Abstract
Context: The thionamide antithyroid drugs methimazole and propylthiouracil are the mainstay of pharmacologic therapy for Graves’ disease. However, little is known about the rate of use of these drugs and the prescribing practices of physicians treating hyperthyroidism.
Objective: The objective of the study was to examine the frequency of methimazole and propylthiouracil use from years 1991 to 2008.
Methods: The data were acquired by the U.S. Food and Drug Administration’s Division of Epidemiology through two databases: IMS National Sales Perspectives and the Surveillance Data, Inc. Vector One: National database.
Results: There was a 9-fold increase in the annual number of methimazole prescriptions during the study period, from 158,000 to 1.36 million per year. There was a 19% increase in the annual number of propylthiouracil prescriptions, from 348,000 to 415,000 per year. Propylthiouracil, which held two thirds of the market from 1991 to 1995, was surpassed by methimazole in 1996. Patient demographic data indicated that although 72% of methimazole prescriptions were for females, males were more likely to be on methimazole (82%) than females (74%) (P < 0.001, two tailed χ2 test). The only demographic group in which methimazole use decreased was women of child-bearing age (5% decrease, P < 0.001, two tailed χ2). The incidence of hyperthyroidism in 2008 was estimated based on the number of new prescriptions of thionamides by age group and data from the 2008 U.S. census: 0.44 per 1000 for ages 0–11 yr, 0.26 per 1000 for ages 12–17 yr, 0.59 per 1000 for ages 18–44 yr, 0.78 per 1000 for ages 45–64 yr, and 1.01 per 1000 for ages 65+ yr.
Conclusions: Methimazole has become the most frequently prescribed antithyroid drug. The remarkable increase in the total number of dispensed thionamide prescriptions over the last 18 yr may indicate a trend toward pharmacological treatment as primary treatment of Graves’ disease in the United States.
Methimazole has been the antithyroid drug of choice in the United States since 1996, when propylthiouracil became a second-line agent, as evidenced by physicians’ prescribing practices
Since their introduction in the mid 1940s, the thionamide antithyroid drugs propylthiouracil (PTU) and methimazole (MMI) have continued to be the mainstay of pharmacologic treatment of Graves’ disease in the United States (1). Thionamides exert their antithyroid effects primarily by inhibiting thyroid hormone synthesis through interference with the oxidation and organic binding of iodide into thyroglobulin (2). In addition, PTU, but not MMI, inhibits the peripheral conversion of T4 to T3 by type 1 deiodinase (3,4). There are also incompletely understood effects of both PTU and MMI on the immune system, which may partly account for the remissions seen after a course of antithyroid drug therapy (5), and the blunting of the rise in anti-TSH receptor antibody levels after radioiodine therapy in MMI-pretreated patients (6).
MMI and PTU can be used as primary therapy for Graves’ disease or as preparation for surgery or radioactive iodine treatment. Although radioactive iodine treatment is the preferred treatment modality of hyperthyroidism in the United States for most adults (7), primary therapy with PTU and MMI continue to be common alternatives, with many patients remaining on them for years (8). Data from the early 1990s suggested that PTU was favored over MMI by endocrinologists in the United States (9). However, given its superior side effect profile, greater efficacy in severely hyperthyroid patients (10), and more favorable adherence rate related to its once-daily dosing (11), MMI has been recommended as the first-line agent, with PTU use relegated to specific clinical circumstances: allergy to MMI, Graves’ disease in pregnancy, and possibly life-threatening thyrotoxicosis (thyroid storm) (2). The recommendation against the use of PTU as the first-line agent in children or adults has recently been strengthened as a result of increasing numbers of reports of severe liver toxicity, in some cases leading to transplantation and in a few, death from fulminant hepatic failure (12,13,14). Despite the recommendations to use MMI as the primary thionamide, the prescribing habits of clinicians caring for patients with hyperthyroidism are not known.
In the present study, we report the prescribing practices for PTU and MMI from 1991 to 2008. Using two pharmacy databases, we were able to estimate the total annual number of dispensed prescriptions for PTU and MMI during that time period, as well as the total number of newly dispensed thionamide prescriptions.
Materials and Methods
The data reported in this paper were acquired by the Division of Epidemiology, Office of Surveillance and Epidemiology of the U.S. Food and Drug Administration (FDA). Two databases were used: The IMS Health, IMS National Sales Perspectives database and the Surveillance Data, Inc. Vector One: National database. The IMS Health, IMS National Sales Perspectives database was used to estimate the sales of PTU and MMI in the United States in 2008. This database compiles the sales of drugs from manufacturers to retail markets, which include chain drug stores, independent drug stores, mass merchandisers, food stores, and mail service. Use from mail order pharmacies (6–7% of sales) was not included in this analysis.
