Abstract
Context: Somatostatin receptor subtype 2 (sst2) is widely expressed in neuroendocrine tumors and can be visualized immunohistochemically at the cell membrane for diagnostic purposes. Recently, it has been demonstrated in animal sst2 tumor models in vivo that somatostatin analog treatment was able to induce a complete internalization of the tumor sst2.
Patients and Methods: In the present study, we evaluated whether sst2 expressed in neuroendocrine tumors of patients treated with octreotide are also internalized. Tumor samples were assessed in patients that were treated with various octreotide modalities before and during surgery and compared with tumor samples from untreated patients. Sst2 immunohistochemistry was performed in all samples with three different sst2 antibodies (R2-88, UMB-1, and SS-800). Sst2 receptor expression was confirmed by immunoblotting and in vitro receptor autoradiography.
Results: Patients receiving a high dose of octreotide showed predominantly internalized sst2, and patients with a low dose of octreotide had a variable ratio of internalized vs. membranous sst2, whereas untreated patients had exclusively membranous sst2. The internalized sst2 receptor corresponded to a single sst2 band in immunoblots and to sst2 receptors in in vitro receptor autoradiography. Although generally found in endosome-like structures, internalized sst2 receptors were also identified to a small extent in lysosomes, as seen in colocalization experiments.
Conclusion: It is the first evidence showing that sst2 receptors can be internalized in sst2-expressing neuroendocrine tumors in patients under octreotide therapy, providing clues about sst2 receptor biology and trafficking dynamics in patients.
Somatostatin sst2 receptors can be internalized in sst2-expressing neuroendocrine tumors in patients under octreotide therapy, providing clues about sst2 receptor biology and trafficking dynamics in patients.
One of the general physiological feature of G protein-coupled receptors is that agonist binding to the receptor triggers an internalization of the receptor-ligand complex into the cells (1,2,3,4). This occurs physiologically after binding of endogenous ligand to the receptor, as shown in the elegant study by Mantyh et al. (5,6) on substance P receptor internalization after nerve stimulation. It also occurs after binding of exogenous ligands to the receptor, as illustrated by the field of peptide receptor tumor targeting, where receptor-mediated internalization of radiolabeled peptides is used for diagnostic and therapeutic applications (7,8,9). The best examples in this regard are the somatostatin receptors: the high degree of internalization of somatostatin radioligands into somatostatin receptor subtype 2 (sst2)-expressing tumor cells is a powerful mechanism of radioactivity accumulation within the tumor that may permit the successful imaging of tumors in patients as well as targeted tumor radiotherapy (8,10,11).
Although most of the internalization studies have been performed in vitro (12,13,14,15), it was reported recently by Waser et al. (16) that internalization of the sst2 receptor could also be observed in vivo in animal tumor models after iv application of the somatostatin agonist [Tyr3] octreotate. It was found that the agonist-induced sst2 internalization was extremely rapid and powerful and that almost all sst2 receptors moved from the cell membrane to endosome-like cellular structures within the cytoplasm, giving a characteristic immunohistochemical pattern of receptor distribution (16).
Because the in vivo sst2 internalization process after agonist application is so potent and prominent, we were interested to know whether internalized sst2 could also be detected in resected tumor tissue from patients treated with the somatostatin agonist octreotide immediately before or during surgery. Using sst2 immunohistochemistry, a method shown previously to identify sst2 receptors on formalin-fixed, paraffin-embedded tumors (17,18,19,20,21), we have analyzed in the present study the tumor tissue samples from patients treated with octreotide [including octreotide long-acting repeatable (LAR), octreotide infusion during surgery, or sc octreotide application at beginning of surgery] and compared with tumor samples from patients that had not been treated with octreotide. Additional confirmatory proofs for sst2 expression in these samples were obtained with in vitro somatostatin receptor autoradiography and immunoblotting.
Materials and Methods
Cell line
The HEK293 cell line expressing the human T7-epitope-tagged sst2 receptor (HEK-sst2) was cultured at 37 C and 5% CO2 in DMEM with GlutaMax I containing 10% (vol/vol) fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 500 μg/ml G418. All culture reagents were from Life Technologies, Inc. (Grand Island, NY).
Tissues
Formalin-fixed and fresh-frozen tissue samples from surgically resected neuroendocrine tumors expressing sst2 receptors were used. The samples were divided into three groups depending on the type of octreotide treatment received during surgical tumor resection: 1) tumors from patients that had not been in contact with octreotide before or during surgery, 2) tumors from patients that had received 200 μg octreotide sc at the start of surgery, and 3) tumors from patients that had received an iv octreotide infusion (200 μg/h) during the time of surgical resection plus 20 mg octreotide LAR less than 3 wk before surgery. For each patient, a specific clinical rationale was the basis for the particular perioperative octreotide treatment, namely to avoid preoperative complications in functional neuroendocrine tumors (22) or to improve the postoperative outcome of pancreatic interventions as suggested by a previous study (23). Limitations due to patient-based and surgery-dependent type of investigation included variable route of application of octreotide (iv injection, sc injection, and LAR) and variable intervals between octreotide injection and tumor removal. Written informed consent was available for all patients. The present study conformed to the ethical guidelines of the Institute of Pathology of the University and the University Hospital of Berne and was reviewed by the Institutional Review Board.
