Abstract
Context: Prenatal androgen excess can cause a phenocopy of polycystic ovary syndrome (PCOS) in mammals. Retrospective studies have suggested that girls at risk for PCOS have low birth weight, and prospective studies have suggested an increased prevalence of small-for-gestational-age offspring in women with PCOS.
Objective: The objective of the study was to determine whether infants of women with PCOS have reduced birth weight or increased intrauterine androgen levels.
Design: This was a prospective case-control study.
Participants: Thirty-nine PCOS and 31 control women and their infants participated in the study.
Main Outcome Measures: Birth weight and mixed cord blood testosterone, androstenedione (A), dehydroepiandrosterone, 17-hydroxyprogesterone, estradiol (E2), and dihydrotestosterone levels were measured.
Results: Mean birth weight did not differ, but there was a significant increase in the prevalence of large-for-gestational-age infants in the PCOS group. Cord blood E2 and A levels were lower (P < 0.05), but testosterone to E2 ratios did not differ in female PCOS compared with control offspring. There was no difference in E2 and A levels in the male PCOS and control offspring. There was no difference in 17-hydroxyprogesterone or other androgen levels in either male or female PCOS offspring compared with their respective control group.
Conclusion: Infants of women with PCOS were more likely to be large for gestational age. Female offspring of affected women have lower cord blood A levels; other cord blood androgen levels do not differ compared with female control offspring. Cord blood E2 levels are also significantly decreased in PCOS, without any difference in the testosterone to E2 ratio, suggesting decreased fetal or placental production of steroids.
Infants of women with PCOS tend to be larger for gestational age and female PCOS offspring have lower cord blood androstenedione and estradiol levels.
Polycystic ovary syndrome (PCOS) is among the most common disorders of premenopausal women (1). It is characterized by its reproductive phenotype of hyperandrogenism and disordered gonadotropin secretion (1). PCOS is also frequently associated with insulin resistance, pancreatic β-cell dysfunction, and obesity; abnormalities that confer a markedly increased risk for metabolic syndrome and type 2 diabetes in affected women (1). PCOS is a complex genetic disease and the phenotype is likely the result of the interplay of variation in a number of different genes with environmental factors. We identified PCOS risk alleles that are linked and/or associated with hyperandrogenemia (2,3). Indeed, hyperandrogenemia appears to be a final common path to the female reproductive phenotype (4) and to contribute to the pathogenesis of the associated metabolic abnormalities (5,6).
It has been suggested that the intrauterine environment plays an important role in health status. Decreased birth weight has been associated in epidemiologic studies with the development of type 2 diabetes and cardiovascular disease in adult life (7). Maternal obesity and diabetes have been associated with large fetal size (8) and risk for obesity and glucose intolerance in the offspring (9). Animal studies in monkey, sheep, and rodent models have shown the development of a PCOS phenotype in offspring after maternal prenatal exposure to androgens (10). Retrospective studies have shown that girls with premature pubarche and features of PCOS have a history of being significantly smaller for gestational age (11,12). Furthermore, a prospective study of infants born to women with PCOS showed an increase in the prevalence of small for gestational age (SGA) infants compared with infants of reproductively normal control women (13). These findings suggest that PCOS may have developmental origins (10,14). However, a prospective study comparing insulin-resistant and non-insulin-resistant PCOS women with control subjects failed to identify a difference in birth weight between PCOS and control subjects (15). Several retrospective studies (16,17,18) examined perinatal outcomes in PCOS women and again failed to confirm an association between PCOS and alterations in birth weight in singleton pregnancies. The possibility that intrauterine androgen excess contributes to PCOS remains largely unexplored. We performed this study to prospectively investigate whether there were alterations in fetal growth or evidence for intrauterine androgen excess in the offspring of women with PCOS.
Subjects and Methods
The Institutional Review Board of the Feinberg School of Medicine, Northwestern University, approved this study. Written informed consent was obtained from all participants. Pregnant non-Hispanic white women aged 18–40 yr were recruited. Subjects were enrolled at any stage of their pregnancies. Subjects were included if the fetus was known to be female or if the sex of the fetus was unknown. Subjects with a known male fetus were excluded because the study was primarily focused on female offspring.