We examined prescription use trends for MMI and PTU for the years 1991 through 2008 using Surveillance Data’s Vector One: National (VONA). Surveillance Data’s VONA measures retail dispensing of medications to consumers via formal prescriptions. Information is available on physician specialty, the patient’s age and gender, and estimates of the numbers of patients who are either continuing therapy or who are initiating therapy. The Vector One database integrates prescription activity from a variety of sources, including national retail chains, mass merchandisers, mail order pharmacies, pharmacy benefits managers and their data systems, and provider groups. Vector One receives more than 2.0 billion prescription claims per year, representing more than 160 million unique patients. Prescriptions are captured from a sample of approximately 59,000 pharmacies throughout the United States. The pharmacies in the database account for nearly all retail pharmacies and represent nearly half of retail prescriptions dispensed nationwide. Using these data, estimates for national drug prescription claims and demographic factors at the national level are derived by Surveillance Data.
Data on patient demographics were available for the years 2002–2008. Data on the frequency of new-to-therapy prescriptions were available for years 2005–2008. Prescriptions were classified as new-to-therapy prescriptions from the date of dispensing if no prescriptions were dispensed to a patient for either MMI or PTU in the previous 6 months. We used the number of new PTU and MMI prescriptions dispensed as a surrogate in the calculation of the annual incidence of hyperthyroidism. The total number of new-to-therapy PTU and MMI prescriptions for each age group in the FDA data (0–11, 12–17, 18–44, 45–64, and 65+ yr) were divided by the respective population from the projected 2008 census data on population by age groups (www.census.gov/popest/national/asrh/NC_EST2008-sa.html). Because the census data were reported in 21 age groups, we converted the information into five age groups, similar to the FDA report age distribution (0–9, 10–19, 20–44, 45–64, and 65+ yr). We report the estimated incidence per 1000 persons.
Finally, the databases also provided information on dispensed prescriptions by prescriber specialty.
Results
Based on the IMS Health, IMS National Sales Perspectives data, it can be estimated that PTU and MMI are distributed primarily in outpatient settings. In 2008 approximately 78% of MMI and 83% of PTU were distributed through retail pharmacies.
MMI and PTU dispensed outpatient prescriptions
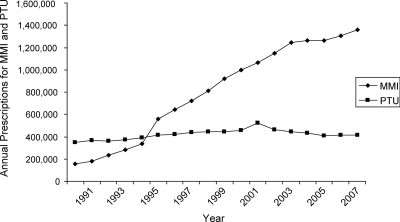
Over the 18-yr period from 1991 to 2008, the total number of prescriptions dispensed annually for MMI increased nearly 9-fold, from approximately 158,000 in 1991 to nearly 1.36 million prescriptions in 2008 (Fig. 1). The total number of prescriptions includes new prescriptions, renewed prescriptions, and prescription refills. Annual dispensed prescriptions for PTU, on the other hand, increased only 19%, going from approximately 348,000 prescriptions in 1991 to approximately 415,000 prescriptions in 2008. PTU held approximately one half to two thirds of the market from 1991 through 1995; in 1996 dispensed prescriptions for MMI surpassed those for PTU, and MMI remained the most prescribed drug through the end of the study period. By 2008 MMI had about 77% of the market share.
Figure 1.
Total number of prescriptions dispensed for MMI and PTU from outpatient retail pharmacies, 1991–2008, according to Surveillance Data, Vector One: National Database, extracted March 2009.
