Abstract
Context: Low energy and fatigue are frequent complaints in subjects with GH deficiency (GHD). Because interrelations between sleep and GH regulation are well documented, these complaints could partly reflect alterations of sleep quality.
Objective: The objective of the study was to determine objective and subjective sleep quality and daytime sleepiness in adult GHD patients.
Subjects: Thirty patients, aged 19–74 yr, with untreated GHD (primary pituitary defects confirmed or likely in 26 patients, hypothalamic origin in four patients), and 30 healthy controls individually matched for gender, age, and body mass index participated in the study. Patients with associated pituitary deficiencies (n = 28) were on hormonal replacement therapy.
Methods: Polygraphic sleep recordings, assessment of Pittsburgh Sleep Quality Index, and Quality of Life Assessment for GHD in Adults were measured.
Results: Irrespective of etiology, GHD patients had a Pittsburgh Sleep Quality Index score above the clinical cutoff for poor sleep and lower Quality of Life Assessment for GHD in Adults scores than controls, with tiredness being the most affected domain. Patients with pituitary GHD spent more time in slow-wave sleep (SWS) and had a higher intensity of SWS than their controls. Among these patients, older individuals obtained less total sleep than controls, and their late sleep was more fragmented. Contrasting with pituitary GHD, the four patients with hypothalamic GHD had lower intensity of SWS than their controls.
Conclusions: GHD is associated with sleep disorders that may be caused by specific hormonal alterations as well as with poor subjective sleep quality and daytime sleepiness. Disturbed sleep is likely to be partly responsible for increased tiredness, a major component of quality of life in GHD.
GH deficiency may be associated with major sleep alterations.
Adults with GH deficiency (GHD) often complain of impaired quality of life (QoL) (1), with frequent fatigue and easy exhaustion. Recent evidence indicates that in many chronic disorders, reduced QoL is associated with sleep disturbances (2). The possibility that tiredness complaints in adult GHD could partly reflect sleep alterations is supported by the well-documented interrelations between sleep and the somatotropic axis (3,4), including modulatory effects of hypothalamic GHRH and GH itself on sleep quality (3,4). Animal (4,5,6) and human (7,8,9,10) studies have demonstrated that GHRH has hypnotic properties and specifically increases the duration and/or the intensity of deep nonrapid eye movement sleep, commonly referred to as slow-wave sleep (SWS), even in the absence of GH (6). These data suggest that excessive hypothalamic GHRH activity may result in increased pressure for sleep with daytime fatigue. Overactivity of the hypothalamic GHRH system may occur in untreated GHD of primary pituitary origin because of the lack of negative feedback inhibition by GH. In addition, findings in rodents (5,6) and humans (11,12) indicate that GH itself may stimulate rapid eye movement (REM) sleep. Thus, there is evidence to support the hypothesis that patients with GHD may suffer from sleep disturbances that could cause daytime fatigue and contribute to reduced QoL. These sleep disturbances may differ according to the origin of GHD, i.e. pituitary with potentially excessive hypothalamic GHRH activity vs. hypothalamic with potentially insufficient GHRH activity.
Very few studies have objectively characterized sleep in GHD (13,14,15) and the results have been inconclusive. Not a single study has assessed subjective sleep quality or daytime sleepiness in GHD as compared with normal controls.
The identification of the factors contributing to impaired QoL in GHD may have important implications for the management of GHD. The purpose of our study was therefore to characterize objective sleep quality as well as subjective sleep quality, daytime sleepiness, and QoL in a large cohort of untreated adult patients with GHD, individually matched for gender, age, and body mass index (BMI) with control subjects.
Subjects and Methods
Patients
Thirty adults with GHD were recruited in four centers (University of Chicago; Université Libre de Bruxelles and Université de Liège, Belgium; Università di Pisa, Italy). GHD was diagnosed based on an iv insulin tolerance test performed within the last 5 yr, with a maximum GH response less than 3 μg/liter. GHD was present for at least 1 yr. Six patients had childhood-onset idiopathic GHD; the other 24 patients had an adult-onset GHD (see below). The patients had either never received GH therapy or were off GH treatment for at least 6 months at the time of enrollment. Screening included a clinical examination and routine laboratory measurements. Patients with evidence of substance abuse, liver disease, renal insufficiency, heart insufficiency, malignant, chronic infectious, neurological or psychiatric disease, clinically significant hyperprolactinemia, or diabetes requiring administration of insulin or sulfonylurea were excluded. Individuals employed as shift workers within the last 3 months and subjects having traveled across more than two time zones within the last 2 wk were not included. All subjects were off hypnotic drugs for at least 3 months.
