Abstract
Context: Data on the presence, extent, and reversibility of cardiovascular disease in primary hyperparathyroidism (PHPT) are conflicting.
Objective: To evaluate the heart in PHPT, we assessed cardiac structure and diastolic function in patients with mild PHPT compared with age- and sex-matched controls.
Design: This was a case-control study.
Settings: The study was conducted in a university hospital Metabolic Bone Diseases Unit.
Participants: Fifty-four men and women with PHPT and 76 controls without PHPT participated in the study.
Outcome Measures: We measured left ventricular mass index (LVMI), the presence of mitral annular calcification, the ratio of early to late diastolic mitral inflow velocities (E/A), and early diastolic velocity of the lateral mitral annulus using Doppler tissue imaging (tissue Doppler e′).
Results: Patients had mild disease with mean (±sd) serum calcium 10.5 ± 0.5 mg/dl and PTH 96 ± 45 pg/ml. LVMI and diastolic function were normal in PHPT. There was no difference in LVMI (98 ± 23 vs. 96 ± 24 g/m2, P = 0.69) or the frequency of mitral annular calcification between PHPT cases and controls. Diastolic function variables (E/A and tissue Doppler e′) were higher (better) in cases compared with controls, although both were within the reference range. PHPT patients with low E/A had higher serum PTH (121 ± 36 vs. 89 ± 46 pg/ml, P = 0.03) and calcium (10.8 ± 0.4 vs. 10.5 ± 0.5 mg/dl, P = 0.05) than those with normal values. Finally, we found LVMI to be inversely associated with serum 25-hydroxyvitamin D in PHPT (r = −0.29, P < 0.05). All findings persisted after adjustment for group differences in cardiovascular risk factors.
Conclusions: Patients with biochemically mild PHPT do not have evidence of increased left ventricular mass, diastolic dysfunction, or increased valvular calcifications. However, the data support an association between low vitamin D levels and the development of left ventricular hypertrophy in this disorder. Finally, the increased serum calcium and PTH levels in those with diastolic dysfunction suggest that disease severity may determine the presence of cardiac manifestations in PHPT.
In mild primary hyperparathyroidism, there is no increased left ventricular mass, diastolic dysfunction, or increased valvular calcifications, but low vitamin D levels may predispose to the development of left ventricular hypertrophy.
Conflicting data concerning the cardiovascular manifestations of primary hyperparathyroidism (PHPT) may be explained by the decrease in disease severity observed over the past 30 yr. Whereas cardiovascular mortality is clearly increased in PHPT associated with moderate to severe hypercalcemia (1,2,3,4,5,6,7), data from the single epidemiologic study assessing patients with mild hypercalcemia found reduced cardiovascular mortality in affected patients (8). In milder disease, it is therefore important to assess for potential deleterious cardiovascular consequences short of mortality.
Increased left ventricular (LV) mass is a strong, independent predictor of cardiovascular mortality (9,10,11) and has been observed in PHPT in many (12,13,14,15,16,17), but not all (18,19,20), studies across a wide range of calcium levels. Likewise, diastolic dysfunction, also a predictor of cardiac death (21), has been documented in some studies of patients with severe PHPT (20,22) as well as patients with moderate hypercalcemia (13,14). However, many of these studies were uncontrolled and others did not take into account group differences in important cardiovascular risk factors, such as hypertension and others.
The association of hypertension with PHPT, whether a true result of PHPT or due to selection bias, raises the possibility that any LV hypertrophy (LVH) or diastolic dysfunction observed in PHPT could be attributable to elevations in blood pressure. Because few studies have controlled for hypertension, it is unclear whether LVH and diastolic dysfunction are part of the clinical picture of modern PHPT and, if present, whether they are secondary to hypertension or perhaps a direct effect of parathyroid hormone, calcium or other factors (23,24).