MMI and PTU prescription distribution by gender and age
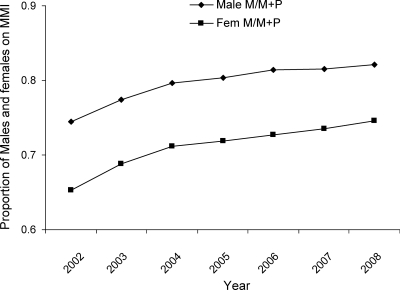
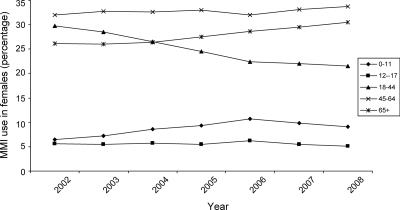
Patient demographic data were available for 2002 through 2008. Table 1 depicts total numbers of prescriptions dispensed annually (new prescriptions and refills). The distribution of prescription use showed that nearly three quarters of MMI dispensed prescriptions were for females (Table 1). For example, in 2008, of approximately 1.36 million dispensed MMI prescriptions, 986,753 were for female patients (72%) and 348,544 for males (28%). Table 2 shows the numbers of individual unique patients receiving prescriptions in each calendar year. Whereas MMI use predominated in both sexes, MMI had a slightly lower proportion of use in the female population, compared with PTU from 2002 to 2008. For example, in 2008 proportionally fewer women (75%; 250,590 females on MMI/334,507 females, which is the total sum of females on both MMI and PTU) received MMI compared with males (82%; 91,912 males on MMI/111,563, which is the total sum of males on both MMI and PTU) (P < 0.001, two tailed χ2 test) (Fig. 2). The data for PTU over this 7-yr period revealed that the number of dispensed prescriptions and patients receiving PTU decreased in all age groups, irrespective of gender (Tables 1 and 2), which is the opposite of what occurred in terms of MMI use. However, the use of MMI decreased in females in the 18- to 44-yr age group (Table 1). Unlike the other female age groups, in this subgroup there was a 5% decrease in dispensed prescriptions in year 2008 compared with the baseline, which is year 2002 (P < 0.001, two tailed χ2) (Fig. 3).
Table 1.
Total number of PTU and MMI prescriptions dispensed from retail pharmacies
| 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | |
|---|---|---|---|---|---|---|---|
| PTU female total | 418,380 | 373,223 | 354,192 | 346,491 | 329,896 | 330,256 | 332,857 |
| Years | |||||||
| 0–11 | 3,350 | 2,947 | 2,822 | 2,725 | 1,798 | 1,736 | 1,917 |
| 12–17 | 8,254 | 7,093 | 6,138 | 5,500 | 4,556 | 4,873 | 4,364 |
| 18–44 | 163,295 | 141,147 | 129,991 | 123,143 | 114,387 | 112,900 | 112,709 |
| 45–64 | 128,579 | 116,759 | 112,381 | 112,209 | 108,370 | 108,456 | 108,714 |
| 65+ | 113,055 | 104,071 | 101,610 | 102,184 | 100,152 | 101,707 | 104,700 |
| Unspecified | 1,847 | 1,206 | 1,250 | 730 | 633 | 584 | 453 |
| MMI female total | 765,223 | 811,918 | 863,280 | 889,730 | 886,073 | 926,117 | 986,753 |
| Years | |||||||
| 0–11 | 47,519 | 55,808 | 71,233 | 81,329 | 93,189 | 89,143 | 88,911 |
| 12–17 | 41,161 | 42,387 | 47,174 | 47,766 | 53,582 | 50,214 | 49,382 |
| 18–44 | 219,257 | 219,953 | 218,573 | 212,374 | 193,680 | 200,771 | 209,131 |
| 45–64 | 235,345 | 252,102 | 269,142 | 286,574 | 277,593 | 300,928 | 328,011 |
| 65+ | 192,070 | 200,464 | 217,058 | 239,123 | 247,740 | 268,365 | 296,554 |
| Unspecified | 29,871 | 41,204 | 40,100 | 22,564 | 20,289 | 16,696 | 14,764 |
| PTU male total | 101,863 | 91,129 | 86,716 | 84,640 | 80,006 | 81,480 | 80,498 |
| Years | |||||||
| 0–11 | 1,544 | 1,276 | 1,075 | 955 | 826 | 675 | 634 |
| 12–17 | 2,908 | 2,372 | 1,854 | 1,913 | 1,706 | 1,691 | 1,330 |
| 18–44 | 31,947 | 27,224 | 25,015 | 23,006 | 21,771 | 21,692 | 20,050 |
| 45–64 | 38,156 | 35,373 | 34,402 | 34,003 | 32,570 | 33,097 | 32,802 |
| 65+ | 26,265 | 24,308 | 23,764 | 24,433 | 22,814 | 23,863 | 24,939 |
| Unspecified | 1,043 | 576 | 606 | 330 | 319 | 462 | 743 |
| MMI male total | 287,930 | 301,084 | 324,211 | 332,867 | 337,518 | 347,654 | 348,544 |
| Years | |||||||
| 0–11 | 38,158 | 41,472 | 50,101 | 55,097 | 63,171 | 62,282 | 54,972 |
| 12–17 | 24,215 | 26,688 | 28,676 | 27,747 | 31,793 | 30,467 | 27,098 |
| 18–44 | 66,894 | 69,138 | 70,999 | 69,397 | 66,741 | 71,248 | 71,586 |
| 45–64 | 74,778 | 78,753 | 83,747 | 91,686 | 88,076 | 94,132 | 101,832 |
| 65+ | 54,424 | 53,144 | 59,331 | 66,027 | 65,318 | 70,206 | 76,831 |
| Unspecified | 29,461 | 31,889 | 31,357 | 22,913 | 22,419 | 19,319 | 16,225 |
Data source was Surveillance Data, VONA, extracted in 03/2009.