In 12 of the 30 patients (referred to as pure pituitary), the origin of the adult-onset GHD was a primary pituitary defect without suprapituitary involvement: surgical removal of a pituitary tumor without radiotherapy (10 patients); spontaneous necrosis of a pituitary tumor (one patient); pituitary stalk section (one patient). None of them presented with diabetes insipidus. In six additional patients (referred to as pituitary with possible hypothalamic involvement), the existence of primary pituitary lesions was confirmed, but a suprapituitary involvement could not be excluded: surgical removal of a pituitary tumor with diabetes insipidus (two patients); radiotherapy for a pituitary tumor (one patient); surgical removal of a craniopharyngioma, without any adherence to the hypothalamus or the optic chiasma, with presence of diabetes insipidus (two patients); spontaneous necrosis of a pituitary tumor with diabetes insipidus (one patient). Both pituitary and hypothalamic lesions were possible in two other patients (one neurosarcoidosis, one histiocytosis), who were also included in the pituitary with possible hypothalamic involvement group. Consistent with known genetic causes for idiopathic pituitary deficiencies (16), a primary pituitary origin of GHD was considered very likely in the six patients with childhood-onset idiopathic GHD. A primary pituitary defect was excluded in the remaining four patients (referred to as hypothalamic): one brain tumor (surgery and radiotherapy, diabetes insipidus), one brain thrombosis, one olfactory duct tumor (surgery), one pineal gland tumor (radiotherapy, diabetes insipidus).
Twenty-eight of the 30 patients had associated pituitary hormonal deficiencies and received stable replacement therapy, as assessed by at least two clinical and biological evaluations performed at intervals of at least 3 months. All six female patients were on estrogen (four also on progesterone/progestogen) replacement therapy; 16 male patients with gonadal insufficiency were treated with testosterone (im injections of 200–250 mg every 2–3 wk or transdermal applications of 50 mg/d); 21 patients with thyroid insufficiency received oral l-thyroxine (50–250 μg/d); 24 patients with adrenal insufficiency received oral hydrocortisone (20–30 mg/d) or cortisone acetate (25–37.5 mg/d), averaging 12.0 ± 0.7 mg/m2 · d (median 11.2 mg/m2 · d, range 7.0–21.6 mg/m2 · d) hydrocortisone or the equipotent dose of cortisone; seven patients with diabetes insipidus were treated with desmopressin.
Controls
Thirty healthy controls were individually matched with the patients for gender, age, and BMI. Women were also matched for formulations of estrogens and progesterone/progestogens. Whenever applicable, inclusion criteria were the same as for the patients.
Experimental protocol
The protocol was approved by the institutional review boards of all participating universities. Written informed consent was obtained from all participants.
The outpatient visit and inpatient study were performed in laboratories at the Universities of Chicago, Brussels (including the patients recruited at the University of Liège), and Pisa. The same equipment, instruments, and recording techniques were used at each site.
Outpatient visit
All GHD and control subjects had an initial outpatient admission. This visit included physical examination, administration of the QoL scale [Quality of Life Assessment for GHD in Adults (QoL-AGHDA)] (17), administration of the Pittsburgh Sleep Quality questionnaire (PSQI) (18), and determination of plasma levels of IGF-I, free T4, Na, K, and glucose.
Inpatient study
The inpatient study was performed after 6 d of ambulatory sleep monitoring by wrist actigraphy (Actiwatch; Philips Respironics, Bend, OR), a method providing accurate estimations of sleep onset and offset (19,20). The median habitual bedtimes from these recordings were used to individually design the bedtime schedule during the inpatient study.
Within 1 wk after the end of ambulatory monitoring, the subjects were admitted to the laboratory between 1700 and 1900 h on d 1 and remained in the laboratory until discharge in the morning of d 3. Regular hospital meals were served at 0800, 1230, and 1900 h. Lights were turned off 5 min before scheduled bedtime and turned on 5 min after scheduled wake time. During bedtimes, sleep was polygraphically recorded (DigiTrace Care Services, Boston, MA).
Upon awakening on d 2, a blood sample was taken for measurement of plasma IGF-I. Thereafter subjects were maintained under normal indoor light (±300 lux) until bedtime. During waking hours, they had sedentary activities (reading, watching TV, and simple neurobehavioral tests) and were free to ambulate around the unit. Naps were not allowed.
During the second night, all experimental conditions were identical with those in the first night.
QoL measures
QoL was measured by the global score of the QoL-AGHDA scale (17). The AGHDA scale includes 25 yes-or-no questions relative to a specific complaint. A higher score corresponds to lower QoL. The 25 complaints may be clustered in five domains: tiredness (seven questions), memory and concentration (six questions), tenseness (three questions), social isolation (five questions), and self-confidence (four questions) (21). For each domain, the mean score per question was calculated.
Subjective sleep quality and daytime sleepiness
Subjective sleep quality over the month preceding the outpatient visit was assessed using the PSQI (18), a validated 19-item questionnaire that produces a global sleep quality score that ranges from 0 to 21 derived from seven component scores. A global score greater than 5 distinguishes poor sleepers from good sleepers (18). One of the seven components assessed by the PSQI is daytime sleepiness.