At enrollment, subjects completed a personal and family medical history questionnaire. All subjects had no underlying medical conditions and no complications during pregnancy. No subject had a current or past history of gestational diabetes mellitus. All women with PCOS had a prior diagnosis of PCOS by a physician, a history of less than six menstrual cycles per year and fulfilled National Institute of Child Health and Human Development criteria (19): hyperandrogenism and/or hyperandrogenemia, oligoovulation after the exclusion of related disorders. Control subjects all had a history of regular menses (∼12 cycles per year) and no history of signs or symptoms of androgen excess by questionnaire. Control subjects were not eligible if they had a first-degree relative with diabetes, including gestational diabetes mellitus. Thirty-nine PCOS and 31 control women met these criteria. Self-reported height and prepregnancy weight were obtained from the questionnaire, as previously validated (2). Weight at delivery was reported from the subject’s final doctor’s visit or on admission to the hospital.
Mixed arterial and venous cord blood was collected from the placenta after delivery into plain, no-additive tubes. Cord blood was available from 29 PCOS and 21 control infants. The delivery hospital performed serum separation and preparation for transport. Specimens were shipped on ice to Northwestern University for storage at −80 C until analysis.
Laboratory assays
All assays were performed at the Laboratory of Medicine and Pathology of Mayo Clinic (Rochester, MN). Steroid levels were determined by liquid chromatography-mass spectrometry. The intra- and interassay coefficients of variation and lower limit of detectability were: testosterone (T) 5 and 6% and 7 ng/dl; dihydrotestosterone (DHT) 6, and 12% and 50 pg/ml; dehydroepiandrosterone (DHEA) 5 and 9% and 0.5 ng/ml; 17-hydroxyprogesterone (17-OHP) 3 and 7% and 15 ng/dl; androstenedione (A) 5, and 5% and 15 ng/dl; and estradiol (E2) 7 and 6% and 10 pg/ml.
Calculations and statistical analysis
Body mass index [BMI; weight in kilograms/(height in meters)2] was calculated. World Health Organization cutoff points were used for BMI categories: ideal, 18.5–24.9 kg/m2; overweight, 25.0–29.9 kg/m2; obese, 30.0 kg/m2 or greater. Gestational weight gain was calculated from weight at delivery minus prepregnant weight. Gestation at time of delivery was calculated as completed weeks based on expected date of delivery as declared by the subject’s obstetrician. Infant weight and length were measured at the hospital of delivery and used to calculate neonatal ponderal index (birth weight/crown-heel length3 × 100). Two infants were not included in the ponderal index analysis because birth length data were missing. National normative data were used to standardize birth weight for gestation, sex, race, and birth order (20). Infants were stratified into categories based on birth weight percentiles: SGA included infants with weight less than the 10th percentile for gestational age; appropriate for gestational age included infants with weight 10th percentile or greater and 90th percentile or less for gestational age; and large for gestational age (LGA) infants were greater than the 90th percentile for gestational age.
Steroid values below the limits of detectability were assigned the limit of detectability as a value for the purpose of statistical analysis. Group comparisons were made using t tests or nonparametric tests according to the normality of the data. Log transformations were performed as necessary to achieve homogeneity of variance. Associations between continuous variables were analyzed using Pearson product-moment correlation. Categorical data were analyzed using χ2 analyses. Statistical analysis was performed using SAS 9.2 (SAS Institute, Inc., Cary, NC) and PASW Statistic 17.0 (SPSS, Inc., Chicago, IL). All data are presented as untransformed mean ± sd with the level of significance set at P ≤ 0.05.
Results
Thirty-nine PCOS and 31 control women gave birth to 43 female (25 PCOS and 18 control) and 27 male (14 PCOS and 13 control) infants. The clinical data on the mothers and infants are summarized in Table 1. The women with PCOS were slightly but significantly younger than the control women. BMI was higher in PCOS women than control women, both before pregnancy and at delivery. There were significantly more overweight and obese women in the PCOS group compared with the control group: seventy-four percent of the control women and 28% of the PCOS women had ideal BMI, 7% control and 26% PCOS women were overweight based on BMI, and 19% control and 46% PCOS women were obese (P < 0.001). However, there was no difference in gestational weight gain between the two groups. Delivery weight data were missing for four PCOS subjects. Sixty-four percent of the PCOS women were nulliparous compared with 39% in the control group (P < 0.05), as would be expected in a condition with impaired fertility. Significantly more PCOS women (77%) required ovulation induction or in vitro fertilization to achieve a pregnancy, compared with 6% in control women (P < 0.005).
