Abstract
Objective: We tested the hypothesis that skeletal muscle lipid content (SMLC) is higher in obese black adolescents compared with their white peers and assessed the relationship between SMLC and insulin sensitivity (IS).
Methods: Subjects were healthy obese black (n = 42) and white (n = 38) adolescents. Measurements included an oral glucose tolerance test, IS by a 3-h hyperinsulinemic-euglycemic clamp, abdominal adipose tissue (AT) by magnetic resonance imaging and midthigh SMLC by computed tomography.
Results: All measures of SMLC including intermuscular AT (IMAT), low-density muscle, and thigh sc AT increased (P < 0.05) with increasing total adiposity independent of race. For a given total body adiposity or thigh circumference, SMLC did not differ between black and white adolescents; however, for a given visceral adipose tissue, IMAT was higher in blacks. Consistent with prior observations, IS did not differ between black and white obese adolescents despite lower visceral fat in blacks. In whites, all markers of SMLC were associated (P < 0.05) with IS, whereas in blacks, only IMAT correlated (P < 0.05) with IS. However, in both races, these relationships did not remain significant after accounting for total fat (kilograms).
Conclusions: SMLC is not different between black and white obese adolescents who have similar total body adiposity but lower visceral fat in blacks. The lack of association between IS and SMLC after adjusting for total adiposity suggest that muscle fat does not make a unique contribution to IS in this group of obese adolescents regardless of race.
Skeletal muscle lipid content by CT is not different between black and white obese adolescents and does not per se make a unique contribution to insulin sensitivity.
It is well established that visceral adipose tissue (VAT) is an independent risk factor for insulin resistance and type 2 diabetes mellitus (T2DM) in children and adolescents (1,2,3,4). As in adults, racial differences in abdominal adipose tissue (AT) distribution have been noted in youth, such that for a given body mass index (BMI) or total adiposity, black youth have lower VAT than their white peers (2,5,6). Despite lower VAT, black youth are hyperinsulinemic and insulin resistant and are at increased risk of developing T2DM compared with their white counterparts (2,7). The underlying mechanism(s) responsible for the increased diabetogenic risk among black youth remain unclear.
Skeletal muscle is the primary site of glucose disposal during hyperinsulinemic euglycemic conditions (8). Accordingly, alteration in skeletal muscle metabolism is implicated to play a role in the pathogenesis of insulin resistance (9). In adults, muscle biopsy studies have shown that increased skeletal muscle lipid is closely linked with insulin resistance in obesity and T2DM (10,11,12). These findings are similar to studies using noninvasive imaging techniques to determine skeletal muscle composition (13,14). Using computed tomography (CT), Goodpaster et al. (14) demonstrated that the skeletal muscle of obese or T2DM adults is characterized by reduced midthigh muscle attenuations (e.g. a marker of lipid infiltration within muscle) and increased intermuscular AT (IMAT) areas (e.g. lipid accumulation between muscle bundles beneath the fascia) compared with lean controls. In addition, they observed that both reduced muscle attenuation and increased IMAT are associated with lower insulin sensitivity in obesity and T2DM (14). Whether altered skeletal muscle composition by CT is a marker of insulin resistance is unknown in the pediatric age group.
In adults, several studies (15,16,17,18) demonstrated racial differences in skeletal muscle lipid content (SMLC) between blacks and whites. Using whole-body magnetic resonance imaging (MRI) technique, Gallagher et al. (16) reported that with increasing total AT (kilograms), black men and women had a greater increment in total IMAT (kilograms) compared with their white peers. Currently the data regarding racial comparison in SMLC and its relationship to insulin sensitivity are limited in youth. Therefore, the purpose of this study was to test the hypothesis that black obese adolescents, who have lower VAT compared with their white peers but similar total body adiposity (2), have higher SMLC than white obese adolescents and assess the relationship between SMLC and insulin sensitivity within each racial group.