Objective sleep analysis
Polygraphic recordings were visually scored at 30-sec intervals in stages wake, I, II, III, IV, and REM using standardized criteria (22) by the same experienced scorer who was blind to the subject’s condition. Sleep onset and morning awakening were defined as, respectively, the times of the first and last 30-sec intervals scored II, III, IV, or REM. The sleep period was defined as the time separating sleep onset from final morning awakening. Total sleep time was defined as the sleep period minus the total duration of wake after sleep onset (WASO). Sleep latency was defined as the time from lights off until sleep onset. Sleep efficiency was calculated as the total sleep time, expressed as percentage of the time allocated to sleep.
A spectral analysis on the central electroencephalographic (EEG) lead was performed (PRANA; PhiTools, Strasbourg, France) (23). Muscular, ocular, and movement artifacts were eliminated before spectral analysis. Delta, theta, and alpha activities were calculated as the absolute spectral power in the frequency bands 0.5–4, 4.5–8, and 8.5–12 Hz, respectively. Mean power per 30-sec epoch was calculated for each band. Mean delta power in non-REM (NREM) sleep quantifies the intensity of SWS.
Technical artifacts prevented sleep scoring for 3 of the 120 nights of recordings and spectral analysis for 9 of the 120 nights. With very few exceptions due to technical failures, comparisons between patients and controls used the second rather than the first night of polysomnography because all patients and controls were habituated to the experimental procedures and spent the preceding day in the same standardized and controlled environment.
Statistical analysis
The analysis principally compared the 26 GHD patients with confirmed or likely pituitary defects and their individually matched controls. Because sleep quality changes in the course of normal aging (24), we performed a median split of this group according to age (younger: 29 ± 2 yr, range 19–43 yr, n = 13; older: 60 ± 3 yr, range: 47–74 yr, n = 13) and compared patients and controls by ANOVA for repeated measures with age group as a between-subject factor. Differences in the prevalence of QoL symptoms were tested by the χ2 test with Yates’ correction for continuity. Correlations were explored using the Spearman coefficient.
We also compared the 12 patients with pure primary pituitary GHD, the eight patients classified as pituitary GHD with possible hypothalamic involvement, the six patients with childhood-onset idiopathic GHD, and the four hypothalamic GHD patients. Differences between patients and their individually matched controls were compared by ANOVA with etiology of GHD as factor and age as covariate.
All group values are expressed as means ± sem.
Results
Clinical characteristics for each GHD group and matched controls are shown in Table 1. IGF-I values were lower in all GHD patients than in individually matched controls, averaging 72 ± 7 vs. 194 ± 11 μg/liter (P < 0.001). GHD patients had normal plasma levels of fasting glucose, sodium, and potassium. Plasma free T4 levels averaged 86 ± 5% of the median value of the normal range.
Table 1.
Clinical characteristics in GHD patients and matched controls (group values are expressed as mean ± sem)
| Groups | M/F ratio | GHD patients
|
Controls
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | BMI (kg/m2) | Associated deficits (% subjects)
|
|||||||
| TSH | ACTH | LH/FSH | ADH | Age (yr) | BMI (kg/m2) | ||||
| Pure pituitary (n = 12) | 9/3 | 54.5 ± 5.4 | 26 ± 1 | 67 | 92 | 67 | 0 | 54.3 ± 5.2 | 25 ± 1 |
| Pituitary + possible hypothalamic (n = 8) | 7/1 | 39.3 ± 4.5 | 31 ± 2 | 100 | 100 | 100 | 63 | 39.1 ± 4.5 | 31 ± 2 |
| Idiopathic (n = 6) | 6/0 | 32.8 ± 4.6 | 26 ± 3 | 67 | 67 | 67 | 0 | 33.0 ± 4.5 | 25 ± 3 |
| Hypothalamic (n = 4) | 2/2 | 44.3 ± 11.5 | 28 ± 2 | 50 | 25 | 50 | 25 | 44.5 ± 10.6 | 26 ± 2 |
All associated deficits were on appropriate and stable replacement therapy (see Subjects and Methods). ADH, Antidiuretic hormone.
We first present detailed analyses for the 26 patients with pure pituitary defects, pituitary defects with possible hypothalamic involvement, or likely pituitary defects. An exploratory analysis of differences with patients with hypothalamic GHD follows.
Objective sleep quality (Table 2 and Figs. 1 and 2)
Table 2.