Table 1.
Clinical features of all mothers and infants
| Control (n = 31)
|
PCOS (n = 39)
|
P value | |||
|---|---|---|---|---|---|
| n | Mean ± sd (range) | n | Mean ± sd (range) | ||
| Maternal age at delivery (yr) | 31 | 32.4 ± 4.4 (23.5–42.5) | 39 | 30.1 ± 3.9 (21.7–41.2) | 0.020 |
| Maternal prepregnancy BMI (kg/m2) | 31 | 25.1 ± 5.7 (19.4–41.5) | 39 | 30.8 ± 8.9 (18.6–54.1) | 0.002 |
| Maternal delivery BMI (kg/m2) | 31 | 30.8 ± 5.6 (23–45.1) | 35 | 36.4 ± 8.2 (23.7–59.4) | 0.002 |
| Maternal gestational weight gain (kg) | 31 | 15.3 ± 6.2 (5.4–30) | 35 | 14.2 ± 6.8 (−2.7–27.3) | 0.479 |
| Infant gestational age (wk) | 31 | 39.3 ± 1.2 (35–41) | 39 | 38.9 ± 1.3 (36–41) | 0.158 |
| Infant birth weight (g) | 31 | 3571 ± 389 (2580–4421) | 39 | 3539 ± 597 (2410–5580) | 0.787 |
| Infant birth weight percentile | 31 | 59 ± 23 (6–95) | 39 | 56 ± 32 (2–99) | 0.625 |
| Infant birth weight z-score | 31 | 0.29 ± 0.7 (−1.5–1.6) | 39 | 0.28 ± 1.1 (−2.2–2.2) | 0.976 |
| Infant ponderal index | 31 | 2.6 ± 0.3 (1.9–3.4) | 37 | 2.6 ± 0.3 (2–3.2) | 0.465 |
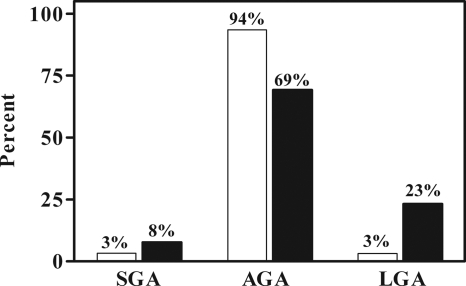
All infants were born after at least 35 wk gestation, and there was no difference in gestational age between PCOS and control offspring. There was no difference in ponderal index, birth weight percentile, or z-score adjusted for gestational age, sex, birth order, and race in PCOS compared with control offspring. A subanalysis by sex also failed to show any significant differences in birth weight parameters between PCOS and control offspring. However, stratification of infants into birth weight categories based on percentiles (Fig. 1) demonstrated a significant (P < 0.05) increase in LGA infants in the PCOS group. There was no significant increase in the prevalence of SGA infants in the PCOS group (Fig. 1). There was no significant association between maternal prepregnant BMI and infant ponderal index within the cohort (r = 0.22, P = NS). However, there was a significant association between maternal BMI at term and infant ponderal index (r = 0.282, P < 0.05), although this association was not found with birth weight percentile or z-score.
Figure 1.
The prevalence of SGA, appropriate for gestational age (AGA), and LGA infants in PCOS (black bars) compared with control (open bars) offspring. There is a significant increase in PCOS LGA infants (P < 0.05).
There was no difference in delivery method between groups or sex, and the majority (66%) of the total subjects had a vaginal delivery. Women with PCOS had a higher rate of cesarean section (33%) than control women (26%), but this difference was not statistically significant. Labor duration did not differ between all PCOS and control mothers, however; female infants of women with PCOS were born after a significantly shorter mean duration of labor than the male infants of women with PCOS (P < 0.05). This significant difference in labor duration persisted between all female and male infants (P < 0.05), regardless of PCOS status.