Table 2.
Total number of patients receiving a prescription for PTU or MMI
| Projected patient count
|
|||||||
|---|---|---|---|---|---|---|---|
| 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | |
| PTU female total | 110,333 | 101,874 | 91,154 | 87,570 | 83,732 | 82,754 | 83,917 |
| Years | |||||||
| 0–11 | 829 | 779 | 625 | 612 | 476 | 440 | 467 |
| 12–44 | 53,429 | 47,170 | 41,298 | 39,048 | 36,474 | 35,632 | 35,442 |
| 45–64 | 31,853 | 30,136 | 27,298 | 26,621 | 25,723 | 25,506 | 26,084 |
| 65+ | 23,201 | 22,130 | 20,244 | 19,781 | 19,623 | 19,689 | 20,532 |
| Unspecified | 1,021 | 1,659 | 1,689 | 1,508 | 1,436 | 1,487 | 1,392 |
| MMI female total | 209,321 | 235,978 | 235,419 | 230,568 | 230,257 | 234,823 | 250,590 |
| Years | |||||||
| 0–11 | 14,410 | 15,702 | 18,951 | 20,703 | 24,324 | 22,943 | 23,543 |
| 12–44 | 83,631 | 81,927 | 77,955 | 75,954 | 72,098 | 72,449 | 74,639 |
| 45–64 | 64,099 | 67,649 | 67,711 | 70,250 | 67,901 | 72,227 | 79,562 |
| 65+ | 45,286 | 46,582 | 46,616 | 48,779 | 51,112 | 54,364 | 61,326 |
| Unspecified | 1,895 | 24,118 | 24,186 | 14,882 | 14,822 | 12,840 | 11,520 |
| PTU male total | 25,349 | 24,098 | 21,745 | 20,668 | 19,638 | 19,845 | 19,651 |
| Years | |||||||
| 0–11 | 394 | 341 | 308 | 208 | 204 | 173 | 172 |
| 12–44 | 9,618 | 8,541 | 7,559 | 6,921 | 6,515 | 6,385 | 5,957 |
| 45–64 | 9,275 | 9,004 | 8,159 | 7,992 | 7,666 | 7,680 | 7,640 |
| 65+ | 5,923 | 5,788 | 5,290 | 5,203 | 4,924 | 5,201 | 5,389 |
| Unspecified | 139 | 424 | 429 | 344 | 329 | 406 | 493 |
| MMI male total | 74,428 | 87,011 | 89,075 | 87,505 | 90,493 | 91,159 | 91,912 |
| Years | |||||||
| 0–11 | 11,753 | 11,604 | 13,575 | 14,041 | 16,759 | 16,187 | 15,094 |
| 12–44 | 27,452 | 27,570 | 27,118 | 26,109 | 26,637 | 27,133 | 27,538 |
| 45–64 | 20,123 | 21,187 | 20,849 | 22,119 | 21,297 | 22,325 | 24,169 |
| 65+ | 14,548 | 13,892 | 14,138 | 15,277 | 15,029 | 16,105 | 17,977 |
| Unspecified | 552 | 12,758 | 13,395 | 9,959 | 10,771 | 9,409 | 7,134 |
Data source was Surveillance Data, Total Patient Tracker, extracted March 2009.
Figure 2.
Proportion of males and females taking antithyroid drugs who were using MMI from years 2002–2008, Surveillance Data, VONA database, extracted March 2009.
Figure 3.
Percentage of MMI use among females by age group from 2002 to 2008, Surveillance Data, VONA database, extracted March 2009.
