Abstract
Context: Ghrelin, an endogenous ligand for the GH secretagogue receptor, is an orexigenic peptide hormone produced primarily by the stomach. Recent studies suggest significant differences in the specificity of currently available ghrelin assays.
Objective: The aim of the study was to compare four ghrelin assays (two commercially available and two developed by our group) of differing specificity, each used on the same set of more than 800 plasma samples from a human study.
Design: Thirteen volunteers were sampled every 20 min for 6 h after consumption of one of three isocaloric drinks consisting of either 80% fat, 80% carbohydrate, or 80% protein. The samples were assayed by RIA for total and active ghrelin, as well as by sandwich assays for acyl and des-acyl ghrelin. The ghrelin profiles for each individual were smoothed using a statistical algorithm to lessen the effects of pulsatility and noise.
Results: The sandwich assays for acyl and des-acyl ghrelin yielded ghrelin values that were lower than those from the corresponding RIAs. The ghrelin profiles after nutrient ingestion were similar, yet key differences among the four assays were apparent; in particular, percentage changes were significantly greater in the sandwich assays.
Conclusions: The lower levels and greater relative changes in ghrelin values reported by the sandwich assays are consistent with greater assay specificity. When applied to the nutrient study, the sandwich assays were better able to distinguish the different responses to different nutrients than were the RIAs.
Consistent with greater assay specificity, two-site sandwich assays for ghrelin give lower values for circulating levels than RIAs, but report larger and sharper relative changes.
Ghrelin is a 28-residue peptide hormone discovered through its activity as an endogenous ligand of the GH secretagogue receptor (GHS-R1a) (1). Ghrelin is produced primarily in the stomach, where the enzyme ghrelin O-acyl transferase (2,3) acylates the serine3 hydroxyl side chain, predominantly by n-octanoic acid. Ghrelin is found in the circulation in both acylated and des-acylated forms. Only the acylated form of ghrelin can act at the GHS-R1a to affect appetite, GH release, and metabolism. Ghrelin is the only known peptide hormone modified with a fatty acid acylation, although octanoylated homoserine lactone molecules with structural similarities to ghrelin are known to serve signaling functions in bacteria (1,4). Although des-acyl ghrelin was originally described as inactive (1), later studies suggest that it can share or antagonize certain activities with its acyl counterpart, including modulation of cell proliferation, adipogenesis, and blood pressure, acting through unknown mechanisms distinct from the GHS-R1a (5,6).
Ghrelin levels fluctuate, exhibiting a diurnal variation in addition to rising preprandially and falling upon food intake (7,8,9,10). Most published studies examining ghrelin levels have used commercial RIAs based on a single recognition site for the primary antibody; however, other assay methods such as in-house RIAs, commercial and in-house single site acyls, and two-site sandwich ELISAs and immunocomplex transfer-enzyme immunoassays have also been developed (9,11,12). Single-site assays can be less specific as a result of interference from peptide fragments or cross-reactivity at a single epitope (13), whereas sandwich assays require recognition of two epitopes using two different antisera, thus eliminating many interferences and lowering nonspecific background. Published data suggest that ghrelin assays using different methodologies do not always agree with one another (11,14,15). An alternative approach to ghrelin assay is to analyze extracted or deproteinized samples by mass spectrometry. This approach can provide exquisite specificity but has disadvantages in cost and throughput (16).
In the present study, two recently described high-throughput two-site sandwich ELISAs, one specific for acyl ghrelin and one for des-acyl ghrelin, were used to measure a set of more than 800 samples that were also each assayed by single-site RIAs for active and total ghrelin. The samples assayed are from a time-course study in human volunteers that examined the effect of consumption of fat, protein, and carbohydrate beverages on both plasma acyl-ghrelin and total ghrelin levels measured at 20-min intervals for 6 h after nutrient consumption (10). That study (10) included data from two of the four assays presented here. The effects of macronutrient type on ghrelin profiles seen in these data are consistent with those of previous studies in rats (17). Additional experiments were carried out to further investigate the observed differences between the ELISA and RIA. These suggest greater specificity in the two-site assays. A statistical comparison of the results obtained by each assay demonstrates that the relative changes over time in response to nutrient ingestion were significantly greater with either of the two-site ELISAs relative to the RIA methods.