Cord blood steroids
Cord blood was obtained from 29 PCOS and 21 control infants. The PCOS group included 17 female and 12 male infants, and the control group contained 14 female and seven male infants. Table 2 shows the results of the cord blood analyses. E2 and A levels were lower (P < 0.05) in female PCOS compared with control offspring. There was no significant difference in E2 and A levels in the male offspring. T, 17-OHP, DHT, and DHEA levels did not differ in either the female or male PCOS offspring compared with their respective same-sex control group. However, many of the T and DHT values were below the limits of detectability for the assay. Seventy-one percent of the female control and 88% of the female PCOS T results were less than the lower limit of 7 ng/dl. Sixty-two percent of the female control and 50% of the female PCOS DHT results were less than the lower limit of 50 pg/ml. Fifty-seven percent of the male control and 25% of the male PCOS T results were less than the lower limit of 7 ng/dl. Sixty-seven percent of the male control and 40% of the male PCOS DHT results were less than the lower limit of 50 pg/ml. Both the T to E2 and the A to E2 ratios did not differ between groups in either the female or male offspring.
Table 2.
Cord blood steroid hormone levels for offspring
| Female offspring | Control (n = 14)
|
PCOS (n = 17)
|
P value | ||
|---|---|---|---|---|---|
| n | Mean ± sd (range) | n | Mean ± sd (range) | ||
| E2 (pg/ml) | 12 | 18606 ± 18845 (2850–59100) | 17 | 6467 ± 3989 (936–14600) | 0.049 |
| A (ng/dl) | 14 | 146 ± 104 (44–426) | 17 | 87 ± 46 (34–187) | 0.042 |
| T (ng/dl) | 14 | 19 ± 29 (7–90) | 16 | 7 ± 1 (7–12) | 0.156 |
| 17-OHP (ng/dl) | 14 | 2990 ± 1883 (110–5900) | 17 | 2777 ± 2103 (490–9660) | 0.770 |
| DHT (pg/ml) | 13 | 88 ± 75 (50–311) | 14 | 137 ± 162 (50–650) | 0.325 |
| DHEA (ng/ml) | 11 | 5 ± 4 (1–15) | 10 | 3 ± 2 (1–7) | 0.168 |
| T to E2 | 12 | 0.012 ± 0.008 (0.001–0.023) | 16 | 0.018 ± 0.018 (0.005–0.071) | 0.227 |
| A to E2 | 12 | 0.114 ± 0.059 (0.020–0.208) | 17 | 0.174 ± 0.119 (0.043–0.453) | 0.087 |
| Male offspring | Control (n = 7) | PCOS (n = 12) | |||
|---|---|---|---|---|---|
| E2 (pg/ml) | 7 | 10067 ± 4527 (4870–17700) | 10 | 13192 ± 9470 (4290–31000) | 0.433 |
| A (ng/dl) | 7 | 128 ± 105 (42–356) | 12 | 114 ± 44 (75–234) | 0.752 |
| T (ng/dl) | 7 | 14 ± 10 (7–34) | 12 | 15 ± 15 (7–60) | 0.863 |
| 17-OHP (ng/dl) | 7 | 3872 ± 1576 (541–5200) | 12 | 3073 ± 1324 (1510–5330) | 0.252 |
| DHT (pg/ml) | 6 | 60 ± 20 (50–100) | 5 | 77 ± 25 (50–100) | 0.254 |
| DHEA (ng/ml) | 6 | 3 ± 3 (1–9) | 8 | 3 ± 1 (2–5) | 0.843 |
| T to E2 | 7 | 0.050 ± 0.033 (0.019–0.100) | 10 | 0.059 ± 0.073 (0.010–0.232) | 0.770 |
| A to E2 | 7 | 0.447 ± 0.191 (0.259–1.00) | 10 | 0.366 ± 0.163 (0.136–0.700) | 0.460 |
Discussion
We found no evidence for fetal growth restriction or intrauterine androgen excess in the offspring of women with PCOS. A and E2 levels were significantly decreased in mixed cord blood from female PCOS compared with control infants. The mechanism for this decrease remains unclear. It is possible that alterations in steroidogenesis in the fetal ovary or adrenal gland or an abnormality in placental steroidogenesis contributed to the observed findings.