Subjects and Methods
Subjects
Subjects were overweight/obese otherwise healthy (BMI ≥85 percentile for age and gender) black (n = 42) and white (n = 38) adolescents, except for seven who had impaired glucose tolerance (IGT). Subjects were recruited via newspaper advertisements in the greater Pittsburgh area, flyers posted in the city public transportation, posters placed on campus, and the Weight Management and Wellness Center at Children’s Hospital of Pittsburgh. The investigation was approved by the University of Pittsburgh Institutional Review Board. Parental informed consent and child assent were obtained from all participants before participation. Inclusion criteria required that the subjects be 12–18 yr of age in Tanner II-V, healthy (no syndromic obesity, chronic medical conditions), and nonsmokers and led a sedentary lifestyle (no structured physical activity >2 times per week for past 6 months). Subjects were excluded if they have been dieting or have experienced significant weight change in the preceding months. None of the subjects were taking medications known to affect body composition or glucose metabolism. Racial background was verified by self-identity in three generations on both sides of the parents. All participants underwent a complete physical examination and routine hematological and biochemical tests at the Pediatric Clinical and Translational Research Center (PCTRC). All subjects were admitted to the PCTRC for the evaluations described below.
Anthropometric measurements
Body weight and height were measured to the nearest 0.1 kg and 0.1 cm using a fixed wall stadiometer and a balanced scale. Waist circumference was obtained either at the midpoint (n = 69) between the lowest rib and the iliac crest or at umbilicus (n = 11). Thigh circumference was measured on the right leg at the midpoint between the inguinal crease and superior edge of the patella. The average of two measurements was used in the analyses.
Assessment of insulin sensitivity
Forty-five (23 black and 22 white) participants underwent a 3-h hyperinsulinemic (80 mU/m2 · min)-euglycemic clamp after a 10- to 12-h overnight fast, as reported previously (2). In one black girl, the clamp test did not complete due to difficulty with iv access. Thus, insulin sensitivity data are reported for 22 blacks and 22 whites. Plasma glucose was clamped at 5.6 mmol/liter with a variable-rate infusion of 20% dextrose based on arterialized plasma glucose determinations every 5 min. Insulin-stimulated glucose disposal rate was calculated using the average exogenous glucose infusion rate during the final 30 min of the clamp. Insulin sensitivity (milligram per kilogram per minute per microunit per milliliter × 100) was calculated by dividing insulin-stimulated glucose disposal rate by the steady-state insulin levels during the last 30 min of the clamp as described previously (19). All 80 participants underwent a 2-h oral glucose tolerance test (OGTT; 1.75 g/kg, maximum 75 g) after a 10- to 12-h overnight fast. Blood samples were obtained at −15, 0, 15, 30, 60, 90, and 120 min for determination of glucose and insulin levels. Insulin sensitivity was calculated using the whole-body insulin sensitivity index [WBISI; 10,000/√([fasting glucose × fasting insulin] × [mean glucose × mean insulin during OGTT])] (20). Insulin secretion was calculated during the OGTT as ΔInsulin15/ΔGlucose15 and ΔInsulin30/ΔGlucose30 (21).
Skeletal muscle composition by CT
CT images were obtained on a GE CTI-Helical scanner (GE Medical Systems, Milwaukee, WI) using 170 mA, 120 kV, a 512 × 512 matrix, and 48-cm field of view. Using external landmark, one axial image (10 mm thick) was acquired at the midpoint between the inguinal crease and superior edge of the patella. As shown previously, mean skeletal muscle attenuation was determined as the mean attenuation value for all pixels within the range of 0–150 HU using commercially available software (Slice-O-Matic; Tomovision Inc., Montréal, Québec, Canada) (22). Skeletal muscle was further subdivided into low (0–30 HU; LDM) and high (31–150 HU) density muscle (22). AT was measured in the range of −200 to 0 HU. A reduced mean muscle attenuation is an indicative of increased AT infiltration within the muscle (23). IMAT area was defined as AT area beneath the fascia lata surrounding skeletal muscle and AT area between muscle bundles as shown previously (14). The intraobserver (S.L.) variability in the CT analyses was 4.0% for IMAT, 0.5% for sc AT (SAT) (0.5%), and 0.2% for mean muscle attenuation in 10 subjects.