Sleep and QoL variables in 26 patients with pituitary GHD and individually matched controls
| GHD (n = 26) (mean ± sem) | Controls (n = 26) (mean ± sem) | Condition GHD vs. controls P level | Age Younger vs. older P level | Age × condition Interaction P level | |
|---|---|---|---|---|---|
| Sleep variables | |||||
| Sleep period time (min) | 488 ± 12 | 482 ± 11 | 0.75 | 0.77 | 0.83 |
| Sleep latency (min) | 32 ± 7 | 27 ± 4 | 0.46 | 0.59 | 0.94 |
| Total sleep time (min) | 432 ± 11 | 447 ± 9 | 0.14 | 0.004 | 0.01 |
| Sleep efficiency (%) | 83 ± 2 | 88 ± 1 | 0.05 | 0.001 | 0.03 |
| WASO (min) | 51 ± 11 | 28 ± 5 | 0.05 | 0.002 | 0.04 |
| REM sleep (min) | 91 ± 7 | 100 ± 5 | 0.28 | 0.002 | 0.05 |
| NREM stages 1 + 2 (min) | 220 ± 11 | 267 ± 14 | 0.02 | 0.93 | 0.70 |
| NREM stage 3 (min) | 47 ± 5 | 37 ± 4 | 0.06 | 0.67 | 0.17 |
| NREM stage 4 (min) | 68 ± 7 | 44 ± 6 | 0.01 | 0.02 | 0.58 |
| Mean delta power (μv2) | 1284 ± 227 | 837 ± 99 | 0.03 | 0.01 | 0.23 |
| Mean theta power (μv2) | 146 ± 27 | 96 ± 12 | 0.05 | 0.47 | 0.94 |
| Mean alpha power (μv2) | 58 ± 9 | 51 ± 6 | 0.53 | 0.95 | 0.27 |
| PSQI (global score) | 6.6 ± 0.7 | 3.6 ± 0.5 | 0.004 | 0.30 | 0.59 |
| QoL variables | |||||
| Global score | 6.85 ± 1.24 | 2.85 ± 0.85 | 0.006 | 0.93 | 0.49 |
| Tiredness | 0.31 ± 0.06 | 0.07 ± 0.03 | 0.001 | 0.94 | 0.93 |
| Memory and concentration | 0.30 ± 0.07 | 0.15 ± 0.05 | 0.03 | 0.81 | 0.11 |
| Tenseness | 0.39 ± 0.07 | 0.22 ± 0.07 | 0.08 | 0.29 | 0.48 |
| Social isolation | 0.20 ± 0.06 | 0.08 ± 0.03 | 0.07 | 0.51 | 0.48 |
| Self-confidence | 0.18 ± 0.05 | 0.11 ± 0.05 | 0.26 | 0.43 | 0.57 |
For each QoLdomain, values indicate the mean score per question. Bold values denote statistically significant P level.
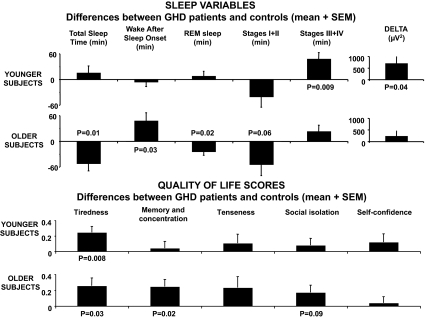
Figure 1.
Top panels, Differences (mean and sem) in total sleep time, sleep stages, and delta activity between 26 GHD patients with pure pituitary defects, pituitary defects with possible hypothalamic involvement, or childhood-onset idiopathic GHD, and their pair-matched healthy controls for the younger (n = 13) and older (n = 13) age groups. Positive values indicate higher levels in GHD patients and negative values lower levels in GHD patients. In each age group, P values denote the results of paired t tests between GHD patients and their matched controls. P ≥ 0.10 is not indicated. Bottom panels, Differences (mean and sem) in QoL scores for the five domains of the QoL-AGHDA scale between GHD patients with pure pituitary defects, pituitary defects with possible hypothalamic involvement, or childhood-onset idiopathic GHD, and their pair-matched healthy controls for the younger (n = 13) and older (n = 13) age groups. Positive values indicate higher levels in GHD patients. In each age group, P values denote the results of paired t tests between GHD patients and their matched controls. P ≥ 0.10 is not indicated.
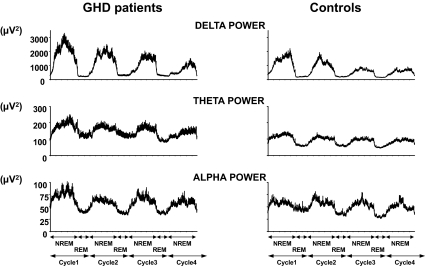
Figure 2.
Mean profiles (+sem) of absolute EEG spectral power in the delta, theta, and alpha ranges during the first four NREM-REM cycles in GHD patients with pure pituitary defects, pituitary defects with possible hypothalamic involvement, or childhood-onset idiopathic GHD (left panels), and in their healthy controls (right panels). The durations of NREM/REM cycles were normalized to account for individual differences as previously described (30). EEG delta power is a marker of the intensity of SWS and reflects an increase in the amplitude of delta waves. In conditions in which the prevalence and amplitude of EEG delta waves are markedly increased, spectral power in the adjacent theta frequency range is also generally elevated. Spectral power in the delta and alpha ranges are independent markers of the synchronization of cortical oscillations in the low- and high-frequency ranges, respectively, and these oscillations have their origin in distinct neuronal networks.