There is little known about the activity of the fetal ovary during gestation. Fetal ovarian steroidogenesis has not been documented at term (21), but expression of steroidogenic enzymes, specifically P450c17 as well as P450scc, has been demonstrated starting in the early part of the second trimester of pregnancy (22). Whereas it is possible that in our study, lower LH levels due to increased maternal androgens (23) suppressed the fetal ovarian steroidogenic enzymes in PCOS pregnancy, this possibility seems unlikely. First, it appears that some of the regulation of fetal ovarian steroidogenesis is gonadotropin independent because near-normal expression of the P450c17 enzyme was seen in the ovaries of anencephalic fetuses compared with controls (22). Furthermore, LH receptors have not been detected in second-trimester ovaries (22), although data beyond that time in gestation are not available.
The fetal adrenal and placenta are classically described as incomplete endocrine organs because they each lack the full complement of steroidogenic enzymes and depend on one another for steroid metabolism (24). The placenta is dependent on maternal and fetal steroid hormone precursors for the production of its major hormones, progesterone and estrogen. The fetal adrenal glands are highly active in utero, producing elevated levels of DHEA sulfate (DHEAS) (24). DHEAS from the fetal adrenal is acted on by a placental sulfatase to produce DHEA, which is metabolized by the placenta to produce A. A may be aromatized directly to estrone or may be converted to T, which is then aromatized to E2. Thus, the resulting estrogen and androgen levels are dependent on both fetal adrenal and placental steroidogenesis. Lower A and E2 levels in our female PCOS cohort could be due to an abnormality in placental steroidogenesis. An aromatization defect is unlikely because the T to E2 and A to E2 ratios were not altered. It is possible that there is a more proximal defect in either the placenta or the fetal adrenal in the women with PCOS.
Mixed cord blood steroid levels reflect both fetal and maternal steroidogenesis. It contains both the blood leaving the fetus to enter the placenta via the paired umbilical arteries and the blood leaving the placenta to the fetus via the umbilical vein. It is unclear whether umbilical venous or arterial blood better represents the fetal hormonal environment. A correlation between umbilical venous and arterial concentrations of E2 and estriol has been established (25). There are fewer data available for androgens, perhaps because the action of aromatase in the placenta must be considered. DHEAS levels have been correlated between the umbilical vein and artery (26), as have A and T levels (21).
Studies have proposed that amniotic fluid gives an accurate representation of the fetal androgen environment (27,28). Due to the risks involved with amniocentesis and the fact that it is generally reserved for high-risk pregnancies, amniotic fluid measurements were not feasible in our study. In addition, in a study that compared amniotic fluid levels to mixed cord blood, the cord blood A levels correlated with amniotic fluid A levels in both male and female fetuses, and cord blood and amniotic fluid T levels correlated in the female fetuses (28). The measured cord blood steroid hormone levels in our control subjects are in ranges similar to the published data of other investigators using extraction and chromatography hormone assay methods (26,29).
A recent prospective cohort study of pregnant women evaluated female offspring in adolescence for PCOS in whom cord blood had been collected at birth (30). No association was found between cord blood T levels and PCOS. Furthermore, there was no association between birth weight and cord blood androgen levels, nor was birth weight associated with PCOS. There was also no difference in the prevalence of PCOS in women from same-sex compared with opposite-sex twin pairs (31), arguing against a role for developmental exposure to androgens in the pathogenesis of PCOS. These studies differ from our study because the pregnant women were not selected on the basis of PCOS. Nevertheless, the present and previous studies fail to support the hypothesis that intrauterine androgen excess contributes to the development of PCOS.
The second finding in this study was no evidence in our cohort for fetal growth restriction in the infants of women with PCOS. On the contrary, we found a significant increase in the prevalence of LGA in the PCOS offspring. Studies of birth weight in PCOS have yielded conflicting results. Two previous studies, one retrospective (32) and one prospective (33), also reported an association between PCOS and increased birth weight. On the other hand, retrospective studies of girls with features of PCOS (11,12) and a prospective study of the offspring of women with PCOS (13) found an increased prevalence of SGA offspring. However, an epidemiological (34), a prospective (15), and three retrospective studies (17,18,19), including our own, failed to find an association between fetal growth restriction and PCOS. A meta-analysis of perinatal outcomes in PCOS (35) failed to find an association between PCOS and lower birth weight when only highly valid studies were included.