Total body composition by dual-energy x-ray absorptiometry and abdominal AT by MRI
Body composition was assessed by dual-energy x-ray absorptiometry performed at the PCTRC. Abdominal AT was measured at L4-L5 with a 1.5-Tesla (GE Medical Systems) or 3.0-Tesla magnetic resonance scanners (Siemens Medical Systems, Erlangen, Germany) at the University of Pittsburgh Magnetic Resonance Research Center. The 1.5-Tesla images were obtained in 70 adolescents, using T1-weighted spin-echo sequence (210 msec repetition time and 17 msec echo time) with a 48 × 36 field of view and a 256 × 256 matrix during which time the subjects were asked to hold their breath for 26 sec to minimize the respiratory motion artifacts (24). The 3.0-Tesla images were obtained in 10 boys, who were initially recruited for a lifestyle intervention study, using T1-weighted spin-echo sequence (700 msec repetition time and 5.5 msec echo time) with a 48 × 36 field of view and a 320 × 240 matrix during a 17-sec breath hold. To examine the test-retest variability between 1.5 vs. 3.0 Tesla magnetic resonance, images were obtained in two subjects at 1.5 and 3.0 Tesla on the same day. The coefficient of variation between 1.5- and 3.0-Tesla images with the same observer analyzing the images were 3.9% for VAT and 2.1% for abdominal SAT. Once acquired, the MRI data were transferred electronically to a stand-alone computer for analysis using specially designed image analysis software (Tomovision), the procedures for which are described elsewhere (24).
Statistical analyses
Independent t tests were used to compare racial differences in subject characteristics. General linear models were used to examine race differences in slope and intercept of the regression lines between percent body fat, thigh circumference, and SMLC. Relationships between SMLC, total and abdominal AT, and metabolic markers were examined using partial correlation coefficients after adjusting for Tanner group. Statistical procedures were performed using SPSS (version 15; SPSS, Inc., Chicago, IL). Data are presented as mean ± sem with significance level at P < 0.05.
Results
Subject characteristics are shown in Table 1. Black and white obese adolescents did not differ (P > 0.1) in age, BMI, fat mass, percent body fat, and Tanner distribution. As expected, white adolescents had significantly (P < 0.05) higher VAT (square centimeters) than their black peers. IMAT and skeletal muscle density (LDM and mean muscle attenuation) were not different (P > 0.1) between black and white adolescents independent of gender. Consistent with our previous observations (2), there were no differences between black and white obese adolescents in regard to insulin sensitivity and insulin levels, but blacks had significantly (P < 0.05) lower cholesterol, triglycerides, low-density lipoprotein, and very low-density lipoprotein.
Table 1.