New-to-therapy prescriptions
Newly dispensed MMI and PTU prescriptions were categorized as new to therapy by age group from years 2005 to 2008 (Table 3). Overall, in 2008 there were 190,327 new-to-therapy prescriptions dispensed for antithyroid drugs, which, assuming that most patients receive a prescription for an antithyroid drug at the onset of their disease, may represent a very rough estimate of the total number of new cases of hyperthyroidism in the United States in that year. There were 41,551 new-to-therapy prescriptions for PTU, or approximately 22% of the total. However, this fraction varied with age. For example, in young children aged 0–11 yr, the proportion receiving PTU was only 1.7% of the total, whereas in young and middle-aged women aged 18–44 yr, the proportion was 32%. Assuming that most hyperthyroid patients receive primary antithyroid drug therapy or receive antithyroid drugs for a few months before radioiodine therapy or surgery, we calculated an approximate annual incidence of hyperthyroidism. For this calculation, the total number of new PTU and MMI prescriptions per age group for year 2008 was divided by the projected 2008 census numbers for each age group (Table 3). For the 0- to 11-yr age group, the estimated incidence was 0.44 per 1000; in the 12- to 17-yr age group, the incidence was 0.26 per 1000; in the 18- to 44-yr age group, the incidence was 0.59 per 1000; for the 45- to 64-yr age group, the incidence was 0.78 per 1000; and for the 65+ yr age group, the estimated incidence was 1.01 per 1000.
Table 3.
New patient prescriptions for PTU and MMI
| 2005 | 2006 | 2007 | 2008 | |
|---|---|---|---|---|
| MMI total | 144,025 | 135,144 | 140,299 | 148,776 |
| Years | ||||
| 0–11 | 15,756 | 18,010 | 18,311 | 17,714 |
| 12–17 | 8,821 | 9,613 | 9,887 | 9,907 |
| 18–44 | 45,122 | 38,873 | 40,402 | 41,801 |
| 45–64 | 45,349 | 40,909 | 43,749 | 47,864 |
| 65+ | 28,977 | 27,739 | 27,950 | 31,490 |
| PTU total | 46,988 | 43,496 | 42,513 | 41,551 |
| Years | ||||
| 0–11 | 366 | 325 | 297 | 313 |
| 12–17 | 995 | 765 | 775 | 756 |
| 18–44 | 22,750 | 20,687 | 20,350 | 19,808 |
| 45–64 | 14,093 | 13,160 | 13,097 | 12,847 |
| 65+ | 8,784 | 8,559 | 7,994 | 7,827 |
Data source was Surveillance Data, VONA, extracted April 2009.
Dispensed outpatient prescriptions by prescriber specialty
During 2008 endocrinologists accounted for approximately 30% of dispensed prescriptions for MMI, followed by internists with 20% and general practitioners, family medicine physicians, and doctors of osteopathy with 13%. Prescriptions written by pediatricians accounted for approximately 2% of dispensed prescriptions. For PTU, internists and endocrinologists each accounted for approximately 27%, followed by general practitioners, family medicine physicians, and doctors of osteopathy with 24%. Prescriptions written by pediatricians accounted for approximately 2% of dispensed prescriptions for PTU.
Discussion
The present study provides an overview of thionamide use in the United States over almost 2 decades, revealing important and heretofore undocumented trends in prescribing practices. Since 1996 MMI has become the most frequently prescribed thionamide, with an 800% increase in the number of dispensed outpatient prescriptions. On the other hand, the number of dispensed PTU prescriptions reached a plateau in the early 1990s, and its use rate remained virtually unchanged through 2008. The reason for this dramatic change in clinical practice is likely due to a combination of factors. First, the side effect profile favors MMI. Although agranulocytosis is a potential adverse effect of both thionamides, it is dose related for MMI and less so for PTU (15). PTU is the cause of rare but potentially life-threatening hepatotoxicity, whereas severe hepatotoxic reactions related to MMI are extraordinarily rare (12,16,17). Also, PTU is a far more common cause of ANCA-positive vasculitis than is MMI (18,19). The more convenient dosing of MMI, translated into enhanced adherence, also makes it preferable to PTU. Finally, MMI is more effective than PTU in controlling severe hyperthyroidism (10). These advantages are unlikely to be anything more than a minor explanation for the change in prescribing practices because the differences in toxicity, adherence, and efficacy had been well known before the 1990s when PTU use still dominated the market.