Sst2 immunohistochemistry
Sst2 receptors were detected using immunohistochemistry, as described before (16,17,18,24,25). All samples were tested with the polyclonal sst2-specific antibody R2-88 (generously provided by Dr. A. Schonbrunn, Houston, TX), with the commercially available polyclonal sst2-specific antibody SS-800 (Gramsch Laboratories, Schwabhausen, Germany) and with the rabbit monoclonal antibody UMB-1 (SS-8000RM; Biotrend GmbH, Cologne, Germany) (17,21). Four-micrometer-thick formalin-fixed, paraffin-embedded tissue sections were used. As reported previously (18), the best antigen retrieval method for R2-88 and UMB-1 immunohistochemistry was boiling in the microwave in presence of a 5% urea buffer (pH 9.5). For SS-800 immunohistochemistry, boiling in the microwave in the presence of a 10 mm citrate buffer (pH 6.0) was preferable (17,21). Both R2-88 and SS-800 were applied in a 1:1000 dilution, whereas a 1:100 dilution was necessary for UMB-1. The secondary antibody was a biotinylated goat antirabbit Ig (1:200 dilution). Antibody binding was visualized using the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA). Staining was carried out with diaminobenzidine and counterstaining with hemalum. For specificity control, the primary antibodies were preadsorbed with 100 nm of the corresponding antigen peptide. In all experiments, a well-characterized gastro-enteropancreatic neuroendocrine tumor strongly expressing sst2 as determined by receptor autoradiography was included as positive control. As controls for membranous or internalized sst2, AR42J tumor tissue and pancreas from rats injected either with saline or with [Tyr3] octreotate, respectively (16), were analyzed in parallel.
Receptor autoradiography
In all cases with available fresh-frozen tumor tissues, somatostatin receptor autoradiography was carried out as described before (26). Briefly, 20-μm-thick frozen tissue sections were cut from each of the collected tumor samples and were incubated for 2 h at room temperature with the sst2-preferring somatostatin radioligand 125I-labeled [Tyr3] octreotide (26). Nonspecific binding was determined in serial tissue sections incubated with the radioligand in the presence of 100 nm octreotide. The slides were subsequently exposed to Kodak Biomax MR films for 7 d at 4 C. Receptor quantification was performed as previously described (26).
Immunoblotting
Approximately 50–100 mg fresh-frozen neuroendocrine tumors was cut into small pieces, partially thawed, and washed in ice-cold PBS containing protease inhibitors (1 mm phenylmethylsulfonyl fluoride, 10 μg/ml soybean trypsin inhibitor, 10 μg/ml leupeptin, 5 μg/ml aprotinin, and 50 μg/ml bacitracin), incubated for 30 min at 4C in ice-cold homogenization buffer [20 mm HEPES, 150 mm NaCl, 5 mm EDTA, 3 mm EGTA, and 4 mg/ml dodecyl β-d-maltoside (pH 7.6)] containing protease inhibitors, and then homogenized and lysed in a precooled Dounce homogenizer. The lysate was subsequently clarified by centrifugation at 16,000 × g and 4 C for 30 min and then subjected to partial purification by wheat germ agglutinin-agarose treatment. The adsorbed glycoproteins were eluted at 60 C for 15 min in SDS-PAGE sample buffer containing 10% 2-mercaptoethanol and then resolved on a 10% SDS-polyacrylamide gel. Resolved proteins were transferred to polyvinylidene difluoride membrane. The membrane was blocked for 2 h with 5% nonfat dry milk in Tris buffered saline-Tween [TBS-T: 20 mm Tris-HCl, 150 mm NaCl, 0.1% Tween 20 (pH 7.4)], and then incubated over night at 4 C with the sst2-specific antibody R2-88 (1:10,000) in TBS-T. Immunoreactive proteins were detected with a donkey antirabbit-F(ab′)2 conjugated with horseradish peroxidase (1:10,000) in TBS-T, and the ECL chemiluminescent antibody detection system (GE Healthcare, Little Chalfont, UK). As positive control, lysates of HEK-sst2 cells were used and treated as described above for the lysates of the neuroendocrine tumors.
