most significant SNP in the current study, rs73503752 (TDT χ2 = 10.84; P = 0.001), was not genotyped by Urbanek et al. (10), whereas Fin55b (rs17160149), Fin55c (an insertion/deletion variant rs55990065), and Fin55d were genotyped only in Urbanek et al. and found not to be significant.
The D19S884 region of FBN3 continues to have the most convincing evidence of association with PCOS; however, many other PCOS association studies have reported significant evidence for the role of other candidate genes in susceptibility to PCOS, some of which we have followed up in the current study. We found that SNPs POMC, ACVR2A, FEM1B, and SGTA were nominally significant.
POMC, a small 7.8-kb gene on chromosome 2p23.3, encodes a polypeptide hormone that is alternatively cleaved to form up to 10 different peptide molecules in a tissue-specific manner. These include α-, β-, and γ-MSH; β- and γ-lipotrophin; ACTH; and β-endorphin. These molecules are involved in different processes including steroidogenesis, energy metabolism, obesity, and lipolysis, all of which potentially play a role in PCOS (37). SNP rs12473543, for which we found significant association with PCOS, is located 329 bp 3′ of exon 2, which encodes the signal peptide, and 2.6 kb 5′ of exon 3, which encodes the peptide hormones. Because this SNP resides in an intron and is not in LD with any of the other nearby SNPs, its functional significance in PCOS has yet to be determined.
ACVR2A is the receptor for activin A, a member of the TGFβ superfamily. The involvement of activin A and ACVR2A in follicular development, regulation of FSH-β biosynthesis, as well as glucose metabolism (38) make ACVR2A a potential candidate for playing a role in PCOS. Seven SNPs in the 83.6-kb ACVR2A gene were genotyped, and three were found to be nominally significant in the TDT analysis. The most significant SNP, rs1424941, is found in intron 1 (χ2 = 6.9; P = 0.009).
Few of the PCOS genetic associations reported in the literature have been followed up in replication studies. Short of biological proof of the role of putative causal SNPs in the development of PCOS, replication of association results in independent samples remains the gold standard of confirmation. Here, we followed up candidate genes identified in 12 case-control studies in Caucasian women. We confirmed that SNPs in FEM1B and SGTA are associated with PCOS risk (Table 4).
We were not able to replicate many of the other published association results. The major differences between our study and the published studies are sample size (the largest number of cases studied was 287, compared with 453 probands plus 49 sisters with PCOS in our study) and diagnostic criteria. PCOS is a heterogeneous disease with multiple diagnostic traits, and different combinations of these traits are often present in different women. We have attempted to limit the heterogeneous nature by phenotyping both probands and sisters based solely on elevated testosterone levels (total testosterone >58 ng/dl or non-SHBG-bound testosterone >15 ng/dl) and a history of six or fewer menses per year (3). In other studies, PCOS diagnosis can include a requirement for polycystic ovaries or use clinical hyperandrogenism rather than elevated serum testosterone levels. Thus, it is possible that our narrower definition of PCOS compared with that used in some other studies could contribute to the failure to replicate these results.
Several of the PCOS candidate genes evaluated in this study are expressed in human theca cells, including ACVR2, FEM1B, SGTA, and POMC (Supplemental Fig. 4). Because the theca cells in PCOS have been shown to have distinct biochemical and molecular phenotypes (39,40,41), the fact that these genes are expressed in the thecal compartment is of interest with respect to their potential pathophysiological roles in contributing to hyperandrogenemia of ovarian origin.
Our observations provide further support for a PCOS gene in the region of D19S884, although the nature of this gene or the mechanism by which genetic variation in this region contributes to PCOS phenotypes remains to be elucidated. In addition, POMC should be considered as a candidate of interest in future studies.
Supplementary Material
Acknowledgments
The authors thank subjects and their families for participating in this study. We also thank the study coordinators (B. Scheetz, S. Ward, and J. Schindler) and the nursing staff of Pennsylvania State University, Brigham and Women’s Hospital, and Northwestern University General Clinical Research Centers for their assistance.