Bedtimes, sleep period, and sleep latencies were similar in GHD patients with primary pituitary lesions and controls, irrespective of age. Whereas total duration of NREM sleep was not modified, GHD patients presented a shift of NREM sleep toward deeper stages, with lower amounts of stages I + II and a more than 50% increase in the duration of stage IV. Differences between GHD patients and controls for total sleep time, sleep efficiency, WASO, and REM were dependent on age (as revealed by a significant interaction between age and condition). Figure 1 (top panels) illustrates the differences between GHD patients and controls for the sleep variables and the two age groups. The elevation in SWS (stages III + IV) and delta power relative to controls was significant in the entire group of GHD patients and in the younger group but failed to reach statistical significance in the older group. Total sleep time was decreased and sleep fragmentation (as quantified by sleep efficiency and WASO) was increased in older but not younger GHD patients, compared with controls. The increase in WASO in older subjects was not significant during the first 3 h of sleep (GHD patients: 23 ± 7 min; controls: 16 ± 6 min; P = 0.39) and reflected mainly sleep fragmentation during the later part of the night (h 3–6 of sleep: 39 ± 9 vs. 13 ± 2 min, P = 0.009). The reduction in total sleep time in older GHD patients was associated with lower amounts of REM sleep.
Mean profiles of delta, theta, and alpha activities in both groups of 26 subjects are shown in Fig. 2. The normal homeostatic decline of delta activity occurred across the night in both groups. However, delta and theta activities were markedly higher in GHD patients than in controls. Throughout the first 6 h after sleep onset, mean delta activity in NREM sleep was increased by more than 50% in GHD patients compared with controls (Table 1). EEG spectral power in the alpha range was not affected by GHD.
Subjective sleep quality and daytime sleepiness (Table 2)
On average, the global PSQI was indicative of poor sleep (i.e. above the clinical cutoff of 5) in the 26 GHD patients with primary pituitary lesions, whereas it was consistent with normal sleep in their controls (Table 2). The component assessing daytime sleepiness was also elevated in these patients compared with their controls (1.1 ± 0.2 vs. 0.5 ± 0.1, P = 0.03 by paired Wilcoxon test).
No significant correlations were found between the global PSQI score and polysomnography-derived sleep variables. However, the daytime sleepiness component of the PSQI was associated with reduced sleep efficiency (rs = −0.55, P = 0.006) and longer WASO (rs = 0.47, P = 0.02).
QoL (Table 2 and Fig. 1)
Group data for QoL variables for the 26 patients with primary pituitary lesions and their matched controls are given in Table 2.
QoL scores were similar in U.S. and European patients. GHD patients had significantly higher total QoL-AGHDA scores than their controls, irrespective of age, but the scores were not indicative of a major impairment. Tiredness was the most frequent complaint and reached the highest score in both age groups. Only 30% of patients (eight of 26), compared with 73% of controls (19 of 26), did not report any tiredness complaint (negative answer to each of the seven questions; P < 0.01). Memory problems were also more frequent in GHD patients than controls.
Figure 1 (bottom panels) illustrates the differences between GHD patients and controls for the five domains of QoL and the two age groups. When each age group was analyzed separately, tiredness was the only domain significantly affected in young patients, whereas in older patients, deficits in memory and concentration and a trend for more social problems were also found.
In the entire group of 26 patients, the score on the tiredness domain of QoL was associated with the daytime sleepiness component of the PSQI (rs = 0.41, P = 0.04). In younger patients (n = 13), a trend for a correlation between the tiredness score and the amount of SWS was detectable (rs = 0.51, P < 0.08). No other correlations between sleep variables and global or partial QoL scores were found.
Impact of etiology of GHD on objective sleep variables, subjective sleep quality, and QOL (Figs. 3 and 4)
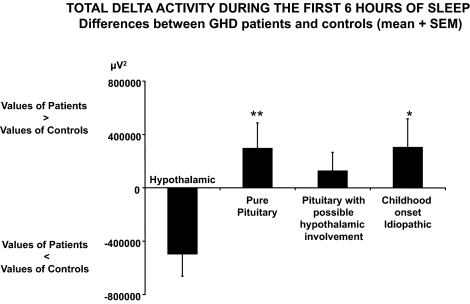
Figure 3.
Differences (mean ± sem) between patients and controls in total delta activity during the first 6 h of sleep for patients with pure pituitary GHD (n = 12); patients with pituitary GHD with possible hypothalamic involvement (n = 7; spectral analysis could not be performed for one patient); patients with childhood-onset idiopathic GHD (n = 6); or patients with hypothalamic GHD (n = 4). Positive values indicate higher levels in GHD patients, negative values lower levels in GHD patients. **, P < 0.005 for difference between pure pituitary GHD and hypothalamic GHD, after controlling for age; *, P = 0.05 for difference between idiopathic GHD and hypothalamic GHD, after controlling for age.
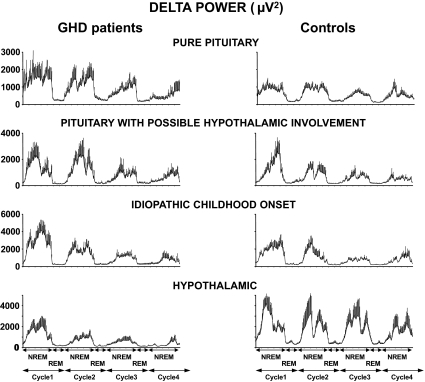
Figure 4.