There is a known association between prepregnant BMI and birth weight (36), and a prepregnant BMI of 25 kg/m2 or greater is associated with an increased risk for a macrosomic infant (37). The mean BMI and prevalence of overweight and obesity were significantly increased in our PCOS compared with our control cohort. Furthermore, the BMI of these women with PCOS was higher than that in other studies in the literature (13,15,18). The increased proportion of PCOS women who were overweight and obese may have contributed to the significant increase in LGA infants in the PCOS group. However, in the study by Sir-Petermann et al. (13), the PCOS women were overweight (mean BMI 27.5 kg/m2), and they still found an increased prevalence of SGA. Elevated levels of insulin and glucose have been documented in pregnant women with PCOS (33,38), abnormalities that are directly correlated with fetal size (39). Perhaps these factors also contributed to the increase in LGA in the PCOS group.
Our study has several limitations. Despite the fact that highly sensitive methods were used to measure steroid levels (3), the levels of T and DHT were below the detection limits of the assay in a number of samples. With a more sensitive assay, perhaps we would have detected significant changes in T levels, similar to those seen with A and E2. Cord blood levels from female infants at birth may not reflect androgen exposure during the second trimester of human development, when serum T levels can be elevated into the male range in 40% of female fetuses (40). Because other studies failed to detect differences in androgen levels between male and female infants at term, it is possible that cord blood measurements are not ideal for evaluation of prenatal androgen exposure. Furthermore, differences in the stress of delivery may have affected the cord blood hormone levels. However, there was no significant difference in delivery methods, and there was no difference between PCOS and control female infants in duration of labor. Although gestational weight gain did not differ between groups, the higher mean BMI in PCOS subjects may have contributed to the increased fetal size.
Conclusion
There is no evidence for decreased birth weight or increased androgen levels in the offspring of women with PCOS. In our cohort, the infants of women with PCOS were more likely to be LGA. Cord blood A and E2 levels were lower in female PCOS offspring, whereas the T to E2 ratio remained unaltered.
Footnotes
This work was supported by National Institutes of Health Grant P50 HD44405.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 12, 2010
Abbreviations: A, Androstenedione; BMI, body mass index; DHEA, dehydroepiandrosterone; DHEAS, DHEA sulfate; DHT, dihydrotestosterone; E2, estradiol; LGA, large for gestational age; 17-OHP, 17-hydroxyprogesterone; PCOS, polycystic ovary syndrome; SGA, small for gestational age; T, testosterone.
References
- Ehrmann DA 2005 Polycystic ovary syndrome. N Engl J Med 352:1223–1236 [DOI] [PubMed] [Google Scholar]
- Urbanek M, Sam S, Legro RS, Dunaif A 2007 Identification of a polycystic ovary syndrome susceptibility variant in fibrillin-3 and association with a metabolic phenotype. J Clin Endocrinol Metab 92:4191–4198 [DOI] [PubMed] [Google Scholar]
- Biyasheva A, Legro RS, Dunaif A, Urbanek M 2009 Evidence for association between polycystic ovary syndrome (PCOS) and TCF7L2 and glucose intolerance in women with PCOS and TCF7L2. J Clin Endocrinol Metab 94:2617–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC 2000 Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 85:4047–4052 [DOI] [PubMed] [Google Scholar]
- Coviello AD, Legro RS, Dunaif A 2006 Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab 91:492–497 [DOI] [PubMed] [Google Scholar]