Confocal double-labeling immunofluorescence: histochemistry
Selected sst2-expressing neuroendocrine tumor samples were used for colocalization studies using confocal immunohistochemistry. The samples were analyzed for colocalization with the sst2-specific antibody R2-88 and with a monoclonal antibody raised against the lysosomal marker protein cathepsin D (ab6319; Abcam Ltd., Cambridge, UK). Four-micrometer-thick formalin-fixed, paraffin-embedded tissue sections were used and treated as described above for sst2 immunohistochemistry. The best antigen retrieval method for R2-88 and anti-cathepsin D antibody immunohistochemistry was boiling in the microwave in the presence of a 5% urea buffer (pH 9.5). Both antibodies, R2-88 and the anti-cathepsin D antibody, were applied together in a dilution of 1:1000 overnight at room temperature. The secondary antibody for R2-88 was a goat antirabbit IgG Alexa 488 conjugate (Molecular Probes, Eugene, OR), whereas the secondary antibody for the anti-cathepsin D antibody was a goat antimouse IgG Alexa 594 conjugate (Molecular Probes). Both secondary antibodies were applied together in a dilution of 1:300 for 45 min at room temperature and in the dark. Slides were subsequently mounted with a polyvinyl-alcohol- and glycerine-based mountant containing 1,4-diazabicyclo(2,2,2)octane (polydabco). Confocal imaging was performed on an Olympus FluoView FV1000 scanning unit with the IX81 inverted microscope (Olympus, Tokyo, Japan). All confocal images processed for analysis were collected using a ×60 oil immersion lens.
Confocal double-labeling immunofluorescence: cytochemistry
HEK-sst2 cells were grown on poly-d-lysine-coated (20 μg/ml) (Sigma-Aldrich, St. Louis, MO) 10-mm glass coverslips in 24-well plates for 24 h at 37 C and with 5% CO2. Cells were then treated with 1 μm [Tyr3] octreotide (Novartis, Basel, Switzerland) at 37 C and with 5% CO2 in growth medium for 30 min and then processed for immunofluorescence microscopy as described previously (12,13) using as primary antibodies the sst2-specific antibody R2-88 and the monoclonal antibody raised against the lysosomal marker protein cathepsin D (ab6319; Abcam Ltd., Cambridge UK). Both antibodies, R2-88 (1:1000 dilution) and the anti-cathepsin D antibody (1:500 dilution), were applied together for 1 h at room temperature. The secondary antibody for R2-88 was a goat antirabbit IgG Alexa 488 conjugate (Molecular Probes), whereas the secondary antibody for the anti-cathepsin D antibody was a goat antimouse IgG Alexa 594 conjugate (Molecular Probes). Both secondary antibodies were applied together in a dilution of 1:600 for 45 min at room temperature and in the dark. Slides were subsequently mounted with polydabco. Confocal imaging was performed on an Olympus FluoView FV1000 scanning unit with the IX81 inverted microscope (Olympus, Tokyo, Japan). All confocal images processed for analysis were collected using a ×60 oil immersion lens.
Results
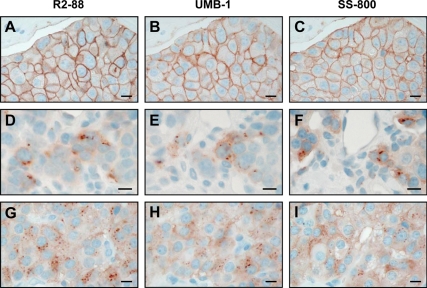
The immunohistochemical data are summarized in Table 1 and in Figs. 1, 2, and 4. Tumor samples of patients that had not received an octreotide therapy exhibited exclusively a membranous sst2 staining. In contrast, the tumor samples from octreotide-treated patients showed internalized sst2 receptor in variable amounts (Table 1 and Figs. 1 and 2). The two cases (patients 1 and 2) with 200 μg/h octreotide infusion and 20 mg octreotide LAR therapy, reflecting a high octreotide dose (22), showed predominantly internalized sst2 receptor with few focal areas with membranous sst2. The cases with sc octreotide application (200 μg single injection) at the beginning of the surgery showed internalized sst2 as well, but the ratio between internalized and membranous sst2 varied considerably between individuals. Some cases had predominantly internalized sst2 receptors, others a mixture of internalized and membranous receptors, and few had preferably membranous receptors (Table 1). Moreover, as reported previously for octreotide-naive patients (17), the present study repeatedly identified heterogeneous receptor-expressing tumor areas, i.e. containing sst2-positive tumor cells adjacent to sst2-negative ones. This is illustrated in Fig. 1 (D–F) in samples of octreotide-treated patients, showing adjacent tumor cells with and without internalized sst2. To provide strong immunohistochemical proof, we used three different sst2-specific antibodies, recognizing C-terminal sst2 epitopes on successive sections of each of the tumor samples. Comparative qualitative results were obtained for each of the three antibodies. Figure 1 shows examples of neuroendocrine tumor samples revealing a comparable immunostaining with R2-88, UMB-1, or SS-800 in adjacent sections, with sst2 receptors internalized in two octreotide-treated cases and a membranous sst2 staining in one control sample.
Table 1.