Footnotes
This research was supported by National Institutes of Health (NIH) Grants U54 HD034449 (to J.F.S. and R.S.S.); the Division of Intramural Research of the National Human Genome Research Institute (to D.R.S.); NIH Grants 5R01HD033852, 5R01HD058300 (to J.M.M.), P50 HD44405 (to M.U. and A.D.), U54 HD34449 (to A.D.), RR10732 and C06 RR016499 [to Pennsylvania State University General Clinical Research Center (GCRC)], M01 RR00048 (to Northwestern University GCRC), M01 RR10732, and M01 RR02635 (to Brigham and Women’s Hospital GCRC). K.M.B. is supported by the Physician Scientist training Program of Virginia Commonwealth University School of Medicine.
Disclosure Summary: K.G.E., D.R.S., W.A., M.U., J.M.M., C.C., K.M.B., S.C.J.P., E.H.M., R.S.L., A.D., J.F.S., and R.S.S. have nothing to declare.
First Published Online March 3, 2010
For editorial see page 2058
Abbreviations: A8, Allelle 8; FBN3, fibrillin 3; INSR, insulin receptor; LD, linkage disequilibrium; PCOS, polycystic ovary syndrome; SNP, single nucleotide polymorphism; TDT, transmission disequilibrium test.
References
- Ehrmann DA 2005 Polycystic ovary syndrome. N Engl J Med 352:1223–1236 [DOI] [PubMed] [Google Scholar]
- Sam S, Dunaif A 2003 Polycystic ovary syndrome: syndrome XX? Trends Endocrinol Metab 14:365–370 [DOI] [PubMed] [Google Scholar]
- Legro RS, Driscoll D, Strauss 3rd JF, Fox J, Dunaif A 1998 Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci USA 95:14956–14960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke MN, Strauss 3rd JF 2007 Genetic approaches to polycystic ovarian syndrome. Curr Opin Obstet Gynecol 19:355–359 [DOI] [PubMed] [Google Scholar]
- Urbanek M 2007 The genetics of the polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab 3:103–111 [DOI] [PubMed] [Google Scholar]
- Simoni M, Tempfer CB, Destenaves B, Fauser BC 2008 Functional genetic polymorphisms and female reproductive disorders. Part I: polycystic ovary syndrome and ovarian response. Hum Reprod Update 14:459–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanek M, Legro RS, Driscoll DA, Azziz R, Ehrmann DA, Norman RJ, Strauss 3rd JF, Spielman RS, Dunaif A 1999 Thirty-seven candidate genes for polycystic ovary syndrome: strongest evidence for linkage is with follistatin. Proc Natl Acad Sci USA 96:8573–8578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DR, Dombroski BA, Urbanek M, Ankener W, Ewens KG, Wood JR, Legro RS, Strauss 3rd JF, Dunaif A, Spielman RS 2006 Fine mapping of genetic susceptibility to polycystic ovary syndrome on chromosome 19p13.2 and tests for regulatory activity. J Clin Endocrinol Metab 91:4112–4117 [DOI] [PubMed] [Google Scholar]
- Urbanek M, Woodroffe A, Ewens KG, Diamanti-Kandarakis E, Legro RS, Strauss 3rd JF, Dunaif A, Spielman RS 2005 Candidate gene region for polycystic ovary syndrome on chromosome 19p13.2. J Clin Endocrinol Metab 90:6623–6629 [DOI] [PubMed] [Google Scholar]
- Urbanek M, Sam S, Legro RS, Dunaif A 2007 Identification of a polycystic ovary syndrome susceptibility variant in fibrillin-3 and association with a metabolic phenotype. J Clin Endocrinol Metab 92:4191–4198 [DOI] [PubMed] [Google Scholar]
- Grigorescu F, New genetic strategies in understanding insulin resistance of polycystic ovary syndrome in human populations. Proc 11th World Congress of Gynecological Endocrinology, Florence, Italy, 2004 (Abstract SS30) [Google Scholar]