Mean (+sem) of absolute EEG spectral power in the delta range during the first four NREM-REM cycles in GHD patients compared with their matched controls for the four diagnostic categories. From top to bottom, Pure pituitary GHD (n = 12), pituitary GHD with possible hypothalamic involvement (n = 7), childhood-onset idiopathic GHD (n = 6), and hypothalamic GHD patients (n = 4). As in Fig. 2, the durations of NREM/REM cycles were normalized to account for individual differences as previously described (30). Note differences of scales in the different categories. Differences in levels of delta activity across the four control groups are due to differences in sex, age, and BMI distribution. For each diagnostic category, patients and controls are pair matched for sex, age, and BMI.
Irrespective of GHD etiology (i.e. pure pituitary, pituitary with possible hypothalamic involvement, idiopathic childhood onset, hypothalamic), differences between patients and controls were qualitatively similar for all sleep variables derived from sleep staging as well as for subjective sleep quality and QoL. However, EEG spectral analysis revealed striking differences in the microarchitecture of NREM sleep in patients with hypothalamic GHD compared with those with pituitary GHD. Indeed, as illustrated in Fig. 4, the etiology of GHD was a significant predictor of the difference in delta activity between patients and controls after adjusting for age (P = 0.05). In the small group of patients with hypothalamic GHD, the findings regarding delta activity were opposite from those observed in patients with pure pituitary GHD (P = 0.005) or patients with childhood-onset idiopathic GHD (P = 0.05), with markedly decreased, rather than increased, delta activity in patients compared with controls.
Discussion
The present study, performed in a large group of adult GHD patients individually matched for gender, age, and BMI with control subjects, indicates that GHD due to a confirmed or likely primary pituitary defect is associated with an excess of high intensity SWS, poor subjective sleep quality, and daytime sleepiness. Excessive amounts of SWS (which were also observed in night 1, i.e. the habituation night; data not shown) represent an unusual form of sleep disturbance as the vast majority of sleep disorders are characterized by reduced, rather than enhanced, SWS. The enhancement of EEG power was specific to the delta and theta ranges as alpha power was similar in patients and controls. One possible mechanism underlying this enhancement of SWS and delta activity could be an exaggeration of the well-documented stimulatory effect of GHRH on delta activity resulting from a lack of negative feedback regulation of GHRH by circulating GH. Consistent with this putative mechanism, up-regulation of hypothalamic GHRH activity, due to the absence of inhibitory control by GH, associated with increased amounts of NREM sleep has indeed been demonstrated in an animal model of GHD, the spontaneous dwarf rat (25,26). A reduction in the inhibitory control of hypothalamic GHRH by GH might contribute to an incomplete inhibition of SWS-generating mechanisms during wake as well as during sleep and result in daytime sleepiness. Among our younger patients, those with higher amounts of SWS tended to have higher tiredness scores. In all our pituitary GHD patients, tiredness was the most consistent complaint on the QoL-AGHDA scale and was correlated with daytime sleepiness as assessed by a validated questionnaire that is not specific of GHD. Although these associations do not indicate causality, they suggest that sleep disturbances may contribute to daytime sleepiness and tiredness complaints.
Older adults with pituitary GHD had less REM sleep. This loss of REM sleep, possibly due to GHD (5,6,11,12), could be involved in the emergence of memory problems, which were reported more frequently by older patients than their controls. There is indeed a growing body of evidence linking REM sleep and memory (27,28). Older adults with pituitary GHD also had more sleep fragmentation in the later part of the night and obtained less total sleep time than control subjects. Whereas increased number and duration of awakenings, decreased total sleep time, and decreased amounts of REM sleep are typical of normal adults in late life (24), pituitary GHD appears associated with an exacerbation of these sleep disturbances, suggestive of a more rapid senescence of sleep-regulating mechanisms in the absence of GH. The combination of increased duration and intensity of SWS in the early part of the night with increased sleep fragmentation in the later part of the night represents a peculiar sleep disorder as higher levels of SWS are generally associated with more consolidated sleep.
In remarkable contrast to findings in the two groups of patients in whom there was no evidence for a suprapituitary involvement in the etiology of GHD (i.e. pure pituitary and childhood onset idiopathic), the patients with hypothalamic GHD had shallow NREM sleep with markedly lower delta power than their controls, consistent with reduced GHRH activity. Although these limited results await confirmation in a larger group of patients, they are consistent with a central role of GHRH activity in modulating sleep quality in GHD. Findings in patients with pituitary defects and possible hypothalamic involvement were intermediate, consistent with a mixed etiology of GHD.