Immunohistochemical data
| Patients | Tumor type | OCT long-term therapy | OCT pre-/peri-OP | Sst2 IHCa | ARG |
|---|---|---|---|---|---|
| Octreotide-treated | |||||
| 1 | NET (pancreatic) | LAR 20 mg 3 wk pre-OP | 200 μg/h infusion peri-OP | Mostly internal. sst2 | +++ |
| 2 | NET (bronchial; heart-met) | LAR 20 mg 3 wk pre-OP | 200 μg/h infusion peri-OP | Mostly internal. sst2 | NT |
| 3a | NET (pancreatic) | No | 200 μg sc | Mostly internal. sst2 | +++ |
| 3b | NET (pancreatic) | No | 200 μg sc | Mostly internal. sst2 | +++ |
| 4a | NET (pancreatic) | No | 200 μg sc | Internal. = membr. sst2 | NT |
| 4b | NET (pancreatic) | No | 200 μg sc | Internal. = membr. sst2 | NT |
| 5a | NET (pancreatic; primary) | No | 200 μg sc | Internal. = membr. sst2 | +++ |
| 5b | NET (pancreatic; liver-met) | No | 200 μg sc | Internal. = membr. sst2 | +++ |
| 6 | NET (pancreatic) | No | 200 μg sc | Membr. > internal. sst2 | +++ |
| 7 | NET (pancreatic) | No | 200 μg sc | Mostly membr. sst2 | +++ |
| 8 | NET (pancreatic) | No | 200 μg sc | Mostly membr. sst2 | +++ |
| 9 | NET (pancreatic) | No | 200 μg sc | Mostly membr. sst2 | +++ |
| Untreated | |||||
| 10 | NET (bile duct) | No | No | Membr. sst2 | ++ |
| 11a | NET (pancreatic) | No | No | Membr. sst2 | +++ |
| 11b | NET (pancreatic) | No | No | Membr. sst2 | +++ |
| 12 | NET (bronchial) | No | No | Membr. sst2 | NT |
| 13 | NET (mesenteric) | No | No | Membr. sst2 | +++ |
| 14 | NET (pancreatic; liver-met) | No | No | Membr. sst2 | +++ |
| 15 | NET (pancreatic) | No | No | Membr. sst2 | +++ |
ARG, Somatostatin receptor autoradiography; IHC, immunohistochemistry; internal., internalized; membr., membranous; NET, neuroendocrine tumor; OCT, octreotide; OP, operation; met, metastasis; NT, not tested. +++ indicates high density of somatostatin receptors (>8000 dpm/mg tissue); ++ represents a density of 4567 dpm/mg tissue.
All three sst2 antibodies (R2-88, UMB-1, and SS-800) were tested in each case with comparable results.
Figure 1.
Sst2 immunohistochemistry with three different sst2-specific antibodies: R2-88 (A, D, and G), UMB-1 (B, E, and H), and SS-800 (C, F, and I). Bars, 0.01 mm. A–C, Tumor tissue from patient 11b not treated with octreotide (control) showing membranous sst2 staining with all three antibodies. D–F, Tumor tissue from patient 2 treated with 20 mg octreotide LAR plus 200 μg/h octreotide infusion perioperatively, showing intracellular sst2 staining with all three antibodies. G–I, Tumor tissue from patient 3a treated with 200 μg octreotide sc at start of surgery, showing intracellular sst2 staining with all three antibodies.
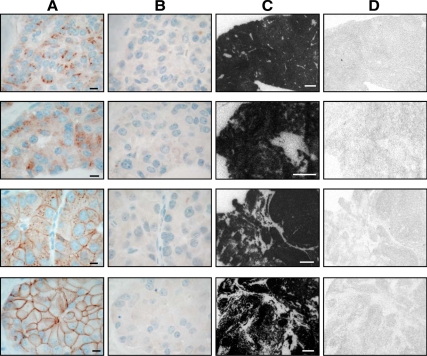
Figure 2.
Sst2 immunohistochemistry with R2-88 (columns A and B) in neuroendocrine tumor samples from four patients treated with octreotide is compared with sst2 expression measured in the same tissue samples by in vivo receptor autoradiography (columns C and D). Column A shows the great individual variability of sst2 internalization among the four cases, ranging from predominantly internalized sst2 (top) to predominantly membranous sst2 (bottom). Bars, 0.01 mm. Column B shows that the sst2 immunostaining is specific in all cases because preadsorption with excess antigen peptide abolishes all staining. Column C shows autoradiograms showing total binding of 125I-labeled [Tyr3] octreotide in the four different tumors. All show a very high density of somatostatin receptors. Bars, 1 mm. Column D shows autoradiograms showing nonspecific binding of 125I-labeled [Tyr3] octreotide (in the presence of 10−6 m octreotide). The four rows from top to bottom correspond to the samples of patients 3a, 1, 5b, and 8, respectively.
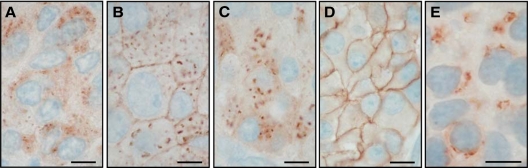
Figure 4.