- Gambineri A, Patton L, Vaccina A, Cacciari M, Morselli-Labate AM, Cavazza C, Pagotto U, Pasquali R 2006 Treatment with flutamide, metformin and their combination added to hypocaloric diet in overweight-obese women with polycystic ovary syndrome: a randomized, 12-month, placebo-controlled study. J Clin Endocrinol Metab 91:3970–3980 [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ 2001 The thrifty phenotype hypothesis. Br Med Bull 60:5–20 [DOI] [PubMed] [Google Scholar]
- Ehrenberg HM, Mercer BM, Catalano PM 2004 The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol 191:964–968 [DOI] [PubMed] [Google Scholar]
- Silverman BL, Rizzo TA, Cho NH, Metzger BE 1998 Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care 21(Suppl 2):B142–B149 [PubMed] [Google Scholar]
- Franks S 2009 Do animal models of polycystic ovary syndrome help to understand its pathogenesis and management? Yes, but their limitations should be recognized. Endocrinology 150:3983–3985 [DOI] [PubMed] [Google Scholar]
- Ibáñez L, Jiménez R, de Zegher F 2006 Early puberty-menarche after precocious pubarche: relation to prenatal growth. Pediatrics 117:117–121 [DOI] [PubMed] [Google Scholar]
- Ibáñez L, Jaramillo A, Enriquez G, Miró E, López-Bermejo A, Dunger D, de Zegher F 2007 Polycystic ovaries after precocious pubarche: relation to prenatal growth. Hum Reprod 22:395–400 [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Hitchsfeld C, Maliqueo M, Codner E, Echiburú B, Gazitúa R, Recabarren S, Cassorla F 2005 Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum Reprod 20:2122–2126 [DOI] [PubMed] [Google Scholar]
- Abbott DH, Barnett DK, Bruns CM, Dumesic DA 2005 Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update 11:357–374 [DOI] [PubMed] [Google Scholar]
- Bjercke S, Dale PO, Tanbo T, Storeng R, Ertzeid G, Abyholm T 2002 Impact of insulin resistance on pregnancy complications and outcome in women with polycystic ovary syndrome. Gynecol Obstet Invest 54:94–98 [DOI] [PubMed] [Google Scholar]
- Fridström M, Nisell H, Sjöblom P, Hillensjö T 1999 Are women with polycystic ovary syndrome at an increased risk of pregnancy-induced hypertension and/or preeclampsia. Hypertens Pregnancy 18:73–80 [DOI] [PubMed] [Google Scholar]
- Haakova L, Cibula D, Rezabek K, Hill M, Fanta M, Zivny J 2003 Pregnancy outcome in women with PCOS and in controls matched by age and weight. Hum Reprod 18:1438–1441 [DOI] [PubMed] [Google Scholar]
- Mikola M, Hiilesmaa V, Halttunen M, Suhonen L, Tiitinen A 2001 Obstetric outcome in women with polycystic ovarian syndrome. Hum Reprod 16:226–229 [DOI] [PubMed] [Google Scholar]
- Legro RS, Roller RL, Dodson WC, Stetter CM, Kunselman AR, Dunaif A 2010 Associations of birthweight and gestational age with reproductive and metabolic phenotypes in women with polycystic ovarian syndrome and their first-degree relatives. J Clin Endocrinol Metab 95:789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Kleinman KP, Rich-Edwards J, Gillman MW 2003 A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 3:6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapanainen J, Huhtaniemi I, Koivisto M, Kujansuu E, Tuimala R, Vihko R 1984 Hormonal changes during the perinatal period: FSH, prolactin and some steroid hormones in the cord blood and peripheral serum of preterm and full-term female infants. J Steroid Biochem 20:1153–1156 [DOI] [PubMed] [Google Scholar]
- Cole B, Hensinger K, Maciel GA, Chang RJ, Erickson GF 2006 Human fetal ovary development involves the spatiotemporal expression of P450c17 protein. J Clin Endocrinol Metab 91:3654–3661 [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Pérez-Bravo F, Recabarren SE 2002 Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod 17:2573–2579 [DOI] [PubMed] [Google Scholar]
- Kallen CB 2004 Steroid hormone synthesis in pregnancy. Obstet Gynecol Clin North Am 31:795–816, x [DOI] [PubMed] [Google Scholar]