Subject characteristics: physical and skeletal muscle lipid and metabolic data
| Blacks (n = 42) | Whites (n = 38) | P | |
|---|---|---|---|
| Age (yr) | 14.2 ± 0.3 | 14.2 ± 0.3 | NS |
| Gender (male/female) | 22/20 | 24/14 | NS |
| Tanner, II–III/IV–V | 6/36 | 11/27 | NS |
| BMI (kg/m2) | 32.7 ± 0.9 | 31.5 ± 1.0 | NS |
| Waist circumference (cm) | 96.5 ± 2.6 | 98.6 ± 2.5 | NS |
| Thigh circumference (cm) | 62.7 ± 1.2 | 59.9 ± 1.3 | NS |
| Fat mass (kg) | 34.5 ± 2.0 | 35.9 ± 2.2 | NS |
| Fat-free mass (kg) | 51.1 ± 1.6 | 49.8 ± 2.1 | NS |
| Body fat (%) | 38.7 ± 1.4 | 40.1 ± 1.2 | NS |
| VAT (cm2) | 44.2 ± 4.3 | 66.1 ± 4.5 | 0.001 |
| Abdominal SAT (cm2) | 378.3 ± 26.8 | 390.7 ± 27.9 | NS |
| Midthigh skeletal muscle lipid contenta | |||
| IMAT (cm2) | 39.9 ± 3.6 | 34.8 ± 3.1 | NS |
| LDM (cm2) | 35.9 ± 2.6 | 32.8 ± 2.8 | NS |
| Mean muscle attenuation (HU) | 52.3 ± 0.4 | 52.2 ± 0.4 | NS |
| Thigh SAT (cm2) | 324.1 ± 17.1 | 301.3 ± 17.6 | NS |
| Metabolic profiles | |||
| Fasting glucose (mg/dl) | 89.0 ± 1.0 | 93.6 ± 1.2 | 0.003 |
| Fasting insulin (μU/ml) | 21.9 ± 2.1 | 26.5 ± 4.4 | NS |
| In vivo insulin sensitivity (mg/kg · min per μU/ml)b | 2.9 ± 0.3 | 3.1 ± 0.3 | NS |
| WBISI | 2.7 ± 0.2 | 2.3 ± 0.2 | NS |
| OGTT ΔI15/ΔG15 (μU/ml · mg per deciliter) | 4.0 ± 0.7 | 2.8 ± 0.7 | NS |
| OGTT ΔI30/ΔG30 (μU/ml · mg per deciliter) | 4.1 ± 0.5 | 3.1 ± 0.3 | NS |
| Cholesterol (mg/dl) | 142.1 ± 5.1 | 161.8 ± 5.2 | 0.008 |
| Triglycerides (mg/dl) | 75.1 ± 6.0 | 115.0 ± 11.1 | 0.002 |
| HDL (mg/dl) | 43.5 ± 1.4 | 41.8 ± 1.3 | NS |
| LDL (mg/dl) | 84.5 ± 4.2 | 97.5 ± 4.8 | 0.044 |
| VLDL (mg/dl) | 15.0 ± 1.2 | 21.4 ± 1.6 | 0.002 |
Data are means ± SEM. HDL, High-density lipoprotein; LDL, low-density lipoprotein; VLDL, very low-density lipoprotein; NS (not significant), P > 0.05.
39 blacks, 36 whites (CT not measured or data lost, n = 3; wrong measurement site, n = 2);
Twenty-two blacks, 22 whites.
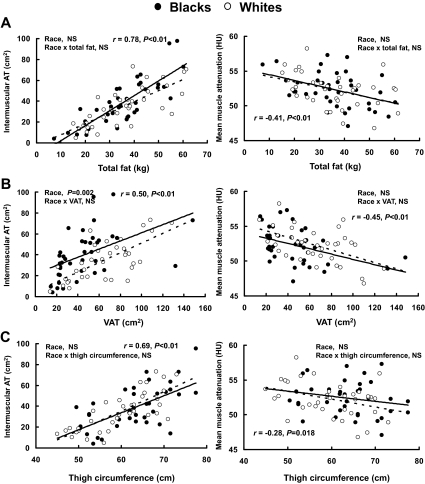
Race and the relationship between SMLC, total fat, VAT, and thigh circumference
For a given total fat (Fig. 1A) or thigh circumference (Fig. 1C), there were no racial differences (P > 0.1) in the relationships between total fat (or thigh circumference) and IMAT or mean muscle attenuation. No significant (P > 0.1) racial differences were found between total fat (or thigh circumference) and LDM or thigh SAT (data not shown). However, there was a significant main effect of race (P < 0.05) on the relationship between IMAT and VAT (Fig. 1B), such that blacks had a higher IMAT than whites for a given VAT. Independent of race, all measures of SMLC were significantly (P < 0.05) associated with total and abdominal adiposity after adjusting for Tanner stage (Table 2).
Figure 1.
Relationships between skeletal muscle lipid and total fat (A), VAT (B), and thigh circumference (C) in black (•) and white (○) adolescents.
Table 2.