Mitral annular calcification is also an independent predictor of stroke, myocardial infarction, and cardiac death (25,26). Myocardial and valvular calcifications have clearly been demonstrated in PHPT patients with marked hypercalcemia (16,27,28). Studies in patients with more modest increases in serum calcium are limited but have not shown increased calcification (13), suggesting that this phenomenon may be related to the level of hypercalcemia.
The purpose of this study was to evaluate left ventricular size, diastolic function, and valvular calcification in patients with biochemically mild PHPT, adjusting for hypertensive status and other cardiovascular risk factors.
Subjects and Methods
This was a case-control study comparing cardiac structure and function in patients with PHPT to normal controls. All patients gave written, informed consent. This study was approved by the Institutional Review Board of Columbia University Medical Center.
Subjects
Participants with PHPT were referred from the Metabolic Bone Diseases Unit at Columbia University Medical Center and represent consecutive cases who agreed to participate in the study between October 2005 and September 2008. Cases were eligible if they were between the ages of 45–75 yr to study those at risk for cardiac disease and because this age range includes the vast majority of patients with PHPT. Patients had PHPT, diagnosed by the presence of hypercalcemia (calcium >10.2 but <12.0 mg/dl to study the presence of cardiovascular findings in those with mild hypercalcemia) and an elevated or inappropriately normal PTH level. Calcium to creatinine clearance ratio was measured to exclude familial hypocalciuric hypercalcemia, and none had thiazide-induced hyperparathyroidism. Exclusion criteria included reported use of bisphosphonates within 2 months and initiation or changes in cholesterol-lowering medications within 2 yr of entry to the study (by patient report). Some patients were on multivitamins, but none were taking more than 600 IU/d of vitamin D supplements.
Controls were from the Northern Manhattan Study (NOMAS), a population-based study designed to investigate cardiovascular risk factors in 3298 individuals. Recruitment and enrollment for NOMAS has been described (29,30). From September 2005 through December 2008, NOMAS subjects older than age 50 yr who agreed to undergo brain MRI and echocardiographic evaluation that included diastolic function assessment were included in the Cardiac Abnormalities and Brain Lesion study. This subset of individuals constitutes the sample from which the control subjects of the present report were drawn. We matched each PHPT case by age, sex, and race- to one (or two when a second match was available) normocalcemic Cardiac Abnormalities and Brain Lesion control(s).
Cardiovascular risk factors
Demographic data, cardiac risk factors, and medical history were obtained from participants. These include race/ethnicity by self-identification; coronary artery disease (history of myocardial infarction, angina, angioplasty or coronary artery bypass surgery); hypercholesterolemia (a physician’s report of elevated lipid levels or being on a lipid lowering medication); hypertension (systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or a patient’s self-report of hypertension or antihypertensive medication use); diabetes mellitus (fasting blood glucose level >126 mg/dl, patient’s self-report of diabetes, or use of insulin or other hypoglycemic medications); cigarette smoking (categorized as nonsmoker or ever smoked).
Biochemical evaluation
Fasting samples for serum calcium, creatinine, and total cholesterol were measured by an automated chemistry analyzer. PTH was measured by immunochemilumometric assay for intact PTH (Scantibodies Laboratories, Inc., Santee, CA), which detects PTH (1-84) and PTH (7-84). Serum 25-hydroxyvitamin D was measured by RIA (Diasorin, Stillwater, MN).
Transthoracic echocardiography
Transthoracic echocardiography was performed and measurements were taken by standard two-dimensional protocols according to guidelines of the American Society of Echocardiography (31). LV diastolic dimension, interventricular septal thickness, and posterior wall thickness were measured. LV mass (LVM) was calculated by the corrected American Society of Echocardiography method: 0.8 [1.04 ([LV diastolic dimension + interventricular septal thickness + posterior wall thickness]3)] + 0.6 (32). LVM index (LVMI) was calculated as LVM divided by body surface area. Abnormal LVMI is defined as greater than 108 g/m2 in women and greater than 131 g/m2 in men (31).