Sst2 immunohistochemistry showing internalized sst2 receptors at high magnification in tumors from three octreotide-treated patients (A–C) as compared with a tumor (D) of an untreated patient showing membranous staining. For comparison, E shows internalized sst2 in an AR42J tumor of a rat treated with 0.21 mg [Tyr3] octreotate given iv 1 h before being killed. Bars, 0.01 mm.
Figure 2 illustrates the inter-individual variability of sst2 internalization and documents the specificity of the immunohistochemical observation. Proof for the specificity of the R2-88 immunostaining in all those cases is given by the control samples showing suppression of the internalized as well as of the membranous immunostaining after adsorption with a high dose of the antigen peptide (Fig. 2). This proof of specificity was also obtained with the two other sst2 antibodies (UMB-1 and SS-800) after antigen peptide treatment. Furthermore, Fig. 2 shows that the strong sst2 receptor expression in the four positively immunostained tumor samples can be confirmed with a completely different method of receptor detection, namely in vitro receptor autoradiographic identification using the sst2-preferring radioligand 125I-labeled [Tyr3] octreotide. Confirmation of sst2 expression by receptor autoradiography was obtained for all cases where fresh tissue was available (Table 1).
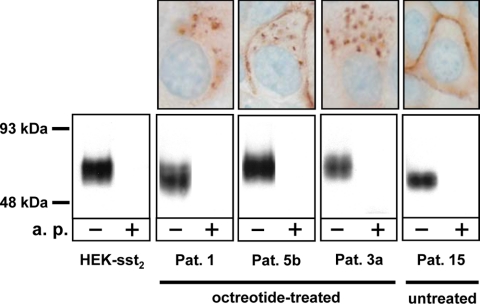
Figure 3 demonstrates in three cases with predominantly internalized sst2 receptors (octreotide-treated patients) that the observed positive R2-88 immunohistochemical staining corresponds to a specific band on an immunoblot using the R2-88 antiserum. A corresponding sst2 band is also seen in a case with membranous sst2 immunohistochemical staining (untreated patient) and in the sst2-expressing HEK-sst2 cell line, used as controls. As specificity control, all bands are shown to be abolished in the presence of the antigen peptide (Fig. 3).
Figure 3.
Confirmation by immunoblotting (bottom row) of the sst2 expression detected with sst2 immunohistochemistry (top row) in tumor samples of octreotide-treated patients (patients 1, 5b, and 3a) compared with controls consisting of sst2-expressing HEK293 cells (HEK-sst2) and a tumor sample from a patient not treated with octreotide (patient 15). In the immunoblot, a broad band centered around 70 kDa is stained by the R2-88 antiserum in all tested samples. This staining is completely abolished by preadsorption of R2-88 with the antigen peptide (a.p.). The variation in apparent molecular mass of the sst2 receptor in the analyzed samples reflects the differences of glycosylation of the receptor. The positive sst2 staining detected with the R2-88 antiserum in the immunohistochemistry samples of patients 1, 5b, and 3a as well as the membranous staining seen in the sample of patient 15 together with the presence of a sst2 receptor band in all samples detected by R2-88 immunoblotting strongly suggests that the granular cytoplasmic structures seen by immunohistochemistry indeed correspond to the internalized sst2 receptor. Pat., Patient.
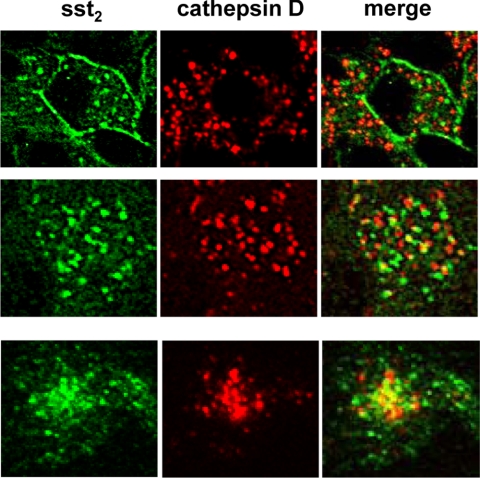
Figure 4 shows at high magnification the localization pattern of the internalized sst2 receptor compared with the membranous sst2 location in an patient not treated with octreotide. The cytoplasmic pattern is compatible with an endosomal localization of the sst2 receptor, as observed in AR42J tumors of [Tyr3] octreotate-treated rats (Fig. 4E). Figure 5 illustrates by confocal double-labeling immunofluorescence microscopy that a small amount of the internalized sst2 receptor is routed from the endosomal system to lysosomes for degradation. This is confirmed by the colocalization of a small population of sst2 receptors, detected by R2-88 antiserum, with the lysosomal marker protein cathepsin D in samples from octreotide-treated patients as well as in the agonist-stimulated control cell line HEK-sst2.
Figure 5.