Correlations between skeletal muscle lipid, adiposity measures, and metabolic parameters
| Blacks
|
Whites
|
|||||||
|---|---|---|---|---|---|---|---|---|
| IMAT | LDM | MA | Thigh SAT | IMAT | LDM | MA | Thigh SAT | |
| BMI | 0.79a | 0.74a | −0.43a | 0.77a | 0.80a | 0.82a | −0.43a | 0.84a |
| Percent body fat | 0.70a | 0.36a | −0.44a | 0.78a | 0.58a | 0.40a | −0.49a | 0.79a |
| Waist circumference | 0.78a | 0.74a | −0.47a | 0.59a | 0.75a | 0.83a | −0.47a | 0.70a |
| VAT | 0.53a | 0.58a | −0.38a | 0.44a | 0.71a | 0.80a | −0.52a | 0.59a |
| Abdominal SAT | 0.81a | 0.60a | −0.43a | 0.72a | 0.69a | 0.68a | −0.42a | 0.84a |
| Triglycerides | 0.03 | 0.01 | 0.07 | 0.03 | 0.27 | 0.45a | −0.17 | 0.04 |
| Δ I15/Δ G15 | 0.11 | −0.05 | −0.05 | −0.07 | 0.35a | 0.45a | −0.13 | 0.14 |
| WBISI | −0.47a | −0.29 | 0.25 | −0.17 | −0.56a | −0.65a | 0.27 | −0.50a |
| In vivo insulin sensitivity | −0.61a | −0.23 | 0.23 | −0.30 | −0.64a | −0.74a | 0.53a | −0.58a |
Correlations are adjusted for Tanner stage (II–III/IV–V). MA, Mean muscle attenuation.
Significant, P < 0.05.
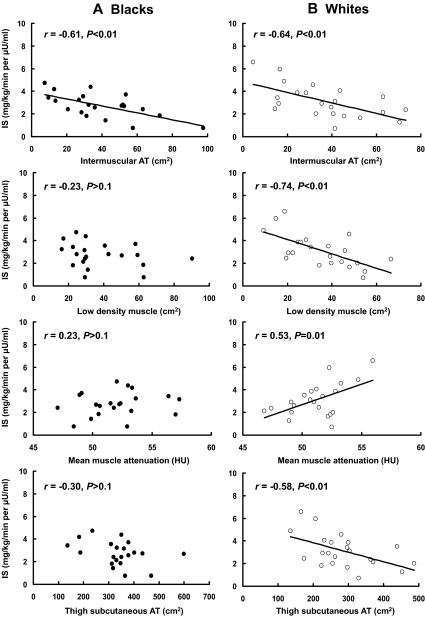
Relationships between SMLC and insulin sensitivity
In black adolescents, IMAT was the only significant correlate of in vivo insulin sensitivity (r = −0.61, P < 0.05) (Fig. 2A) and WBISI (r = −0.47, P < 0.05) (Table 2). Conversely, in white adolescents, all markers of SMLC significantly correlated (P < 0.05) with in vivo insulin sensitivity (Fig. 2B). In addition, IMAT, LDM, and thigh SAT areas were significantly (P < 0.05) associated with WBISI (Table 2).
Figure 2.
Relationships between skeletal muscle lipid and IS by a 3-h hyperinsulinemic-euglycemic clamp in black (A) and white (B) adolescents after adjusting for Tanner stage. In blacks, the relationship between IS and IMAT remained significant (P < 0.05) after further adjustment for visceral fat (r = −0.57) but not after adjustment for total fat mass (r = −0.41, P = 0.08). In whites, none of the relationships remained significant, except the association between IS and thigh-sc AT, which remained near significant (P = 0.054) after controlling for total fat mass (r = 0.44).
In both races, the relationship between insulin sensitivity (IS) and SMLC parameters did not remain significant after adjusting for total fat.
Discussion
The purpose of this study was to test the hypothesis that for similar total body adiposity, SMLC is higher in obese black adolescents compared with their white peers and that increased SMLC in obese black adolescents is associated with an increased risk of insulin resistance despite lower VAT. Contrary to our hypothesis, our observations demonstrate that SMLC is not different between black and white obese adolescents with similar BMI and total body fat. However, for a given VAT, blacks have a higher IMAT than their white peers. Additionally, we found that with increasing total and abdominal adiposity, SMLC increases independent of race and that in white adolescents, in vivo insulin sensitivity is significantly associated with all markers of SMLC whereas in blacks only with IMAT, which is AT deposited underneath the fascia but not with other measures that might reflect intramyocellular lipid (IMCL). This may be an important observation that needs to be further investigated, using magnetic resonance spectroscopy for IMCL, between the two racial groups.