Transmitral diastolic flow was obtained by pulsed-wave Doppler from an apical four-chamber view, with the pulsed Doppler sample volume placed perpendicular to the inflow jet previously identified with the use of color Doppler. LV myocardial velocities were evaluated by tissue Doppler imaging and sampled on the longitudinal axis from the apical four-chamber view. Two-dimensionally guided pulsed-tissue Doppler imaging sample volume was placed at the level of the lateral mitral valve annulus. Doppler gain and wall filter were adjusted to reduce artifacts and velocity scale was set to ±20 cm/sec. Four consecutive beats were recorded at a sweep rate of 100 mm/sec during patient apnea and stored for off-line analysis. Peak velocities of the early and late phase of the mitral inflow pattern from Doppler recordings were measured and their ratio (E/A) was calculated; peak early (e′) diastolic velocity of the lateral mitral annulus by pulsed-tissue Doppler imaging was measured. Normal values for E/A and tissue Doppler e′ were 0.75–1.5 and 7 mm or greater, respectively. Mitral annular calcification (MAC) was defined as an intense echocardiographic-producing structure with highly reflective characteristics that was located at the junction of the atrioventricular groove and the posterior or anterior mitral leaflet.
Statistical analysis
Between-group differences in demographic and cardiovascular risk factors were evaluated by independent two-sided t test, χ2, or Fisher’s exact test as appropriate and adjusted for multiple comparisons as noted. Critical test values were adjusted for unequal variances when variances tested unequal (SAS Stat; SAS Institute, Cary NC). Differences in echocardiographic outcomes (LVMI, MAC, E/A, and tissue Doppler e′) between cases and controls were assessed with repeated-measures ANOVA using the pair as the observation unit and subject within pair as the repeated factor. Because of the differences in mean values for cholesterol, blood pressure, and blood glucose between the cases and controls, hypercholesterolemia, hypertension, and diabetes were entered as categorical variables.
Relationships between calcium, PTH, 25-hydroxyvitamin D, and echocardiographic variables were assessed with Pearson correlation (continuous variables) or logistic regression (categorical variables). Stepwise multiple regression was used to evaluate the influence of cardiovascular risk factors and other biochemistries (PTH, serum calcium, male gender, hypertension, hypercholesterolemia, creatinine, diabetes, coronary artery disease, and smoking) on the relationship between 25-hydroxyvitamin D and LVMI. The stepwise selection process criterion for entry to the model was a univariate P ≤ 0.3 and the criterion for retention in the model was a multivariate P ≤ 0.10.
Serum levels of PTH, calcium, and 25-hydroxyvitamin D in those with normal vs. abnormal LVMI, E/A, and tissue Doppler e′ as well as those with and without MAC were compared using independent two-sided t test. The study had 80% power with a 5% alpha to detect a 0.4-sd between-group difference under the simplifying assumption of a matched-pair t test with 50 pairs. For all analyses, a two-tailed P < 0.05 was considered to indicate statistical significance. Statistical analysis was performed using SAS (version 9.2.3; SAS Institute).
Results
Clinical and biochemical data
Consistent with their diagnosis, the majority of participants were female and had biochemically mild PHPT [serum calcium (mean ± sd): 10.5 ± 0.5 mg/dl, normal 8.7–10.2 mg/dl; PTH: 96 ± 45, normal 10–66 pg/ml; 25-hydroxyvitamin D level: 37.4 ± 14 ng/ml; Table 1]. Whereas the racial distribution was similar among cases and controls, there were more Caucasians of Hispanic ethnicity in the control group. Body mass index (BMI) was higher and hypertension more common in control subjects, and diabetes as well as hypercholesterolemia tended to be seen more frequently in the control group as well (Table 1). However, when adjustment was made for multiple comparisons between groups, only the difference between ethnic groups remained significant. Finally, although the study was not powered to assess this point, patients who met one or more 2002 National Institutes of Health Consensus Workshop guidelines for parathyroidectomy (n = 35) were compared with those who did not meet any surgical criteria (n = 19). There were no differences between these groups in any biochemical, demographic or cardiovascular risk factor listed in Table 1. Of the cardiac structural and functional indices measured, only tissue Doppler e′ differed between the two groups (met guidelines: 11.9 ± 2.9 vs. no guidelines: 10.2 ± 2.4; P < 0.05), and values for both groups were within normal limits.