Comparison of localization of internalized sst2 receptor with the lysosomal marker protein cathepsin D in tumor samples of octreotide-treated patients (top row, patient 5b; middle row, patient 3b) compared with [Tyr3] octreotide-treated, sst2-expressing HEK293 cells (bottom row, HEK-sst2) by confocal double-labeling immunofluorescence imaging. The left column (green) shows the internalized sst2 receptor visualized by the R2-88 antiserum, and the middle column (red) shows the lysosomal staining of cathepsin D. The merged images are shown in the right column. In both tissue samples and in the HEK-sst2 cells, the sst2 receptor shows only a minimal overlap with cathepsin D in the merged images (yellow structures), suggesting that upon agonist-stimulated sst2 receptor internalization, only few sst2 receptors are localized in lysosomal structures.
Discussion
This is the first study indicating that octreotide treatment is associated with agonist-induced internalization of sst2 receptors in human sst2-expressing neuroendocrine tumors. The degree of internalization, i.e. the ratio of internalized sst2 to membranous sst2, varies very much from one patient to the other. A particularly powerful, high-dose octreotide treatment combining octreotide LAR and octreotide infusion (22) during surgical resection triggers a strong and almost complete sst2 internalization. Conversely, with 200 μg sc octreotide given at the start of surgery, a wide range of sst2 internalization pattern is observed, ranging from almost complete receptor internalization in some cases to only focal or even absent internalization next to a predominantly membranous sst2 immunostaining in other individuals. Also, a heterogeneous distribution of tumor cells expressing the internalized receptors may be observed, with cells expressing the sst2 receptors located next to cells without receptors, as reported previously for cells with membrane-bound sst2 (17). The above mentioned diversity in internalization patterns may be due to the fact that the 200 μg sc octreotide is a submaximal dose (22) that may not be sufficient to trigger a complete and long-lasting sst2 internalization in all tumors. The octreotide concentration at the target will indeed depend on numerous systemic and local factors, including the performance of the cardiovascular system as well as local tumor perfusion, tumor sst2 receptor density, and finally, the time elapsing between sc application and tumor resection.
The arguments that the observed cytoplasmic sst2 immunostaining represents true sst2 receptors are 4-fold. First, we observe a comparable immunohistochemical pattern with three distinct sst2-specific antibodies, namely R2-88, SS-800, and UMB-1, that identify C-terminal epitopes. Second, the sst2 immunostaining is always abolished in the presence of an excess of the respective peptide antigens. Third, the samples revealing intracellular immunostaining are found to express a high density of sst2 receptors, as measured by in vitro receptor autoradiography with the sst2-preferring radioligand 125I-labeled [Tyr3] octreotide. Interestingly, all tested samples, including those with predominantly internalized sst2, were found to be receptor positive in autoradiography, as opposed to a previously published single case of somatostatinoma expressing internalized sst2 unidentified by autoradiography (25). Fourth, cases with predominantly internalized sst2 detected by R2-88 immunostaining were submitted to R2-88 immunoblotting and found to exhibit a specific band corresponding to the predicted molecular mass of sst2, strongly indicating that the cytoplasmic granular staining seen by immunohistochemistry indeed represents specific sst2 receptors.
At a high magnification, the internalized sst2 show a cytoplasmic distribution pattern compatible with localization in endosomal structures (12,13,27,28) and comparable with that of internalized sst2 in AR42J tumors from [Tyr3] octreotate-treated rats, as reported previously (16). Furthermore, it appears that the internalized sst2 are not exclusively localized in endosomal structures but that they can be found in smaller amounts also in lysosomes, as shown in the double-labeling immunofluorescence experiments detecting sst2 and the lysosomal marker protein cathepsin D. This suggests that although the internalized sst2 receptors are to a large amount recycled back to the cell surface, as shown previously by the reversibility of the sst2 internalization process in AR42J tumors (16), some of the receptors will nevertheless be routed to the lysosomes and degraded.
Due to the availability of suitable sst2 antibodies, there has been an increased use of sst2 immunohistochemistry for the diagnostic evaluation of sst2 expression in neuroendocrine tumors (17,18,19,21). Up to now, a membranous sst2 localization was considered an accepted criterion for specific sst2 expression and was usually required as a reliable diagnosis of sst2 receptor expression in a tumor (17,18,19,20,21). The present data strongly suggest that the specific pattern of internalized sst2 receptors should also be considered as an additional reliable criterion for specific sst2 expression when it is confirmed that the patient has been on octreotide therapy immediately before or during surgery.
Sst2 receptor desensitization and escape from octreotide treatment, in particular in gastro-enteropancreatic neuroendocrine tumors (29,30) has been an important clinical observation that has been repeatedly debated in the past (31,32,33,34,35). In addition, preoperative management of secreting neuroendocrine tumors is still a challenge. The present data are limited in resolving these problems, because octreotide treatment in the present study cannot be considered a long-term therapy, and the sst2 data obtained on resected tumors represent only a single time point. This type of study, however, may open the possibility to investigate long-term effects of octreotide treatment on the cellular localization of sst2 receptors in tumors in selected patients with and without escape from treatment.