Our findings that CT-measured SMLC increases with increasing total adiposity extend the previous finding by Sinha et al. (25) by adding black/white-related observations. Using proton magnetic resonance spectroscopy (1H-MRS), they (25) demonstrated that both IMCL (lipid inside muscle cell) and extramyocellular lipid (EMCL; lipid outside muscle cells) contents of the soleus muscle were higher in overweight (n = 14) adolescents compared with their lean (n = 8) counterparts (25). Elevated IMCL was also shown in obese adolescents with IGT vs. obese adolescents without IGT (3). In both studies (3,25), IMCL was inversely associated with in vivo insulin sensitivity, findings similar to our observation in white adolescents. However, neither study had sufficient numbers of children to evaluate race-related differences.
In the present study, insulin sensitivity was associated with all measures of skeletal muscle fat in white adolescents but only with IMAT in black adolescents. Previously Miljkovic-Gacic et al. (26) have shown that IMAT increases with advancing age and that IMAT is associated with increased odds of diabetes in men of African ancestry. Furthermore, they observed that skeletal muscles of diabetic individuals of African origin have significantly lower muscle density compared with their nondiabetic counterparts (27). Whereas greater IMAT might not explain racial differences in insulin sensitivity, IMAT may be an important determinant of insulin sensitivity in blacks. Whether increased IMAT is only a marker of increased adiposity or is an independent predictor of insulin resistance is unclear and warrants further investigation in a large pediatric sample.
Previous studies demonstrated increased midthigh LDM areas in black vs. white adults (15,28). Unlike the observations in adults, we did not find significant racial differences in skeletal muscle density in this study. However, our observation is in agreement with a recent study by Liska et al. (29) reporting no significant differences in IMCL or EMCL level between black (n = 17) and white obese adolescents (n = 21). The contrasting findings between adult and pediatric studies may be due to age-related developmental differences in skeletal muscle composition (30). Furthermore, homogeneity of our study sample (e.g. all obese) would likely reduce the magnitude of the associations compared with those observed with a wide range in adiposity as examined in the adult studies (18,28).
In this study, we did not observe differences in insulin sensitivity between black and white obese adolescents. This is consistent with our previous study (2) in obese adolescents but in a different population of black and white participants, and others’ observation (29). However, we and others have observed the black/white difference in insulin sensitivity in normal-weight youths (7,19). We explain these findings by the proposal that obesity has an overriding effect on race-related differences in insulin sensitivity by the mere fact of its magnitude (obese adolescents compared with their normal weight peers have around 50% lower insulin sensitivity, whereas black/white differences in insulin sensitivity in normal weight youth is of the magnitude of ∼25–30%). Thus, once obesity sets in, it overshadows and masks the existing race-related differences in insulin sensitivity.
Limitations of this study warrant mention. The small number of participants may have underpowered the study and may have limited our ability to detect the true relationships between SMLC and insulin sensitivity in black adolescents. For the same reason, we were unable to study the effect of gender on these relationships. However, our study extends previous observations (3,25,29) by specifically examining racial differences in skeletal muscle composition and their associations with in vivo insulin sensitivity using the 3-h hyperinsulinemic-euglycemic clamp in equally obese black and white adolescents. Although CT is unable to differentiate between IMCL and EMCL, it measures a larger muscle group and is more reproducible than 1H-MRS (31). Further studies regarding race-related differences in skeletal muscle composition, using MRS for IMCL and EMCL, are needed in not only obese but also normal-weight adolescents in whom the physiology is not clouded by obesity. Moreover, because of significant racial differences in visceral adiposity, further research into SMLC in black-white VAT-matched youth and insulin sensitivity may yield important information.
In conclusion, SMLC by CT is not different between black and white obese adolescents who have similar total body adiposity, with lower VAT in blacks. In white adolescents both IMAT, fat between muscle bundles beneath the fascia, and skeletal muscle density, fat within muscles, are significantly associated with insulin sensitivity, whereas in blacks only IMAT is associated with insulin sensitivity. However, the absence of an association between IS and SMLC after adjusting for total adiposity may suggest that muscle fat is yet another marker of increased adiposity and does not per se make a unique contribution to IS in this group of obese adolescents, regardless of race. The comparable IS between black and white obese adolescents, despite lower visceral fat in blacks, does not seem to be explained on the basis of race-related differences in SMLC.