Subjects and Methods
Plasma collection
The human study from which these samples were derived has been reported previously (10). Briefly, each volunteer was admitted on three separate occasions. On each admission, three baseline samples were drawn at 0850, 0855, and 0900 h, immediately after which volunteers were given one of three beverages in which 80% of the total calories were derived from either lipid, protein, or carbohydrate. Blood was then drawn at 20-min intervals until 1500 h, yielding a total of 21 samples per volunteer per admission. The order of the beverages was randomly assigned for each subject. Blood samples were collected into chilled tubes containing final concentrations of 1.8 mg/ml EDTA and 4 mm 4-(2-aminoethyl)-benzene sulfonylfluoride (AEBSF). The tubes were left on ice until being centrifuged to fractionate the blood and collect plasma (within 2 h). The plasma was then acidified by addition of sodium monocitrate to a final concentration of 20 mm and stored at −80 C until being assayed. Samples for the total ghrelin RIA were collected on ice as per the manufacturer’s directions without use of AEBSF or acidification. Unlike the samples from the feeding study, the samples for the experiment shown in Fig. 5B were acidified with 200 μl of 1 n HCl per ml plasma instead of citrate, although controls showed that the choice of acid did not affect the assays.
Figure 5.
Samples evaluated by either a single-site ELISA (total ghrelin) or a two-site sandwich assays (acyl + des-acyl ghrelin). A, Older serum samples that had been frozen without preservation. B, Fresh plasma samples preserved with AEBSF and acidification.
RIAs
The RIAs were carried out at the LINCO Research facilities using their Linco active (GHRA-88HK) and total (GHRT-89HK) ghrelin RIA kits according to their protocols. The raw data were kindly provided by John H. Sloan, III, Ph.D. (LINCO Diagnostic Services, Millipore Inc., St. Charles MO) for analysis.
Acyl and des-acyl ghrelin sandwich assays
The acyl and des-acyl ghrelin sandwich assays have been previously described by Liu et al. (9). A polyclonal acyl-specific antiserum raised against ghrelin1-14 was used for the capture step, and a polyclonal antiserum affinity-purified against ghrelin21-27 was biotinylated and used for detection. Sample delivery was by Tecan Genesis (Tecan, Männedorf, Switzerland), and automated reagent deliveries and washing were carried out using a Robbins Hydra 96 (Matrix Liquid Handling Products, Hudson, NH) with a 384-well plate adapter accessory (maximum syringe volume, 580 μl). For the des-acyl sandwich assay, the same ghrelin21-27 affinity-purified antiserum was used for the capture step, and a biotinylated monoclonal antibody specific for the des-acyl ghrelin1-10 N-terminal region was used for detection.
Total ghrelin competitive ELISA
The affinity-purified antiserum directed against the ghrelin21-27 C terminus was coated at 0.5 μg/ml in 50 mm carbonate/bicarbonate (pH 9.5) in an all-black 384-well ELISA plate (Maxisorp; Nunc, Thermo Fisher Scientific, Roskilde, Denmark) overnight at 4 C (50 μl/well). The plate was emptied by inversion and blocked with 100 μl/well blocking buffer (1% BSA in 1X PBS) for 90 min. The plate was washed three times using washing buffer (1X PBS with 0.05% Tween-20), then 25 μl/well neutralization buffer (0.5 m phosphate buffer with 1% BSA, pH 7.4). Thereafter, 25 μl/well ghrelin standards and unknown samples were added. After a brief shaking, the plate was incubated for 2 h at room temperature; then 25 μl/well 1:1,500,000 biotinylated ghrelin in blocking buffer was added. After 1 h of incubation followed by four washes, 50 μl of 1:5000 diluted streptavidin-polyHRP80 in biotin-free 0.15 m phosphate buffer with 1.375% casein and 0.05% Tween-20 was added and incubated for 30 min at room temperature. Finally, the plate was washed six times, and 50 μl/well of 50 μm fluorescent substrate Amplex Red (Molecular Probes, Eugene, OR) was added. After 30 min of development, the plate was read in a Genios plate reader (Phoenix Research Products, Candler, NC) with an excitation wavelength of 535 nm and an emission filter of 590 nm.