- Hercz P, Ungar L, Siklos P, Farquharson RG 1988 Unconjugated 17β-oestradiol and oestriol in maternal serum and in cord vein and artery blood at term and preterm delivery. Eur J Obstet Gynecol Reprod Biol 27:7–12 [DOI] [PubMed] [Google Scholar]
- Troisi R, Potischman N, Roberts JM, Harger G, Markovic N, Cole B, Lykins D, Siiteri P, Hoover RN 2003 Correlation of serum hormone concentrations in maternal and umbilical cord samples. Cancer Epidemiol Biomarkers Prev 12:452–456 [PubMed] [Google Scholar]
- Finegan JA, Bartleman B, Wong PY 1989 A window for the study of prenatal sex hormone influences on postnatal development. J Genet Psychol 150:101–112 [DOI] [PubMed] [Google Scholar]
- van de Beek C, Thijssen JH, Cohen-Kettenis PT, van Goozen SH, Buitelaar JK 2004 Relationships between sex hormones assessed in amniotic fluid, and maternal and umbilical cord serum: what is the best source of information to investigate the effects of fetal hormonal exposure? Horm Behav 46:663–669 [DOI] [PubMed] [Google Scholar]
- Faupel-Badger JM, Hoover RN, Potischman N, Roberts JM, Troisi R 2009 Pregnancy weight gain is not associated with maternal or mixed umbilical cord estrogen and androgen concentrations. Cancer Causes Control 20:263–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey M, Sloboda DM, Atkinson HC, Doherty DA, Franks S, Norman RJ, Newnham JP, Hart R 2009 The relationship between maternal and umbilical cord androgen levels and polycystic ovary syndrome in adolescence: a prospective cohort study. J Clin Endocrinol Metab 94:3714–3720 [DOI] [PubMed] [Google Scholar]
- Kuijper EA, Vink JM, Lambalk CB, Boomsma DI 2009 Prevalence of polycystic ovary syndrome in women from opposite-sex twin pairs. J Clin Endocrinol Metab 94:1987–1990 [DOI] [PubMed] [Google Scholar]
- Cresswell JL, Barker DJ, Osmond C, Egger P, Phillips DI, Fraser RB 1997 Fetal growth, length of gestation, and polycystic ovaries in adult life. Lancet 350:1131–1135 [DOI] [PubMed] [Google Scholar]
- Paradisi G, Fulghesu AM, Ferrazzani S, Moretti S, Proto C, Soranna L, Caruso A, Lanzone A 1998 Endocrine-metabolic features in women with polycystic ovary syndrome during pregnancy. Hum Reprod 13:542–546 [DOI] [PubMed] [Google Scholar]
- Laitinen J, Taponen S, Martikainen H, Pouta A, Millwood I, Hartikainen AL, Ruokonen A, Sovio U, McCarthy MI, Franks S, Järvelin MR 2003 Body size from birth to adulthood as a predictor of self-reported polycystic ovary syndrome symptoms. Int J Obes 27:710–715 [DOI] [PubMed] [Google Scholar]
- Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS 2006 A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update 12:673–683 [DOI] [PubMed] [Google Scholar]
- Abrams BF, Laros Jr RK 1986 Prepregnancy weight, weight gain, and birth weight. Am J Obstet Gynecol 154:503–509. [DOI] [PubMed] [Google Scholar]
- Jolly MC, Sebire NJ, Harris JP, Regan L, Robinson S 2003 Risk factors for macrosomia and its clinical consequences: a study of 350,311 pregnancies. Eur J Obstet Gynecol Reprod Biol 111:9–14 [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Echiburú B, Maliqueo MM, Crisosto N, Sánchez F, Hitschfeld C, Cárcamo M, Amigo P, Pérez-Bravo F 2007 Serum adiponectin and lipid concentrations in pregnant women with polycystic ovary syndrome. Hum Reprod 22:1830–1836 [DOI] [PubMed] [Google Scholar]
- Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA 2008 Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 358:1991– 2002 [Google Scholar]
- Beck-Peccoz P, Padmanabhan V, Baggiani AM, Cortelazzi D, Buscaglia M, Medri G, Marconi AM, Pardi G, Beitins IZ 1991 Maturation of hypothalamic-pituitary-gonadal function in normal human fetuses: circulating levels of gonadotropins, their common α-subunit and free testosterone, and discrepancy between immunological and biological activities of circulating follicle-stimulating hormone. J Clin Endocrinol Metab 73:525–532 [DOI] [PubMed] [Google Scholar]