Table 1.
Demographic and cardiovascular risk factors
| PHPT Mean ± sd (n = 54) | Controls Mean ± sd (n = 76) | P value | |
|---|---|---|---|
| Age (yr) | 62 ± 7 | 63 ± 9 | 0.34 |
| Race | |||
| Caucasian | 98% | 95% | 0.28 |
| Ethnicity: Hispanic | 4% | 44% | <0.0001a |
| African-American | 2% | 5% | 0.32 |
| Male gender | 19% | 20% | 0.86 |
| BMI (kg/m2) | 25.5 ± 4.0 | 27.3 ± 4.0 | 0.02 |
| Hypercholesterolemia | 41% | 57% | 0.08 |
| Coronary artery disease | 7% | 1% | 0.12 |
| Hypertension | 39% | 60% | 0.01 |
| Diabetes | 2% | 9% | 0.06 |
| Ever smoke | 56% | 47% | 0.35 |
| Systolic blood pressure (mm Hg) | 127 ± 20 | 131 ± 16 | 0.29 |
| Diastolic blood pressure (mm Hg) | 75 ± 11 | 77 ± 9 | 0.21 |
| Serum calcium (mg/dl) | 10.5 ± 0.5 | 9.3 ± 0.5 | <0.001a |
| Creatinine (mg/dl) | 0.9 ± 0.3 | 0.8 ± 0.2 | 0.35 |
| Total cholesterol (mg/dl) | 211 ± 34 | 208 ± 34 | 0.62 |
Remains significant after adjusting for multiple comparisons.
Cardiac structure and function
Mean LVMI was normal in PHPT. LVMI was elevated in only 15% of patients, 50% of whom were hypertensive. LVMI did not differ between PHPT and controls (98 ± 23 vs. 96 ± 24 g/m2, P = 0.69; Table 2) with or without adjustment for group differences in cardiovascular risk factors (BMI, hypertension, diabetes, and hypercholesterolemia). Frequency of mitral annular calcification was similar in PHPT vs. controls before (13 vs. 21%; within pair difference 5.9%, P = 0.26) and after adjustment for disparities in cardiovascular risk factors (P = 0.38). Mean diastolic function values (E/A and tissue Doppler e′) were normal in both PHPT and controls, although they were higher (better) in cases within the normal reference range. In PHPT, E/A ratio and tissue Doppler e′ were below the normal cutoff values in only 22 and 6%, respectively.
Table 2.
Echocardiographic studies
| Normal range | PHPT Mean ± sd | Controls Mean ± sd | Within-pair difference ± sd | P value Within-pair difference | P value Within-pair differencea | |
|---|---|---|---|---|---|---|
| Early to late mitral annular velocity (E/A) | 0.75–1.5 | 1.1 ± 0.3 | 0.9 ± 0.3 | 0.13 ± 0.40 | 0.0007 | 0.03 |
| Tissue Doppler e′ (mm) | ≥7 | 11.3 ± 2.6 | 9.6 ± 2.7 | 1.41 ± 3.63 | 0.0005 | 0.006 |
| LVMI (g/m2) | ≤108 for women | 98 ± 23 | 96 ± 24 | 2.8 ± 31.7 | 0.69 | 0.44 |
| ≤131 for men |
Comparison adjusted for body mass index, hypertensive, diabetic and hypercholesterolemic status.