Statistical analysis, smoothing method, curve fitting
To compare ghrelin profiles from different individuals, each measured value was normalized as a percentage of the average of the individual’s three baseline samples, which were drawn a few minutes before nutrient ingestion. A smoothing algorithm using a five-point moving average weighted according to the variance was applied to these normalized values. To compare the assays, the smoothed profiles for the 13 subjects were averaged in groupings of beverage and assay used.
Curve fitting and regression calculations were made using GraphPad Prism version 4.03 for Windows (GraphPad Software, San Diego, CA). To make statistical comparisons among the relative changes in ghrelin seen by different assays in response to the carbohydrate beverage, the normalized, unsmoothed ghrelin data were analyzed by repeated-measures ANOVA comparing the geometric mean values for all 13 subjects among assays and in the first 3 h vs. the second 3 h. Paired-wise assay vs. assay comparison of the nadir and peak ghrelin levels after the carbohydrate beverage (nadirs and peaks picked from the smoothed profile for each subject individually and then averaged) were carried out based on the Tukey’s multiple comparison procedure. The significance level of the Tukey’s procedure was set at 0.05.
Results
More than 800 plasma samples, representing 20-min sampling for 13 subjects from three separate admissions, were each measured using four separate assays. In the sandwich assays for acyl and des-acyl ghrelin, the samples were measured in duplicate in 384-well plates requiring only 25 μl per well, as opposed to the 100 μl per tube needed for the total and active RIAs. According to the product specification, the ranges for the RIAs are 93–6000 pg/ml for the total RIA, and 7.8–2000 pg/ml for the active RIA. One hundred per cent of the samples measured with the RIAs fell into these ranges. For the ELISAs, the assay ranges were 6.7–1000 and 4.6–1000 pg/ml for the acyl and des-acyl assays, respectively, and less than 1% of samples were outside these ranges.
Basal ghrelin levels were determined as the average of three initial samples taken 5 min apart before the test meal was initiated. This was done on each of three admissions for each of the 13 subjects and measured in each of the four assays. The average basal ghrelin was 755.3 ± 263.8 sd, 254.1 ± 109.0 sd, 100.4 ± 76.8 sd, and 207.8 ± 123.4 sd in the total RIA, active RIA, acyl sandwich, and des-acyl sandwich assays, respectively. The range of variation in the basal levels between subjects was approximately 3-fold in the total RIA, approximately 5-fold in the active RIA, and more than 10-fold in the sandwich assays.
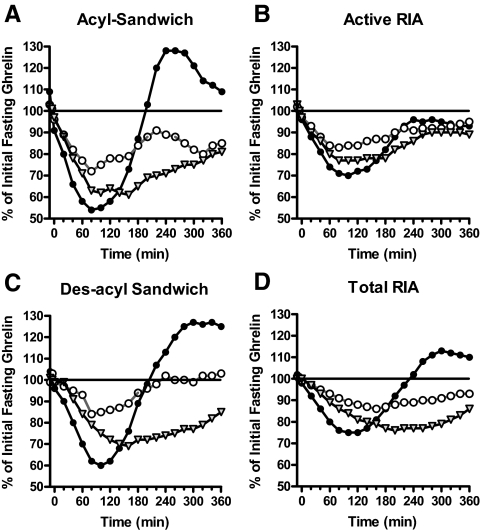
The individual profiles show large fluctuations (Fig. 1A), which may be suggestive of short-term ghrelin pulsatility. Many hormones are secreted in a pulsatile manner including, e.g. insulin, GnRH, FSH, LH, and GH. Natalucci et al. (18) and others (8,9) have demonstrated that ghrelin profiles also exhibit short-term pulsatility. To clarify overall trends without taking into account the apparent short-term pulsatility and to lessen any effect of assay noise, the data sets were smoothed using a statistical algorithm. Because the basal ghrelin levels varied widely among subjects, to make comparisons among the assays, each subject’s smoothed profiles were normalized as a percentage of their individual prebeverage baseline level, and then the 13 normalized profiles for each macronutrient were averaged together. Figure 1, A–C, depicts this process for the carbohydrate admission in the acyl-ghrelin sandwich assay for the first subject. The averaged profiles (n = 13 for each the three admissions) for each of the four assays are shown in Fig. 2. As demonstrated by all four assays, the carbohydrate beverage caused the steepest and most profound but least sustained suppression of both forms of ghrelin (40–100 min), the protein beverage caused the most prolonged suppression (120–160 min), and the fat beverage yielded the least effect overall.