- Tucci S, Futterweit W, Concepcion ES, Greenberg DA, Villanueva R, Davies TF, Tomer Y 2001 Evidence for association of polycystic ovary syndrome in Caucasian women with a marker at the insulin receptor gene locus. J Clin Endocrinol Metab 86:446–449 [DOI] [PubMed] [Google Scholar]
- Villuendas G, Escobar-Morreale HF, Tosi F, Sancho J, Moghetti P, San Millán JL 2003 Association between the D19S884 marker at the insulin receptor gene locus and polycystic ovary syndrome. Fertil Steril 79:219–220 [DOI] [PubMed] [Google Scholar]
- Prodoehl MJ, Hatzirodos N, Irving-Rodgers HF, Zhao ZZ, Painter JN, Hickey TE, Gibson MA, Rainey WE, Carr BR, Mason HD, Norman RJ, Montgomery GW, Rodgers RJ 2009 Genetic and gene expression analyses of the polycystic ovary syndrome candidate gene fibrillin-3 and other fibrillin family members in human ovaries. Mol Hum Reprod 15:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corson GM, Charbonneau NL, Keene DR, Sakai LY 2004 Differential expression of fibrillin-3 adds to microfibril variety in human and avian, but not rodent, connective tissues. Genomics 83:461–472 [DOI] [PubMed] [Google Scholar]
- Isogai Z, Ono RN, Ushiro S, Keene DR, Chen Y, Mazzieri R, Charbonneau NL, Reinhardt DP, Rifkin DB, Sakai LY 2003 Latent transforming growth factor β-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J Biol Chem 278:2750–2757 [DOI] [PubMed] [Google Scholar]
- Rosairo D, Kuyznierewicz I, Findlay J, Drummond A 2008 Transforming growth factor-β: its role in ovarian follicle development. Reproduction 136:799–809 [DOI] [PubMed] [Google Scholar]
- Allen DL, Cleary AS, Speaker KJ, Lindsay SF, Uyenishi J, Reed JM, Madden MC, Mehan RS 2008 Myostatin, activin receptor IIb, and follistatin-like-3 gene expression are altered in adipose tissue and skeletal muscle of obese mice. Am J Physiol Endocrinol Metab 294:E918–E927 [DOI] [PubMed] [Google Scholar]
- Amthor H, Christ B, Rashid-Doubell F, Kemp CF, Lang E, Patel K 2002 Follistatin regulates bone morphogenetic protein-7 (BMP-7) activity to stimulate embryonic muscle growth. Dev Biol 243:115–127 [DOI] [PubMed] [Google Scholar]
- Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, Patel K 2004 Follistatin complexes myostatin and antagonises myostatin-mediated inhibition of myogenesis. Dev Biol 270:19–30 [DOI] [PubMed] [Google Scholar]
- González A, Sáez ME, Aragón MJ, Galán JJ, Vettori P, Molina L, Rubio C, Real LM, Ruiz A, Ramírez-Lorca R 2006 Specific haplotypes of the CALPAIN-5 gene are associated with polycystic ovary syndrome. Hum Reprod 21:943–951 [DOI] [PubMed] [Google Scholar]
- Goodarzi MO, Maher JF, Cui J, Guo X, Taylor KD, Azziz R 2008 FEM1A and FEM1B: novel candidate genes for polycystic ovary syndrome. Hum Reprod 23:2842–2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi MO, Shah NA, Antoine HJ, Pall M, Guo X, Azziz R 2006 Variants in the 5-α-reductase type 1 and type 2 genes are associated with polycystic ovary syndrome and the severity of hirsutism in affected women. J Clin Endocrinol Metab 91:4085–4091 [DOI] [PubMed] [Google Scholar]
- Goodarzi MO, Xu N, Cui J, Guo X, Chen YI, Azziz R 2008 Small glutamine-rich tetratricopeptide repeat-containing protein α (SGTA), a candidate gene for polycystic ovary syndrome. Hum Reprod 23:1214–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen S, Korhonen S, Helisalmi S, Koivunen R, Tapanainen J, Hippeläinen M, Laakso M 2005 Associations between two single nucleotide polymorphisms in the adiponectin gene and polycystic ovary syndrome. Gynecol Endocrinol 21:165–169 [DOI] [PubMed] [Google Scholar]