Acknowledgments
The authors express their gratitude to the study participants and their parents; Sabrina Kadri, project coordinator; Resa Stauffer and Katie McDowell, laboratory research technicians; and the PCTRC nursing staff for their excellent contributions.
Footnotes
This work was supported by Grants 2R01-HD-27503 (to S.A.), 2K24-HD-01357 (to S.A.), UL1 RR024153 CTSA, Richard L. Day Endowed Chair (to S.A.), 7-08-JF-27 (to S.L., American Diabetes Association Junior Faculty Award), and Department of Defense (to S.A. and S.L.).
Disclosure Summary: The authors have nothing to declare.
First Published Online March 10, 2010
Abbreviations: AT, Adipose tissue; BMI, body mass index; CT, computed tomography; EMCL, extramyocellular lipid; IGT, impaired glucose tolerance; IMAT, intermuscular AT; IS, insulin sensitivity; LDM, low-density muscle; MRI, magnetic resonance imaging; 1H-MRS, magnetic resonance spectroscopy; OGTT, oral glucose tolerance test; PCTRC, Pediatric Clinical and Translational Research Center; SAT, sc AT; SMLC, skeletal muscle lipid content; T2DM, type 2 diabetes mellitus; VAT, visceral adipose tissue; WBISI, whole-body insulin sensitivity index.
References
- Lee S, Gungor N, Bacha F, Arslanian S 2007 Insulin resistance: link to the components of the metabolic syndrome and biomarkers of endothelial dysfunction in youth. Diabetes Care 30:2091–2097 [DOI] [PubMed] [Google Scholar]
- Bacha F, Saad R, Gungor N, Janosky J, Arslanian SA 2003 Obesity, regional fat distribution, and syndrome X in obese black versus white adolescents: race differential in diabetogenic and atherogenic risk factors. J Clin Endocrinol Metab 88:2534–2540 [DOI] [PubMed] [Google Scholar]
- Weiss R, Dufour S, Taksali SE, Tamborlane WV, Petersen KF, Bonadonna RC, Boselli L, Barbetta G, Allen K, Rife F, Savoye M, Dziura J, Sherwin R, Shulman GI, Caprio S 2003 Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet 362:951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goran MI, Lane C, Toledo-Corral C, Weigensberg MJ 2008 Persistence of pre-diabetes in overweight and obese Hispanic children: association with progressive insulin resistance, poor β-cell function, and increasing visceral fat. Diabetes 57:3007–3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goran MI, Nagy TR, Treuth MS, Trowbridge C, Dezenberg C, McGloin A, Gower BA 1997 Visceral fat in white and African American prepubertal children. Am J Clin Nutr 65:1703–1708 [DOI] [PubMed] [Google Scholar]
- Owens S, Gutin B, Barbeau P, Litaker M, Allison J, Humphries M, Okuyama T, Le NA 2000 Visceral adipose tissue and markers of the insulin resistance syndrome in obese black and white teenagers. Obes Res 8:287–293 [DOI] [PubMed] [Google Scholar]
- Gower BA, Nagy TR, Goran MI 1999 Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes 48:1515–1521 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Gunnarsson R, Björkman O, Olsson M, Wahren J 1985 Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest 76:149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, Goodpaster BH 2001 Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care 24:933–941 [DOI] [PubMed] [Google Scholar]
- Manco M, Mingrone G, Greco AV, Capristo E, Gniuli D, De Gaetano A, Gasbarrini G 2000 Insulin resistance directly correlates with increased saturated fatty acids in skeletal muscle triglycerides. Metabolism 49:220–224 [DOI] [PubMed] [Google Scholar]
- Levin K, Daa Schroeder H, Alford FP, Beck-Nielsen H 2001 Morphometric documentation of abnormal intramyocellular fat storage and reduced glycogen in obese patients with type II diabetes. Diabetologia 44:824–833 [DOI] [PubMed] [Google Scholar]
- Malenfant P, Joanisse DR, Thériault R, Goodpaster BH, Kelley DE, Simoneau JA 2001 Fat content in individual muscle fibers of lean and obese subjects. Int J Obes Relat Metab Disord 25:1316–1321 [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE 1997 Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 46:1579–1585 [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Thaete FL, Kelley DE 2000 Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 71:885–892 [DOI] [PubMed] [Google Scholar]