Relationship between PTH, calcium, vitamin D, and echocardiographic measures in PHPT
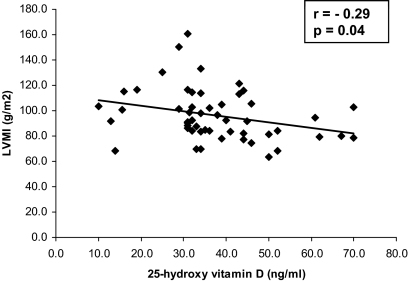
There was no linear association between serum calcium or PTH level and LVMI, valvular calcification, or any diastolic function variable among participants with PHPT. Serum 25-hydroxyvitamin D (25OHD) level, however, negatively correlated with LVMI (r = −0.29, P = 0.04) but not other echocardiographic variables. Removal of the two participants with the highest LVMI values (Fig. 1) did not alter this finding (r = −0.28, P = 0.04). When stepwise multiple regression was used to evaluate the influence of cardiovascular risk factors and other biochemical measurements, only serum 25OHD and creatinine were significant predictors of LVMI. For every 1 ng/ml decrease in 25OHD, LVMI increased by 0.56 g/m2 (P = 0.0086; Table 3). Furthermore, 75% (six of eight) of patients with vitamin D deficiency or insufficiency (25OHD <30 ng/ml) had LVMI above the cohort average of 97, as compared with 37% (16 of 43) of those with 25OHD levels in the vitamin D replete range (above 30 ng/ml). Finally, BMI does not seem to mediate the increase in LVMI. Of the 22 subjects with LVMI above the cohort average (97 g/m2), 18 had BMI between 20–30, whereas two were less than 20 and two had BMI greater than 30 kg/m2.
Figure 1.
Relationship between 25OHD level and LVMI in patients with PHPT.
Table 3.
Multiple regression model of LVMI in PHPT patients
| Variable | β | se | P value | Model R2 |
|---|---|---|---|---|
| 25OHDa | −0.56 | 0.21 | 0.0086 | R2 = 0.18 |
| Creatininea | 23.65 | 10.62 | 0.03 | P = 0.01 |
Selected from a model including PTH, serum calcium, 25OHD, male gender, hypertension, hypercholesterolemia, creatinine, diabetes, coronary artery disease, and smoking as potential variables.
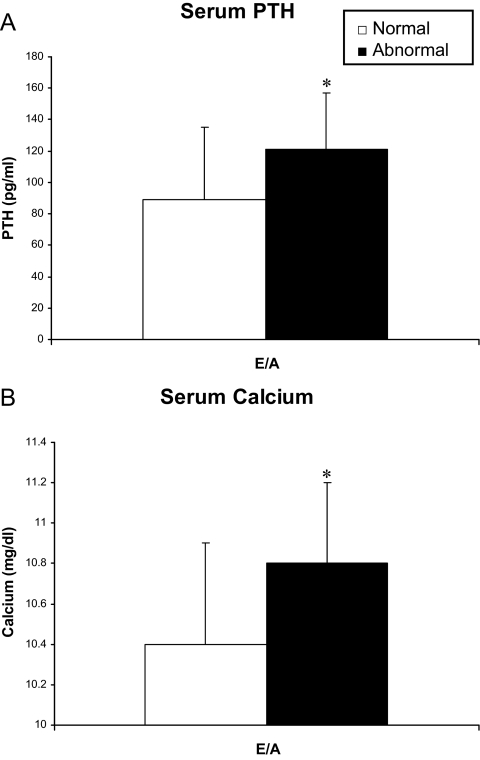
There was no difference in PTH, calcium level, or 25OHD level between PHPT cases with normal vs. abnormal LVMI (PTH: 98 ± 47 vs. 88 ± 36 pg/ml, P = 0.56; calcium: 10.5 ± 0.5 vs. 10.3 ± 0.5 mg/dl, P = 0.23; 25OHD: 38 ± 14 vs. 34 ± 9 ng/ml, P = 0.48) or between those with or without MAC (PTH: 85 ± 50 vs. 98 ± 45 pg/ml, P = 0.47; calcium: 10.6 ± 0.6 vs. 10.5 ± 0.5 mg/dl, P = 0.64; 25OHD: 38 ± 16 vs. 37 ± 13 ng/ml, P = 0.79). Those with low E/A ratio had higher mean serum PTH and calcium levels but not lower vitamin D than those with E/A 0.75 or greater (PTH: 121 ± 36 vs. 89 ± 46 pg/ml, P = 0.03; calcium 10.8 ± 0.4 vs. 10.5 ± 0.5 mg/dl, P = 0.05; 25OHD: 36 ± 9 vs. 38 ± 15 ng/ml, P = 0.75; Fig. 2). There was no difference in PTH, calcium, or 25OHD level in those with low vs. normal tissue Doppler e′ (PTH: 129 ± 38 vs. 94 ± 46 pg/ml, P = 0.20; calcium: 10.8 ± 0.2 vs. 10.5 ± 0.5, P = 0.31; 25OHD: 32 ± 3 vs. 38 ± 13. P = 0.51). Among controls (calcium levels normal; PTH, 25OHD unavailable), there was no difference in calcium level between those with normal vs. abnormal E/A or tissue Doppler e′.