Figure 1.
An example of the smoothing process for data analysis. A, Unsmoothed ghrelin profile, subject 1, carbohydrate admission. B, Smoothed and normalized ghrelin profile, subject 1, carbohydrate admission. C, Mean ± sem of the 13 subjects for the carbohydrate admission.
Figure 2.
The normalized and averaged profiles from the 13 subjects for each of the three beverages. A, Acyl sandwich assay; B, active RIA; C, des-acyl sandwich assay; and D, total RIA. Black circles, Carbohydrate beverage; white circles, fat beverage; gray triangles, protein beverage.
Because the carbohydrate beverage caused the most profound changes in ghrelin levels by all four assays, the carbohydrate data were chosen to execute a statistical comparison of the assay types. Peaks and nadirs found in each subject’s smoothed profiles by different assays were compared, and the first 3 h vs. the second 3 h in the unsmoothed data from different assays were compared.
The smoothed 13-subject average profiles for each of the four assays for the carbohydrate beverage are plotted together in Fig. 3, clearly highlighting the different responses of the four assays. The average of the smoothed nadir and peak values obtained in each subject are shown in Table 1. In the RIA analysis, the suppression of active ghrelin levels after a carbohydrate beverage was 32.8 ± 7.5% compared with baseline, whereas it was 49.7 ± 8.4% with the acyl-ghrelin sandwich assay; the magnitude of suppression was significantly greater in the latter case (P = 0.002 after Tukey’s correction for comparing multiple assays). Similarly, in the acyl-ghrelin sandwich assay, the recovery after meal suppression was sharper and achieved a greater maximum value of 135.2 ± 34%, compared with the active RIA, which showed a rebound of only 103.2 ± 25.3% (P = 0.008 after Tukey’s correction). In comparison, the results from assays of the same technique were not significantly different in the minimum or maximum means (acyl sandwich vs. des-acyl sandwich, P = 0.58 and 0.96, respectively; and active RIA vs. total RIA, P = 0.38 and 0.39, respectively, after Tukey’s correction).
Figure 3.
Comparing the response to the carbohydrate beverage as measured by each of the four ghrelin assays on the same sample set. Black circles, Acyl sandwich; black squares, des-acyl sandwich; open circle, active RIA; and open squares, total RIA.
Table 1.
Summary statistics for the smoothed nadir and peak ghrelin levels (as a percentage of baseline) for each assay type after the carbohydrate beverage
| Group | n | Response | Mean | sd |
|---|---|---|---|---|
| Total RIA | 13 | Nadir | 72.8 | 8.6 |
| Peak | 115.6 | 17.7 | ||
| Active RIA | 13 | Nadir | 67.2 | 7.5 |
| Peak | 103.2 | 25.3 | ||
| Acyl sandwich | 13 | Nadir | 50.3 | 8.4 |
| Peak | 135.2 | 34.0 | ||
| Des-acyl sandwich | 13 | Nadir | 57.2 | 14.3 |
| Peak | 139.1 | 33.6 |
The normalized, unsmoothed ghrelin data for the carbohydrate beverage were analyzed by repeated measures ANOVA comparing the geometric mean values for all subjects among assays in the first 3 h vs. the second 3 h after nutrient ingestion. The geometric means and the 95% confidence intervals for normalized ghrelin concentration (%) during the time intervals 0900–1200 h and 1220–1500 h are displayed in Table 2. All of the assays showed ghrelin concentrations to be significantly suppressed over the 0900–1200 h time interval (P < 0.001 for all). The magnitude of this suppression, however, was greatest when ghrelin was assayed by either of the two sandwich assays. Relative to the total RIA, the acyl-ghrelin sandwich assay and the des-acyl sandwich assay showed ghrelin to be suppressed by an additional 31.3 and 20.0%, respectively (P < 0.001 for both). Relative to the active RIA, the acyl-ghrelin sandwich assay and the des-acyl sandwich assay showed ghrelin to be suppressed by an additional 21.2% (P < 0.001) and 8.3% (P = 0.085), respectively. All of the assays showed a significant rise in ghrelin concentration during the 1220–1500 h time period relative to the ghrelin concentration during the 0900–1200 h time period (P < 0.001 for all). When ghrelin was assayed by the active RIA and the total RIA, the concentrations were on average 120 and 123% greater, respectively, during the second 3 h compared with the first 3 h. Comparatively, the acyl sandwich assay and the des-acyl sandwich assay demonstrated greater changes of 186 and 170%, respectively, during the second time window compared with the first. The percentage increase in ghrelin concentration was not significantly different between the two RIAs (P = 0.650) or between the two sandwich assays (P = 0.119), but it was significantly different between each RIA and each sandwich assay (P < 0.001 for all).