- Ryan AS, Nicklas BJ, Berman DM 2002 Racial differences in insulin resistance and mid-thigh fat deposition in postmenopausal women. Obes Res 10:336–344 [DOI] [PubMed] [Google Scholar]
- Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B, Visser M, Harris TB 2005 Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr 81:903–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albu JB, Kovera AJ, Allen L, Wainwright M, Berk E, Raja-Khan N, Janumala I, Burkey B, Heshka S, Gallagher D 2005 Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr 82:1210–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miljkovic I, Cauley JA, Petit MA, Ensrud KE, Strotmeyer E, Sheu Y, Gordon CL, Goodpaster BH, Bunker CH, Patrick AL, Wheeler VW, Kuller LH, Faulkner KA, Zmuda JM 2009 Greater adipose tissue infiltration in skeletal muscle among older men of African ancestry. J Clin Endocrinol Metab 94:2735–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J 2002 Hyperinsulinemia in African-American children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes 51:3014–3019 [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA 1999 Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470 [DOI] [PubMed] [Google Scholar]
- Bacha F, Gungor N, Arslanian SA 2008 Measures of β-cell function during the oral glucose tolerance test, liquid mixed-meal test, and hyperglycemic clamp test. J Pediatr 152:618–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiszewski PM, Oeffinger KC, Church TS, Dunn AL, Eshelman DA, Victor RG, Brooks S, Turoff AJ, Sinclair E, Murray JC, Bashore L, Ross R 2007 Abdominal obesity, liver fat, and muscle composition in survivors of childhood acute lymphoblastic leukemia. J Clin Endocrinol Metab 92:3816–3821 [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R 2000 Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol 89:104–110 [DOI] [PubMed] [Google Scholar]
- Ross R, Rissanen J, Pedwell H, Clifford J, Shragge P 1996 Influence of diet and exercise on skeletal muscle and visceral adipose tissue in men. J Appl Physiol 81:2445–2455 [DOI] [PubMed] [Google Scholar]
- Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma YZ, Savoye M, Rothman DL, Shulman GI, Caprio S 2002 Assessment of skeletal muscle triglyceride content by (1)H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes 51:1022–1027 [DOI] [PubMed] [Google Scholar]
- Miljkovic-Gacic I, Gordon CL, Goodpaster BH, Bunker CH, Patrick AL, Kuller LH, Wheeler VW, Evans RW, Zmuda JM 2008 Adipose tissue infiltration in skeletal muscle: age patterns and association with diabetes among men of African ancestry. Am J Clin Nutr 87:1590–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miljkovic-Gacic I, Wang X, Kammerer CM, Gordon CL, Bunker CH, Kuller LH, Patrick AL, Wheeler VW, Evans RW, Zmuda JM 2008 Fat infiltration in muscle: new evidence for familial clustering and associations with diabetes. Obesity (Silver Spring) 16:1854–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz J, Gower BA 2003 Relationship between serum leptin concentration and low-density muscle in postmenopausal women. J Clin Endocrinol Metab 88:1157–1161 [DOI] [PubMed] [Google Scholar]
- Liska D, Dufour S, Zern TL, Taksali S, Calí AM, Dziura J, Shulman GI, Pierpont BM, Caprio S 2007 Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS ONE 2:e569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan AS, Nicklas BJ 1999 Age-related changes in fat deposition in mid-thigh muscle in women: relationships with metabolic cardiovascular disease risk factors. Int J Obes Relat Metab Disord 23:126–132 [DOI] [PubMed] [Google Scholar]
- Larson-Meyer DE, Smith SR, Heilbronn LK, Kelley DE, Ravussin E, Newcomer BR 2006 Muscle-associated triglyceride measured by computed tomography and magnetic resonance spectroscopy. Obesity (Silver Spring) 14:73–87 [DOI] [PMC free article] [PubMed] [Google Scholar]