Figure 2.
PTH (A) and calcium (B) levels are higher in PHPT patients with abnormal (black bar) vs. normal (white bar) E/A. Data presented as mean ± sd. *, P < 0.05.
Discussion
Many patients with asymptomatic PHPT are observed without surgical intervention, which heightens the importance of understanding whether cardiovascular abnormalities are associated with biochemically mild PHPT. This echocardiographic study of PHPT patients with mild hypercalcemia (mean <0.5 mg/dl above the upper limit of normal) studied both indices of cardiac structure and function and found no evidence of increased LVMI, mitral annular calcification, or diastolic dysfunction, although we did find that the presence of diastolic dysfunction was associated with higher serum calcium and PTH levels. Additionally, we demonstrated an inverse association between serum 25OHD and LVMI in PHPT.
Previous data on LVM in patients with PHPT have been inconsistent, although all have included subjects with more severe disease and higher mean serum calcium and PTH levels than those in our investigation. Many of the available studies are limited by absence of a control group or lack of consideration of coexisting cardiovascular risk factors (13,14). Indeed, several studies that reported increased LVMI in a PHPT cohort found the result to be driven by those patients with coexisting hypertension (18,19). Others report LVH in PHPT patients, regardless of hypertensive status (16,28), and two studies found a positive association of PTH with LVMI (12,15). Neither study provided data on vitamin D levels, nor did they investigate whether the relationship between PTH and LVMI could be due to lower vitamin D in those with the highest PTH values.
It has long been postulated that LVH, if present in PHPT, may be secondary to PTH excess. There are data suggesting that PTH has trophic effects on cardiomyocytes (24). However, the vitamin D receptor is also present on cardiomyocytes as well as in vascular endothelial and smooth muscle cells. Recent data suggest that vitamin D deficiency activates the renin-angiotensin-aldosterone system, and vitamin D receptor knockout mice have hypertension, cardiac hypertrophy, and fibrosis (33,34,35,36). A recent study in humans found that vitamin D deficiency was associated with incident cardiovascular disease (37). There are no data on whether the cardiovascular effects were secondary to elevated PTH levels, low vitamin D, or both. However, a study in patients with secondary hyperparathyroidism (renal failure) found that higher LVMI was related to a vitamin D receptor polymorphism, rather than PTH levels (38). Vitamin D deficiency and insufficiency are common in PHPT and lower serum 25OHD levels are associated with higher serum PTH levels in PHPT (39,40,41,42), making either or both plausible mediators of deleterious cardiovascular effects in PHPT. That we did not find an increase in LVMI among our patients with PHPT, despite the association between LVMI and vitamin D, may be due to the fact that the majority of patients were vitamin D replete (84%; mean 25OHD 37.4 ng/ml) and/or that the PTH levels were not as high (mean values ±sd: 96 ± 45 vs. 215 ± 178 and 161 ± 73) as those reported in prior studies (15,16).