Table 2.
Normalized mean ghrelin levels (as percentage of baseline) by four different assays
| Time interval | Assay | Geometric mean for normalized ghrelin (%) | Lower 95% confidence limit | Upper 95% confidence limit |
|---|---|---|---|---|
| 0900–1200 h | Active RIA | 72.3 | 66.4 | 78.8 |
| Total RIA | 82.9 | 76.1 | 90.3 | |
| Acyl sandwich | 57.0 | 52.3 | 62.1 | |
| Des-acyl sandwich | 66.3 | 60.8 | 72.2 | |
| 1200–1500 h | Active RIA | 86.7 | 79.5 | 94.7 |
| Total RIA | 101.9 | 93.4 | 111.3 | |
| Acyl sandwich | 105.8 | 96.9 | 115.5 | |
| Des-acyl sandwich | 112.9 | 103.4 | 123.2 |
First and second 3-h intervals after the carbohydrate beverage are shown.
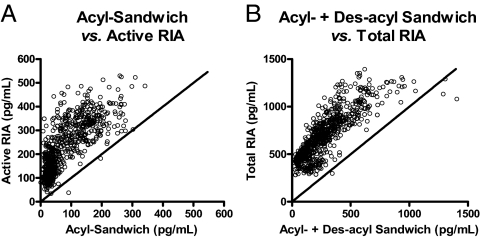
To compare the assays independent of time series and beverage types, the calculated ghrelin levels for comparable assays were correlated graphically in Fig. 4 (total RIA vs. the sum of the sandwich acyl and des-acyl, and the active RIA vs. the sandwich acyl-ghrelin). To provide a visual point of reference between the sandwich assays and the RIAs, the lines where slope equals 1 and y-intercept is 0 were plotted with each data set. Ideally, assays measuring the same analytes would result in data points that fall along this 45° line. Examination of these two correlation plots yielded two immediate observations: 1) the ghrelin levels reported by the RIAs are higher than those reported by the sandwich assay; and 2) the relationship between assays of differing methodologies is nonlinear. The estimated y-intercept for the total ghrelin correlation graph by empirically fitting a nonlinear regression line is approximately 300 pg/ml, and it is 15–30 pg/ml for the active ghrelin correlation plot (data not shown).
Figure 4.
Correlation plots between ghrelin values determined by active RIA vs. acyl-sandwich assay (correlation, r2 = 0.6140; P < 0.0001) (A) and total RIA vs. acyl + des-acyl sandwich assays (correlation, r2 = 0.6815; P < 0.0001) (B).