Footnotes
This work was supported by Grant 3200BO-105726 from the Swiss National Science Foundation.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 12, 2010
Abbreviations: LAR, Long-acting repeatable; sst2, somatostatin receptor subtype 2.
References
- Lefkowitz RJ, Shenoy SK 2005 Transduction of receptor signals by β-arrestins. Science 308:512–517 [DOI] [PubMed] [Google Scholar]
- Hanyaloglu AC, von Zastrow M 2008 Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol 48:537–568 [DOI] [PubMed] [Google Scholar]
- Seachrist JL, Ferguson SS 2003 Regulation of G protein-coupled receptor endocytosis and trafficking by Rab GTPases. Life Sci 74:225–235 [DOI] [PubMed] [Google Scholar]
- Claing A, Laporte SA, Caron MG, Lefkowitz RJ 2002 Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog Neurobiol 66:61–79 [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Allen CJ, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE 1995 Rapid endocytosis of a G protein-coupled receptor: substance P evoked internalization of its receptor in the rat striatum in vivo. Proc Natl Acad Sci USA 92:2622–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh PW, DeMaster E, Malhotra A, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE, Simone, DA 1995 Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science 268:1629–1632 [DOI] [PubMed] [Google Scholar]
- Reubi JC 2003 Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev 24:389–427 [DOI] [PubMed] [Google Scholar]
- Bodei L, Paganelli G, Mariani G 2006 Receptor radionuclide therapy of tumors: a road from basic research to clinical applications. J Nucl Med 47:375–377 [PubMed] [Google Scholar]
- van Essen M, Krenning EP, Kam BL, de Jong M, Valkema R, Kwekkeboom DJ 2009 Peptide-receptor radionuclide therapy for endocrine tumors. Nat Rev Endocrinol 5:382–393 [DOI] [PubMed] [Google Scholar]
- Kwekkeboom DJ, Teunissen JJ, Bakker WH, Kooij PP, de Herder WW, Feelders RA, van Eijck CH, Esser JP, Kam BL, Krenning EP 2005 Radiolabeled somatostatin analog [177Lu-DOTA0, Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol 23:2754–2762 [DOI] [PubMed] [Google Scholar]
- Hofland LJ, Lamberts SW 2003 The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr Rev 24:28–47 [DOI] [PubMed] [Google Scholar]
- Cescato R, Schulz S, Waser B, Eltschinger V, Rivier JE, Wester HJ, Culler M, Ginj M, Liu Q, Schonbrunn A, Reubi JC 2006 Internalization of sst2, sst3 and sst5 receptors: effects of somatostatin agonists and antagonists. J Nucl Med 47:502–511 [PubMed] [Google Scholar]
- Liu Q, Cescato R, Dewi DA, Rivier J, Reubi JC, Schonbrunn A 2005 Receptor signaling and endocytosis are differentially regulated by somatostatin analogs. Mol Pharmacol 68:90–101 [DOI] [PubMed] [Google Scholar]
- Ginj M, Chen J, Walter MA, Eltschinger V, Reubi JC, Maecke HR 2005 Preclinical evaluation of new and highly potent analogues of octreotide for predictive imaging and targeted radiotherapy. Clin Cancer Res 11:1136–1145 [PubMed] [Google Scholar]
- Nock BA, Nikolopoulou A, Galanis A, Cordopatis P, Waser B, Reubi JC, Maina T 2005 Potent bombesin-like peptides for GRP-receptor targeting of tumors with 99mTc: a preclinical study. J Med Chem 48:100–110 [DOI] [PubMed] [Google Scholar]
- Waser B, Tamma ML, Cescato R, Maecke HR, Reubi JC 2009 Highly efficient in vivo agonist-induced internalization of sst2 receptors in somatostatin target tissues. J Nucl Med 50:936–941 [DOI] [PubMed] [Google Scholar]
- Körner M, Eltschinger V, Waser B, Schonbrunn A, Reubi JC 2005 Value of immunohistochemistry for somatostatin receptor subtype sst2A in cancer tissues: lessons from the comparison of anti-sst2A antibodies with somatostatin receptor autoradiography. Am J Surg Pathol 29:1642–1651 [DOI] [PubMed] [Google Scholar]
- Reubi JC, Kappeler A, Waser B, Laissue J, Hipkin RW, Schonbrunn A 1998 Immunohistochemical localization of somatostatin receptors sst2A in human tumors. Am J Pathol 153:233–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volante M, Brizzi MP, Faggiano A, La Rosa S, Rapa I, Ferrero A, Mansueto G, Righi L, Garancini S, Capella C, De Rosa G, Dogliotti L, Colao A, Papotti M 2007 Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod Pathol 20:1172–1182 [DOI] [PubMed] [Google Scholar]