Perhaps the most likely explanation for the dramatic rise in MMI prescribing is the availability of generic MMI in the late 1990s. Previously Tapazole was more expensive than PTU, but now the price of the two generic drugs is roughly similar at comparable effective doses (http://www.destinationrx.com/, accessed August 30, 2009). Finally, the number of case reports of PTU-induced severe liver toxicity more than doubled in the 1980s and 1990s compared with the previous decades (14). Whether this phenomenon is also linked to the changes in prescribing patterns for the thionamides remains unknown.
In our study, the only subgroup in which MMI use did not increase was in women of childbearing age. Indeed, MMI use actually decreased in this age group. This is in accord with the current recommendations contained in the Endocrine Society clinical practice guidelines for the man agement of thyroid disease in pregnancy (20), as well as a recent FDA alert (http://www.fda.gov/Drugs/ucm162701.htm), and an American Thyroid Association statement (21) for use of PTU as the first-line agent in the management of hyperthyroidism during pregnancy. Although The Endocrine Society’s clinical guidelines were published in 2007, they reflected previous expert opinion (22), based on a literature linking the use of MMI during pregnancy to birth defects, including choanal atresia, aplasia cutis, and tracheal-esophageal fistulae (23). In the past, concerns about a greater transplacental transfer of MMI compared with PTU also led to MMI being avoided during pregnancy. However, recent studies showed that PTU is transplacentally transferred at a rate similar to MMI (24,25). This knowledge may have led to declining use of MMI in pregnancy and in women who wish to become pregnant well before the publication of the guidelines and warnings in 2007 and 2009, respectively. Because the proportion of women using PTU in this age group also decreased, it is possible that more women of child-bearing age are choosing radioiodine therapy before pregnancy.
The estimated incidence rates for hyperthyroidism estimated by new-to-therapy prescriptions for antithyroid drugs in 2008 may be a reasonable surrogate for the actual incidence in the American population. Because a large proportion, if not the vast majority, of patients who undergo radioiodine treatment or thyroidectomy for the management of hyperthyroidism are treated with an antithyroid drug at some point in their clinical course, it is likely that these patients have been captured by the databases used in this study. Furthermore, because more than 90% of patients below the age of 50 yr who present with hyperthyroidism have Graves’ disease (26), the estimated incidence of hyperthyroidism presented in our study is indeed a surrogate for Graves’ disease incidence in young to middle-aged individuals. Our rates are slightly higher than the incidence rates previously reported for Western societies. In Sweden, the annual incidence of hyperthyroidism in adults was estimated to be 0.33 per 1000, with an annual incidence of 0.51 and 0.14 per 1000, in females and males, respectively (26). A study performed in the United Kingdom reported an annual incidence of hyperthyroidism of 0.77 per 1000 in women and 0.14 per 1000 in men (27). Data from the Nurse’s Health Study II indicated a much higher incidence of Graves’ disease in women of 4.6 per 1000 (28).
A recent systematic review of the literature on the incidence of autoimmune thyroid disease estimated the incidence of hyperthyroidism to be 0.8 per 1000 in women and 0.08 per 1000 in men, which is surprisingly similar to the overall incidence we presently report of between 0.44 per 1000 in children and 1.01 per 1000 in the elderly (29). Unfortunately, we were unable to estimate incidence in relation to gender because the data on new prescriptions did not include sex distribution. On the other hand, we were able to estimate the incidence of hyperthyroidism in children. Our rates were higher than the previously reported U.S. incidence of 0.04 per 1000 in the population younger than 19 yr (30). Because most children are treated with antithyroid agents, this estimated incidence may be more accurate than the estimates for adults. The adult incidence of hyperthyroidism that we report in our study may be an underestimation because some patients who receive radioiodine are never treated with antithyroid drugs and would not be counted using our methodology.
The data presented in this study should be interpreted in the context of the known limitations of the databases used. For example, the databases provide the total number of prescriptions by age and gender only from 2002 to 2008 and not throughout the study period, which is from 1991 to 2008. Moreover, the database did not provide information on the gender of patients receiving new prescriptions. Finally, it is unclear why the total number of dispensed antithyroid drug prescriptions increased from a few hundred thousand in the early 1990s to more than a million in 2008. Because it is unlikely that the incidence of hyperthyroidism is increasing dramatically, given the relatively stable numbers of new prescriptions dispensed between 2005 and 2008 (Table 3), it is plausible that the remarkable increase in the number of dispensed thionamide prescriptions over the past 2 decades reflects a growing preference for long-term primary pharmacotherapy instead of radioiodine treatment. Whereas it is possible that the database did not capture prescriptions written in the 1990s as accurately as in more recent times, the methods for compiling drug sales by Surveillance Data Vector One has not changed over the study period (personal communication, Timothy McGee, associate director, Client Solutions, Syndicated Analytics at Surveillance Data).