To test the hypothesis that ghrelin fragments might explain the higher overall ghrelin levels seen in single site assays of the nutrient feeding study, we devised experiments using our own in-house single site competitive ghrelin ELISA and samples with and without AEBSF/acid preservation (these samples were not part of the feeding study). The ghrelin concentrations from eight subjects were measured using the two-site sandwich assays and compared with our own single-site competitive ELISA using the same C-terminal antiserum that was employed in both of the sandwich assays. Five of the samples were plasma that had been frozen and stored with no preservation by EDTA, AEBSF, or acidification, and they were therefore expected to contain higher concentrations of fragments or degraded ghrelin peptides. In these samples, full-length acyl and des-acyl ghrelin were both undetectable by the sandwich assays but showed from 150 to 810 pg/ml ghrelin in the competitive single-site total ghrelin ELISA (Fig. 5A). Samples that were preserved with protease and esterase inhibitors as well as treated with HCl had measurable ghrelin levels in the sandwich assays, but also read considerably higher in the single-site assay (Fig. 5B). This is consistent with the results of Akamizu et al. (15) suggesting the presence of ghrelin fragments (see Discussion).
Discussion
Clinical data analyzed by both single-site and two-site assay methodologies showed that carbohydrate-induced changes were larger with the sandwich assays and that the sandwich assays were better able to distinguish between the more modest changes after consumption of fat or protein beverages. The significantly greater percentage changes seen with the sandwich assays are consistent with the expected greater specificity of the sandwich assays. One would expect that in a more specific assay a greater percentage of the total signal is due to the true acyl-ghrelin or true full-length des-acyl ghrelin. In an assay where degradation products or cross-reactivities contribute to the signal, the change in overall signal will be on top of a larger background, and so the percentage change will be less.
The correlation comparison (Fig. 4) of the measured ghrelin values from the two- assay methods clearly shows the higher values reported by the single-site assays. There are several potential explanations for this difference between the sandwich assays and RIAs. For example, factors that might affect the two types of assays to differing degrees include the following: 1) nonspecific competition by plasma proteins or other low-affinity molecules present in the samples that can disrupt the antibody-ghrelin interaction; 2) ghrelin complexed with carrier proteins in the plasma that may not interact with one or more of the antibodies used in the assays lowering the expected signal; or 3) specific competition from ghrelin fragments (degradation products) containing the appropriate epitopes.
Nonspecific competition by plasma proteins can affect both assays; however, it might be expected to have a greater effect on the RIA due to the standard curve being prepared in aqueous buffer. In contrast, the sandwich assay standards were diluted in plasma stripped of endogenous ghrelin by passage over a C-18 column. The stripping procedure removes endogenous peptides but not proteins. Thus, if plasma proteins bind nonspecifically, the standard curve in plasma might be expected to correct for this in the sandwich assays, whereas because the standard curve in the RIAs is in buffer, it does not account for this possible effect.
Studies have found that ghrelin binding proteins present in plasma can interfere with some ghrelin antisera but not others (12,19). Association of ghrelin with a carrier protein is believed to be an insignificant source of error in the sandwich assays due to the acidification of the samples and standards. The acid treatment dissociates ghrelin from its carrier (Gaylinn B. D., and J. Liu, unpublished observations using reverse-phase chromatography). If this type of interference were blocking antibody interactions, however, the sandwich assay standard curve in plasma would be nonlinear at low values; this was not observed. Additionally, experiments spiking ghrelin into plasma show quantitative recovery by both the acyl and the des-acyl sandwich assays, demonstrating that any ghrelin added to the samples is measured in the assays without loss due to binding proteins (9).
Specific interference by ghrelin fragments is the most likely explanation for both the nonlinearity and higher measurements seen in the RIAs, again due to utilization of only a single site for antibody recognition. Ghrelin fragments will affect both assays by competing for binding sites; however, in the RIA this competition is reported as ghrelin, whereas in the sandwich, with only one of the two needed epitopes present, there is little effect except at saturating concentrations when binding sites become limited. The manufacturer’s assay literature states that the total ghrelin RIA is cross-reactive with ghrelin13-28, and the active RIA with acyl-ghrelin1-10.
To examine the hypothesis that the difference between assays could be explained by detection of fragments and that this difference might change systematically in response to nutrients, we looked at each individual subject’s ghrelin profiles and examined the difference between the active RIA value and the acyl sandwich (active − acyl), and also the difference between the total RIA and the sum of the two sandwich assays [total − (acyl + des)]. The differences in ghrelin concentrations on a per-subject basis varied between 45 and 350 pg/ml (active − acyl), and between 180 and 750 pg/ml (total − (acyl +Des)]. These differences are expected due to each subject’s individual metabolism, ghrelin amounts, and differing enzyme profiles. But this difference did not correlate with the magnitude of each individual’s changes in ghrelin, and no clear trends in these “fragment profiles” could be seen in response to the meals (data not shown).