Our findings are at variance with those reported nearly 20 yr ago in eight subjects with isolated GHD (13). In this latter study, controls were matched for sex and age but not for BMI. Bedtimes and sleep periods were not reported, activity during waking hours was not controlled, and daytime naps were not prohibited. Total sleep time was surprisingly long in patients, ranging from 7 h 45 min to 11 h 44 min with a median of nearly 9 h. Extended bedtimes and naps generally result in a shift of NREM sleep toward lighter stages (29), which could explain the discrepancy with our findings. In a separate study, sleep profiles in a group of patients with adult-onset GHD were reported to be comparable with a nonspecified and nonindividually matched healthy control group from the literature (14). Because of the high variability, especially with gender and age, of sleep characteristics among normal individuals, this study design was unlikely to detect differences between GHD patients and controls. Recently increased WASO and increased stage IV sleep were reported in women with GHD due to Sheehan’s syndrome, consistent with the present results (15).
Nearly all GHD patients in our study received chronic replacement therapy (including hydrocortisone/cortisone acetate, l-thyroxine, and sex steroids) to correct associated pituitary hormonal deficits. Although an impact of hormonal deficits and their treatment on sleep quality cannot be excluded, the fact that younger and older patients were similarly treated and sleep was nonetheless more disturbed in older patients does not support a primary role of replacement therapy in the sleep disorders.
In conclusion, the present findings suggest that GHD may be associated with major sleep alterations and that tiredness, a major QoL complaint in GHD, may reflect poor sleep quality and daytime sleepiness. The sleep phenotype of GHD may be dependent on its etiology.
Acknowledgments
Thanks are due to Claire van den Bril, M.S. (Université Libre de Bruxelles), Elisabeth Lallemant, M.S. (CHR-Citadelle, Liège), and the nursing staff of the General Clinical Research Center of the University of Chicago for excellent care of the subjects during the studies. We are grateful to Dr. P. Penev for assistance with the screening of subjects recruited at the University of Chicago and Dr. Barbara Lippe (formerly of Pharmacia Corp.) for helpful advice and suggestions during the development of the protocol.
Footnotes
This work was supported by an investigator-initiated grant from Pharmacia Corp. and partial support from National Institutes of Health (NIH) Grant MO1 RR-00055 (to the General Clinical Research Center of the University of Chicago). During part of the study, A.N. was supported by the Northwestern University-University of Chicago Training Grant for Sleep Research (NIH Grant T32 HL-07909). The analysis of sleep recordings was partly supported by Grant PO1 AG-11412 from the National Institute on Aging.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 23, 2010
Abbreviations: BMI, Body mass index; EEG, electroencephalographic; GHD, GH deficiency; NREM, non-REM; PSQI, Pittsburgh Sleep Quality questionnaire; QoL, quality of life; QoL-AGHDA, QoL Assessment for GHD in Adults; REM, rapid eye movement; SWS, slow-wave sleep; WASO, wake after sleep onset.
References
- Blum WF, Shavrikova EP, Edwards DJ, Rosilio M, Hartman ML, Marín F, Valle D, van der Lely AJ, Attanasio AF, Strasburger CJ, Henrich G, Herschbach P 2003 Decreased quality of life in adult patients with growth hormone deficiency compared with general populations using the new, validated, self-weighted questionnaire, questions on life satisfaction hypopituitarism module. J Clin Endocrinol Metab 88:4158–4167 [DOI] [PubMed] [Google Scholar]
- Verster JC, Pandi-Perumal, SR, Streiner, DL 2008 Sleep and quality of life in clinical medicine. Totowa, NJ: Humana Press [Google Scholar]
- Van Cauter E, Plat L, Copinschi G 1998 Interrelations between sleep and the somatotropic axis. Sleep 21:553–566 [PubMed] [Google Scholar]
- Obal Jr F, Krueger JM 2004 GHRH and sleep. Sleep Med Rev 8:367–377 [DOI] [PubMed] [Google Scholar]
- Obal Jr F, Alföldi P, Cady AB, Johannsen L, Sary G, Krueger JM 1988 Growth hormone-releasing factor enhances sleep in rats and rabbits. Am J Physiol 255:R310–R316 [DOI] [PubMed] [Google Scholar]
- Obál Jr F, Floyd R, Kapás L, Bodosi B, Krueger JM 1996 Effects of systemic GHRH on sleep in intact and hypophysectomized rats. Am J Physiol 270:E230–E237 [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Jarrett DB, Ehlers CL 1991 The effect of GRF on the EEG sleep of normal males. Sleep 14:87–88 [PubMed] [Google Scholar]