It is certain that changes in antithyroid drug prescribing patterns will continue to occur. It is surprising that despite sound evidence concluding that PTU should be used in only certain circumstances and not as a first-line drug that approximately one quarter of new prescriptions for the treatment of hyperthyroidism in 2008 were for PTU. Hopefully, with the new FDA warning, this proportion will continue to fall in the coming years, as it has most dramatically in children. With regard to the treatment of hyperthyroidism during pregnancy, it is unclear how to balance the competing risks of rare MMI embryopathy and MMI-related aplasia cutis in the fetus with the very uncommon occurrence of PTU-related severe hepatotoxicity in the mother. Only future clinical and epidemiological studies will be able to answer this question.
Footnotes
A.B.E. was supported by National Institutes of Health Grant 5 T32 DK007751-09.
Disclosure Summary: The authors have nothing to declare.
First Published Online March 24, 2010
Abbreviations: MMI, Methimazole; PTU, propylthiouracil; VONA, Vector One: National.
References
- Cooper DS 2003 Hyperthyroidism. Lancet 362:459–468 [DOI] [PubMed] [Google Scholar]
- Cooper DS 2005 Antithyroid drugs. N Engl J Med 352:905–917 [DOI] [PubMed] [Google Scholar]
- Cooper DS, Saxe VC, Meskell M, Maloof F, Ridgway EC 1982 Acute effects of propylthiouracil (PTU) on thyroidal iodide organification and peripheral iodothyronine deiodination: correlation with serum PTU levels measured by radioimmunoassay. J Clin Endocrinol Metab 54:101–107 [DOI] [PubMed] [Google Scholar]
- Abuid J, Larsen PR 1974 Triiodothyronine and thyroxine in hyperthyroidism: comparison of the acute changes during therapy with antithyroid agents. J Clin Invest 54:201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tötterman TH, Karlsson FA, Bengtsson M, Mendel-Hartvig I 1987 Induction of circulating activated suppressor-like T cells by methimazole therapy for Graves’ disease. N Engl J Med 316:15–22 [DOI] [PubMed] [Google Scholar]
- Andrade VA, Gross JL, Maia AL 2004 Serum thyrotropin-receptor autoantibodies levels after I therapy in Graves’ patients: effect of pretreatment with methimazole evaluated by a prospective, randomized study. Eur J Endocrinol 151:467–474 [DOI] [PubMed] [Google Scholar]
- Solomon B, Glinoer D, Lagasse R, Wartofsky L 1990 Current trends in the management of Graves’ disease. J Clin Endocrinol Metab 70:1518–1524 [DOI] [PubMed] [Google Scholar]
- Slingerland DW, Burrows BA 1979 Long-term antithyroid treatment in hyperthyroidism. JAMA 242:2408–2410 [PubMed] [Google Scholar]
- Wartofsky L, Glinoer D, Solomon B, Nagataki S, Lagasse R, Nagayama Y, Izumi M 1991 Differences and similarities in the diagnosis and treatment of Graves’ disease in Europe, Japan and the United States. Thyroid 1:129–135 [DOI] [PubMed] [Google Scholar]
- Nakamura H, Noh JY, Itoh K, Fukata S, Miyauchi A, Hamada N 2007 Comparison of methimazole and propylthiouracil in patients with hyperthyroidism caused by Graves’ disease. J Clin Endocrinol Metab 92:2157–2162 [DOI] [PubMed] [Google Scholar]
- Nicholas WC, Fischer RG, Stevenson RA, Bass JD 1995 Single daily dose of methimazole compared to every 8 hours propylthiouracil in the treatment of hyperthyroidism. South Med J 88:973–976 [DOI] [PubMed] [Google Scholar]
- Williams KV, Nayak S, Becker D, Reyes J, Burmeister LA 1997 Fifty years of experience with propylthiouracil-associated hepatotoxicity: what have we learned? J Clin Endocrinol Metab 82:1727–1733 [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Mattison DR 2009 Ending propylthiouracil-induced liver failure in children. N Engl J Med 360:1574–1575 [DOI] [PubMed] [Google Scholar]