As seen in Figs. 4 and 5, ghrelin values measured in the two-site assays are consistently lower than those determined for the same samples with a single site assay. In the previously mentioned study by Akamizu et al. (15), they used their own sandwich assays for acyl and des-acyl ghrelin and concluded that 40 to 60% of the signal in a standard total ghrelin RIA in plasma was actually from inactive C-terminal ghrelin fragments, but they cited the possibility of differing antibodies that could have led to the higher signals from the single-site assays (15). The presence of competing fragments is also supported by data from De Vriese et al. (20) showing a number of proteolytic cleavage sites at the N terminus of ghrelin digested by stomach, liver, and kidney tissue homogenates, although there was little degradative activity in serum other than the conversion of acyl to des-acyl ghrelin. The lower specificity of the RIAs, combined with the presence of an unpredictable number of cross-reactive fragments with unknown clearance rates can result in a flattening of ghrelin profiles over time that can mask smaller changes in the concentrations of full-length ghrelin species, which are considered to be the biologically active forms.
Single-site “total ghrelin” assays have the advantage of measuring both ghrelin and some of its more stable breakdown products. Thus, sample collection and handling methods are not as critical, and higher levels are detected that require a smaller sample size or less assay sensitivity. But the disadvantage of this method is lower specificity. Cases where ghrelin acylation is suppressed without affecting total levels, such as in long-term fasting (9) or pregnancy (21), would be missed, and as shown here (Fig. 3) the dynamic changes in acyl-ghrelin are muted and less visible in the RIAs. Our results illustrate the importance of careful characterization and proper selection of assays within the context of study goals to best support interpretation of hormone data to delineate physiology and pathophysiology accurately.
Acknowledgments
We thank Dr. J. H. Sloan III and LINCO Labs for sharing their data with us and enabling us to complete this comparison of methodologies.
Footnotes
This study was supported in part by National Institutes of Health Grants R01 DK61516 (to D.E.C.) and R01 DK076037 (to M.O.T.). Blood samples were obtained from subjects studied in the General Clinical Research Center at the University of Washington (UW), which is funded by National Center for Research Resources (NCRR) Grant M01-RR-00037. Support was obtained from the UW Diabetes Endocrinology Research Center Clinical Research Core, which is funded by Grant P30 DK17047 from the National Institute of Diabetes and Digestive and Kidney Diseases. K.E.F.-S. is supported by K12 RR023265-03 from the NCRR.
Disclosure Summary: The authors have declared that no conflicts of interest exist. M.O.T. reports that he is a paid consultant for Novo Nordisk and Tercica. Part of this work was supported by an unrestricted grant from Bristol-Myers Squibb.
First Published Online March 1, 2010
Abbreviations: AEBSF, 4-(2-Aminoethyl)-benzene sulfonylfluoride; GHS-R1a, GH secretagogue receptor 1a.