- Steiger A, Guldner J, Hemmeter U, Rothe B, Wiedemann K, Holsboer F 1992 Effects of growth hormone-releasing hormone and somatostatin on sleep EEG and nocturnal hormone secretion in male controls. Neuroendocrinology 56:566–573 [DOI] [PubMed] [Google Scholar]
- Kerkhofs M, Van Cauter E, Van Onderbergen A, Caufriez A, Thorner MO, Copinschi G 1993 Sleep-promoting effects of growth hormone-releasing hormone in normal men. Am J Physiol 264:E594–E598 [DOI] [PubMed] [Google Scholar]
- Marshall L, Mölle M, Böschen G, Steiger A, Fehm HL, Born J 1996 Greater efficacy of episodic than continuous growth hormone-releasing hormone (GHRH) administration in promoting slow-wave sleep (SWS). J Clin Endocrinol Metab 81:1009–1013 [DOI] [PubMed] [Google Scholar]
- Mendelson WB, Slater S, Gold P, Gillin JC 1980 The effect of growth hormone administration on human sleep: a dose-response study. Biol Psychiatry 15:613–618 [PubMed] [Google Scholar]
- Aström C 1995 Interaction between sleep and growth hormone. Evaluated by manual polysomnography and automatic power spectrum analysis. Acta Neurol Scand 92:281–296 [PubMed] [Google Scholar]
- Aströom C, Lindholm J 1990 Growth hormone-deficient young adults have decreased deep sleep. Neuroendocrinology 51:82–84 [DOI] [PubMed] [Google Scholar]
- Schneider HJ, Oertel H, Murck H, Pollmächer T, Stalla GK, Steiger A 2005 Night sleep EEG and daytime sleep propensity in adult hypopituitary patients with growth hormone deficiency before and after six months of growth hormone replacement. Psychoneuroendocrinology 30:29–37 [DOI] [PubMed] [Google Scholar]
- Ismailogullari S, Tanriverdi F, Kelestimur F, Aksu M 2009 Sleep architecture in Sheehan’s syndrome before and 6 months after growth hormone replacement therapy. Psychoneuroendocrinology 34:212–219 [DOI] [PubMed] [Google Scholar]
- Jöstel E, Lissett CA, Shalet SM 2006 Hypopituitarism. In: DeGroot LJ, Jameson JL, eds. Endocrinology. 5th ed. Philadelphia: Elsevier Saunders; 397–409 [Google Scholar]
- McKenna SP, Doward LC, Alonso J, Kohlmann T, Niero M, Prieto L, Wíren L 1999 The QoL-AGHDA: an instrument for the assessment of quality of life in adults with growth hormone deficiency. Qual Life Res 8:373–383 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds 3rd CF, Monk TH, Berman SR, Kupfer DJ 1989 The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28:193–213 [DOI] [PubMed] [Google Scholar]
- Jean-Louis G, von Gizycki H, Zizi F, Spielman A, Hauri P, Taub H 1997 The actigraph data analysis software: I. A novel approach to scoring and interpreting sleep-wake activity. Percept Mot Skills 85:207–216 [DOI] [PubMed] [Google Scholar]
- Jean-Louis G, von Gizycki H, Zizi F, Spielman A, Hauri P, Taub H 1997 The actigraph data analysis software: II. A novel approach to scoring and interpreting sleep-wake activity. Percept Mot Skills 85:219–226 [DOI] [PubMed] [Google Scholar]
- Koltowska-Häggström M, Mattsson AF, Monson JP, Kind P, Badia X, Casanueva FF, Busschbach J, Koppeschaar HP, Johannsson G 2006 Does long-term GH replacement therapy in hypopituitary adults with GH deficiency normalise quality of life? Eur J Endocrinol 155:109–119 [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A 1968 A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute [Google Scholar]
- Latta F, Leproult R, Tasali E, Hofmann E, Van Cauter E 2005 Sex differences in delta and alpha EEG activities in healthy older adults. Sleep 28:1525–1534 [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Leproult R, Plat L 2000 Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA 284:861–868 [DOI] [PubMed] [Google Scholar]
- Kamegai J, Wakabayashi I, Miyamoto K, Unterman TG, Kineman RD, Frohman LA 1998 Growth hormone-dependent regulation of pituitary GH secretagogue receptor (GHS-R) mRNA levels in the spontaneous dwarf rat. Neuroendocrinology 68:312–318 [DOI] [PubMed] [Google Scholar]
- Peterfi Z, Obal Jr F, Taishi P, Gardi J, Kacsoh B, Unterman T, Krueger JM 2006 Sleep in spontaneous dwarf rats. Brain Res 1108:133–146 [DOI] [PubMed] [Google Scholar]
- Hornung OP, Regen F, Danker-Hopfe H, Schredl M, Heuser I 2007 The relationship between REM sleep and memory consolidation in old age and effects of cholinergic medication. Biol Psychiatry 61:750–757 [DOI] [PubMed] [Google Scholar]
- Stickgold R, Walker MP 2007 Sleep-dependent memory consolidation and reconsolidation. Sleep Med 8:331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Colecchia EF, L'Hermite-Balériaux M, Nie Z, Copinschi G, Van Cauter E 2000 Adaptation of the 24-h growth hormone profile to a state of sleep debt. Am J Physiol Regul Integr Comp Physiol 279:R874–R883 [DOI] [PubMed] [Google Scholar]
- Landolt HP, Dijk DJ, Achermann P, Borbély AA 1996 Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res 738:205–212 [DOI] [PubMed] [Google Scholar]