- Cooper DS, Rivkees SA 2009 Putting propylthiouracil in perspective. J Clin Endocrinol Metab 94:1881–1882 [DOI] [PubMed] [Google Scholar]
- Andersohn F, Konzen C, Garbe E 2007 Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med 146:657–665 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kim BH, Han YS, Yang I, Kim KJ, Dong SH, Kim HJ, Chang YW, Lee JI, Chang R 2001 The incidence and clinical characteristics of symptomatic propylthiouracil-induced hepatic injury in patients with hyperthyroidism: a single-center retrospective study. Am J Gastroenterol 96:165–169 [DOI] [PubMed] [Google Scholar]
- Cooper DS 1999 The side effects of antithyroid drugs. Endocrinologist 9:457–478 [Google Scholar]
- Wing SS, Fantus IG 1987 Adverse immunologic effects of antithyroid drugs. CMAJ 136:121–127 [PMC free article] [PubMed] [Google Scholar]
- Noh JY, Yasuda S, Sato S, Matsumoto M, Kunii Y, Noguchi Y, Mukasa K, Ito K, Ito K, Sugiyama O, Kobayashi H, Nihojima S, Okazaki M, Yokoyama S 2009 Clinical characteristics of myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis caused by antithyroid drugs. J Clin Endocrinol Metab 94:2806–2811 [DOI] [PubMed] [Google Scholar]
- Abalovich M, Amino N, Barbour LA, Cobin RH, De Groot LJ, Glinoer D, Mandel SJ, Stagnaro-Green A 2007 Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 92(Suppl 8):S1–S47 [DOI] [PubMed] [Google Scholar]
- Bahn RS, Burch HS, Cooper DS, Garber JR, Greenlee CM, Klein IL, Laurberg P, McDougall IR, Rivkees SA, Ross D, Sosa JA, Stan MN 2009 The role of propylthiouracil in the management of Graves’ disease in adults: report of a meeting jointly sponsored by the American Thyroid Association and the Food and Drug Administration. Thyroid 19:673–674 [DOI] [PubMed] [Google Scholar]
- Mandel SJ, Cooper DS 2001 The use of antithyroid drugs in pregnancy and lactation. J Clin Endocrinol Metab 86:2354–2359 [DOI] [PubMed] [Google Scholar]
- Di Gianantonio E, Schaefer C, Mastroiacovo PP, Cournot MP, Benedicenti F, Reuvers M, Occupati B, Robert E, Bellemin B, Addis A, Arnon J, Clementi M 2001 Adverse effects of prenatal methimazole exposure. Teratology 64:262–266 [DOI] [PubMed] [Google Scholar]
- Mortimer RH, Cannell GR, Addison RS, Johnson LP, Roberts MS, Bernus I 1997 Methimazole and propylthiouracil equally cross the perfused human term placental lobule. J Clin Endocrinol Metab 82:3099–3102 [DOI] [PubMed] [Google Scholar]
- Momotani N, Noh JY, Ishikawa N, Ito K 1997 Effects of propylthiouracil and methimazole on fetal thyroid status in mothers with Graves’ hyperthyroidism. J Clin Endocrinol Metab 82:3633–3636 [DOI] [PubMed] [Google Scholar]
- Abraham-Nordling M, Törring O, Lantz M, Hallengren B, Ohrling H, Lundell G, Calissendorff J, Jörneskog G, Wallin G 2008 Incidence of hyperthyroidism in Stockholm, Sweden, 2003–2005. Eur J Endocrinol 158:823–827 [DOI] [PubMed] [Google Scholar]
- Flynn RW, MacDonald TM, Morris AD, Jung RT, Leese GP 2004 The thyroid epidemiology, audit, and research study: thyroid dysfunction in the general population. J Clin Endocrinol Metab 89:3879–3884 [DOI] [PubMed] [Google Scholar]
- Holm IA, Manson JE, Michels KB, Alexander EK, Willett WC, Utiger RD 2005 Smoking and other lifestyle factors and the risk of Graves’ hyperthyroidism. Arch Intern Med 165:1606–1611 [DOI] [PubMed] [Google Scholar]
- McGrogan A, Seaman HE, Wright JW, de Vries CS 2008 The incidence of autoimmune thyroid disease: a systematic review of the literature. Clin Endocrinol (Oxf) 69:687–696 [DOI] [PubMed] [Google Scholar]
- Furszyfer J, Kurland LT, McConahey WM, Elveback LR 1970 Graves’ disease in Olmsted County, Minnesota, 1935 through 1967. Mayo Clin Proc 45:636–644 [PubMed] [Google Scholar]