- Heinonen S, Korhonen S, Helisalmi S, Koivunen R, Tapanainen JS, Laakso M 2004 The 121Q allele of the plasma cell membrane glycoprotein 1 gene predisposes to polycystic ovary syndrome. Fertil Steril 82:743–745 [DOI] [PubMed] [Google Scholar]
- Jones MR, Italiano L, Wilson SG, Mullin BH, Mead R, Dudbridge F, Watts GF, Stuckey BG 2006 Polymorphism in HSD17B6 is associated with key features of polycystic ovary syndrome. Fertil Steril 86:1438–1446 [DOI] [PubMed] [Google Scholar]
- Kolbus A, Walch K, Nagele F, Wenzl R, Unfried G, Huber JC 2007 Interleukin-1 α but not interleukin-1 β gene polymorphism is associated with polycystic ovary syndrome. J Reprod Immunol 73:188–193 [DOI] [PubMed] [Google Scholar]
- Korhonen S, Romppanen EL, Hiltunen M, Helisalmi S, Punnonen K, Hippeläinen M, Heinonen S 2003 Two exonic single nucleotide polymorphisms in the microsomal epoxide hydrolase gene are associated with polycystic ovary syndrome. Fertil Steril 79:1353–1357 [DOI] [PubMed] [Google Scholar]
- San Millán JL, Botella-Carretero JI, Alvarez-Blasco F, Luque-Ramírez M, Sancho J, Moghetti P, Escobar-Morreale HF 2005 A study of the hexose-6-phosphate dehydrogenase gene R453Q and 11β-hydroxysteroid dehydrogenase type 1 gene 83557insA polymorphisms in the polycystic ovary syndrome. J Clin Endocrinol Metab 90:4157–4162 [DOI] [PubMed] [Google Scholar]
- Abecasis GR, Cherny SS, Cookson WO, Cardon LR 2002 Merlin—rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet 30:97–101 [DOI] [PubMed] [Google Scholar]
- Spielman RS, McGinnis RE, Ewens WJ 1993 Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM). Am J Hum Genet 52:506–516 [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Sun F, Zhao H 2005 HAPLORE: a program for haplotype reconstruction in general pedigrees without recombination. Bioinformatics 21:90–103 [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ 2005 Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21:263–265 [DOI] [PubMed] [Google Scholar]
- Parker SC, Hansen L, Abaan HO, Tullius TD, Margulies EH 2009 Local DNA topography correlates with functional noncoding regions of the human genome. Science 324:389–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum JA, Pang B, Tullius TD 2007 Construction of a genome-scale structural map at single-nucleotide resolution. Genome Res 17:947–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyvazzadeh AD, Pennington KP, Pop-Busui R, Sowers M, Zubieta JK, Smith YR 2009 The role of the endogenous opioid system in polycystic ovary syndrome. Fertil Steril 92:1–12 [DOI] [PubMed] [Google Scholar]
- Xia Y, Schneyer AL 2009 The biology of activin: recent advances in structure, regulation and function. J Endocrinol 202:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson VL, Legro RS, Strauss 3rd JF, McAllister JM 1999 Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol 13:946–957 [DOI] [PubMed] [Google Scholar]
- Wickenheisser JK, Quinn PG, Nelson VL, Legro RS, Strauss 3rd JF, McAllister JM 2000 Differential activity of the cytochrome P450 17 α-hydroxylase and steroidogenic acute regulatory protein gene promoters in normal and polycystic ovary syndrome theca cells. J Clin Endocrinol Metab 85:2304–2311 [DOI] [PubMed] [Google Scholar]
- Wood JR, Nelson VL, Ho C, Jansen E, Wang CY, Urbanek M, McAllister JM, Mosselman S, Strauss 3rd JF 2003 The molecular phenotype of polycystic ovary syndrome (PCOS) theca cells and new candidate PCOS genes defined by microarray analysis. J Biol Chem 278:26380–26390 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.