References
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K 1999 Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660 [DOI] [PubMed] [Google Scholar]
- Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE 2008 Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA 105:6320–6325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL 2008 Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 132:387–396 [DOI] [PubMed] [Google Scholar]
- Tizzano M, Sbarbati A 2006 Hormone fatty acid modifications: Gram negative bacteria and vertebrates demonstrate common structure and function. Med Hypotheses 67:513–516 [DOI] [PubMed] [Google Scholar]
- Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, Malan D, Baj G, Granata R, Broglio F, Papotti M, Surico N, Bussolino F, Isgaard J, Deghenghi R, Sinigaglia F, Prat M, Muccioli G, Ghigo E, Graziani A 2002 Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol 159:1029–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Kangawa K 2005 Ghrelin: structure and function. Physiol Rev 85:495–522 [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS 2001 A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50:1714–1719 [DOI] [PubMed] [Google Scholar]
- Nass R, Farhy LS, Liu J, Prudom CE, Johnson ML, Veldhuis P, Pezzoli SS, Oliveri MC, Gaylinn BD, Geysen HM, Thorner MO 2008 Evidence for acyl-ghrelin modulation of growth hormone release in the fed state. J Clin Endocrinol Metab 93:1988–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Prudom CE, Nass R, Pezzoli SS, Oliveri MC, Johnson ML, Veldhuis P, Gordon DA, Howard AD, Witcher DR, Geysen HM, Gaylinn BD, Thorner MO 2008 Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab 93:1980–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster-Schubert KE, Overduin J, Prudom CE, Liu J, Callahan HS, Gaylinn BD, Thorner MO, Cummings DE 2008 Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J Clin Endocrinol Metab 93:1971–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta M, Ohwada R, Katakami H, Shibasaki T, Hizuka N, Takano K 2004 Plasma levels of intact and degraded ghrelin and their responses to glucose infusion in anorexia nervosa. J Clin Endocrinol Metab 89:5707–5712 [DOI] [PubMed] [Google Scholar]
- Patterson M, Murphy KG, le Roux CW, Ghatei MA, Bloom SR 2005 Characterization of ghrelin-like immunoreactivity in human plasma. J Clin Endocrinol Metab 90:2205–2211 [DOI] [PubMed] [Google Scholar]
- Nussbaum SR, Zahradnik RJ, Lavigne JR, Brennan GL, Nozawa-Ung K, Kim LY, Keutmann HT, Wang CA, Potts Jr JT, Segre GV 1987 Highly sensitive two-site immunoradiometric assay of parathyrin, and its clinical utility in evaluating patients with hypercalcemia. Clin Chem 33:1364–1367 [PubMed] [Google Scholar]
- Gröschl M, Uhr M, Kraus T 2004 Evaluation of the comparability of commercial ghrelin assays. Clin Chem 50:457–458 [DOI] [PubMed] [Google Scholar]
- Akamizu T, Shinomiya T, Irako T, Fukunaga M, Nakai Y, Nakai Y, Kangawa K 2005 Separate measurement of plasma levels of acylated and desacyl ghrelin in healthy subjects using a new direct ELISA assay. J Clin Endocrinol Metab 90:6–9 [DOI] [PubMed] [Google Scholar]
- Rauh M, Gröschl M, Rascher W 2007 Simultaneous quantification of ghrelin and desacyl-ghrelin by liquid chromatography-tandem mass spectrometry in plasma, serum, and cell supernatants. Clin Chem 53:902–910 [DOI] [PubMed] [Google Scholar]
- Overduin J, Frayo RS, Grill HJ, Kaplan JM, Cummings DE 2005 Role of the duodenum and macronutrient type in ghrelin regulation. Endocrinology 146:845–850 [DOI] [PubMed] [Google Scholar]
- Natalucci G, Riedl S, Gleiss A, Zidek T, Frisch H 2005 Spontaneous 24-h ghrelin secretion pattern in fasting subjects: maintenance of a meal-related pattern. Eur J Endocrinol 152:845–850 [DOI] [PubMed] [Google Scholar]
- Beaumont NJ, Skinner VO, Tan TM, Ramesh BS, Byrne DJ, MacColl GS, Keen JN, Bouloux PM, Mikhailidis DP, Bruckdorfer KR, Vanderpump MP, Srai KS 2003 Ghrelin can bind to a species of high density lipoprotein associated with paraoxonase. J Biol Chem 278:8877–8880 [DOI] [PubMed] [Google Scholar]
- De Vriese C, Gregoire F, Lema-Kisoka R, Waelbroeck M, Robberecht P, Delporte C 2004 Ghrelin degradation by serum and tissue homogenates: identification of the cleavage sites. Endocrinology 145:4997–5005 [DOI] [PubMed] [Google Scholar]
- Tham E, Liu J, Innis S, Thompson D, Gaylinn BD, Bogarin R, Haim A, Thorner MO, Chanoine JP 2009 Acylated ghrelin concentrations are markedly decreased during pregnancy in mothers with and without gestational diabetes: relationship with cholinesterase. Am J Physiol Endocrinol Metab 296:E1093–E1100 [DOI] [PMC free article] [PubMed] [Google Scholar]