- Asnacios A, Courbon F, Rochaix P, Bauvin E, Cances-Lauwers V, Susini C, Schulz S, Boneu A, Guimbaud R, Buscail L 2008 Indium-111-pentetreotide scintigraphy and somatostatin receptor subtype 2 expression: new prognostic factors for malignant well-differentiated endocrine tumors. J Clin Oncol 26:963–970 [DOI] [PubMed] [Google Scholar]
- Fischer T, Doll C, Jacobs S, Kolodziej A, Stumm R, Schulz S 2008 Reassessment of sst2 somatostatin receptor expression in human normal and neoplastic tissues using the novel rabbit monoclonal antibody UMB-1. J Clin Endocrinol Metab 93:4519–4524 [DOI] [PubMed] [Google Scholar]
- Oberg K, Kvols L, Caplin M, Delle Fave G, de Herder W, Rindi G, Ruszniewski P, Woltering EA, Wiedenmann B 2004 Consensus report on the use of somatostatin analogs for the management of neuroendocrine tumors of the gastroenteropancreatic system. Ann Oncol 15:966–973 [DOI] [PubMed] [Google Scholar]
- Fiess H, Klempa I, Hermanek P, Sulkowski U, Uhl W, Beger HG, Buchler MW 1994 Prophylaxis of complications after pancreatic surgery: results of a multicenter trial in Germany. Digestion 55(Suppl 1):35–40 [DOI] [PubMed] [Google Scholar]
- Reubi JC, Laissue JA, Waser B, Steffen DL, Hipkin RW, Schonbrunn A 1999 Immunohistochemical detection of somatostatin sst2a receptors in the lymphatic, smooth muscular, and peripheral nervous systems of the human gastrointestinal tract: facts and artifacts. J Clin Endocrinol Metab 84:2942–2950 [DOI] [PubMed] [Google Scholar]
- Reubi JC, Waser B, Liu Q, Laissue JA, Schonbrunn A 2000 Subcellular distribution of somatostatin sst2A receptors in human tumors of the nervous and neuroendocrine systems: membranous versus intracellular location. J Clin Endocrinol Metab 85:3882–3891 [DOI] [PubMed] [Google Scholar]
- Reubi JC, Waser B, Schaer JC, Laissue JA 2001 Somatostatin receptor sst1–sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur J Nucl Med 28:836–846 [DOI] [PubMed] [Google Scholar]
- Tulipano G, Stumm R, Pfeiffer M, Kreienkamp HJ, Höllt V, Schulz S 2004 Differential β-arrestin trafficking and endosomal sorting of somatostatin receptor subtypes. J Biol Chem 279:21374–21382 [DOI] [PubMed] [Google Scholar]
- Mentlein R, Held-Feindt J, Krisch B 2001 Topology of the signal transduction of the G protein-coupled somatostatin receptor sst2 in human glioma cells. Cell Tissue Res 303:27–34 [DOI] [PubMed] [Google Scholar]
- Eriksson B, Oberg K 1999 Summing up 15 years of somatostatin analog therapy in neuroendocrine tumors: future outlook. Ann Oncol 10(Suppl 2):S31–S38 [DOI] [PubMed] [Google Scholar]
- Wynick D, Anderson JV, Williams SJ, Bloom SR 1989 Resistance of metastatic pancreatic endocrine tumours after long-term treatment with the somatostatin analogue octreotide (SMS 201-995). Clin Endocrinol (Oxf) 30:385–388 [DOI] [PubMed] [Google Scholar]
- Hofland LJ, van der Hoek J, Feelders R, van der Lely AJ, de Herder W, Lamberts SW 2005 Pre-clinical and clinical experiences with novel somatostatin ligands: advantages, disadvantages and new prospects. J Endocrinol Invest 28(11 Suppl International):36–42 [PubMed] [Google Scholar]
- Gunn SH, Schwimer JE, Cox M, Anthony CT, O'Dorisio MS, Woltering EA 2006 In vitro modeling of the clinical interactions between octreotide and 111In-pentetreotide: is there evidence of somatostatin receptor downregulation? J Nucl Med 47:354–359 [PubMed] [Google Scholar]
- Wahid ST, Marbach P, Stolz B, Miller M, James RA, Ball SG 2002 Partial tachyphylaxis to somatostatin (SST) analogues in a patient with acromegaly: the role of SST receptor desensitisation and circulating antibodies to SST analogues. Eur J Endocrinol 146:295–302 [DOI] [PubMed] [Google Scholar]
- Froidevaux S, Hintermann E, Török M, Mäcke HR, Beglinger C, Eberle AN 1999 Differential regulation of somatostatin receptor type 2 (sst 2) expression in AR4-2J tumor cells implanted into mice during octreotide treatment. Cancer Res 59:3652–3657 [PubMed] [Google Scholar]
- Anthony CT, Hughey S, Lyons J, Weiss S, Hornick CA, Drouant GJ, Fuselier JA, Coy DH, Murphy WA, Woltering EA 2004 The effect of drug dose and drug exposure time on the binding, internalization, and cytotoxicity of radiolabeled somatostatin analogs. J Surg Res 119:1–13 [DOI] [PubMed] [Google Scholar]