Few studies have studied cardiac calcifications in PHPT. As in our study, no increase in cardiac calcifications was seen at lower mean serum calcium levels (11.1 mg/dl) (13), whereas calcifications are reported in patients with higher calcium levels (12–12.3 mg/dl) (27,28). Myocardial and valvular calcifications have not, however, been shown to correlate with calcium or PTH levels in PHPT (27).
Many studies investigating diastolic function in PHPT are limited by the same issues affecting those examining LVMI, and inevitably results have been contradictory (13,14,15,18,20). Whereas we did not find evidence of diastolic dysfunction in our group of patients with PHPT, our data do indicate that those with low E/A have higher calcium and PTH levels than those with normal diastolic function. A similar association in those with low tissue Doppler e′, another measure of diastolic dysfunction, could have been missed because only three patients had abnormal values. Our results therefore are consistent with the hypothesis that diastolic dysfunction is related to the severity of hypercalcemia and/or PTH elevation in PHPT. This finding could be explained by the fact that myocardial relaxation depends on cytosol clearance of calcium (43), which may be impaired by the hypercalcemic environment in PHPT.
In aggregate, our results along with those of previous studies suggest that increased LVMI, mitral calcification, and diastolic dysfunction are present only in those with biochemically more severe PHPT and/or when hypertension or other cardiovascular risk factors are present and not taken into account. Whereas we did not find evidence for an effect on cardiac structure or function, it is becoming clear that mild hyperparathyroidism has different effects on various aspects of the cardiovascular system. We recently reported that this cohort has abnormal carotid vasculature (44), increased carotid intima-media thickness (IMT), and decreased carotid compliance. Carotid stiffness, strain, and distensibility were associated with PTH levels. This suggests that mild PHPT may have different effects in differing portions of the vascular system, increasing the risk of carotid abnormalities, but not predisposing to abnormalities in cardiac structure or function. The mechanisms that underlie this differential effect on the cardiovascular system in PHPT are unknown. The pathophysiological pathways that influence the development of atherosclerosis in PHPT may be different from those that cause changes in cardiac structure or function. Alternatively, the same pathological factor(s) may be operative, but the vasculature may be more sensitive or respond differently to the effects. Whereas we have yet to examine the relationship between IMT and 25OHD level within our cohort of patients with PHPT, a recent study reported a negative association between internal carotid IMT and 25OHD level, but not PTH, among community-dwelling adults without PHPT (45). Lastly, it is possible that increased carotid wall thickness and arterial wall dysfunction are early manifestations of mild PHPT, whereas changes in cardiac structure and function may develop later in the disease.
Our study has a number of limitations. Although the control group was a random sample of free-living individuals, they had higher BMI and frequency of hypertension, and tended to have more hypercholesterolemia and diabetes than the PHPT patients. We did not find the association of mild PHPT with hypertension, increased BMI, diabetes, or hypercholesterolemia, suggested by others (46,47,48,49,50,51). It is unlikely that the healthier profile of the PHPT cohort is attributable to PHPT disease status (see adjusted P values, Table 1). However, although we adjusted for differences in these important cardiovascular risk factors in our analysis, the fact that they were greater in the control population may have biased against our finding echocardiographic abnormalities in PHPT. We therefore assessed our patients against published normal ranges and did not find any abnormalities using that method either. The study is also limited because it represents a convenience sample of patients with PHPT, including those who met as well as those who did not meet surgical criteria for parathyroidectomy (subgroup analysis precluded by sample size). Finally, we unfortunately did not have vitamin D or PTH data available on our control population, making it impossible to determine whether the association between LVMI and vitamin D status or PTH and E/A is different in PHPT and nonhyperparathyroid populations. The study is also limited by its cross-sectional design: the time course of possible cardiac manifestations could not be examined.
Despite these limitations, the study has several important strengths. We characterized the effect of PHPT on cardiac structure and function in a homogenous group of patients with mild disease, using validated techniques that detect subclinical markers associated with cardiovascular outcomes. The study provides the first data on the association between echocardiographic measures and 25OHD level in PHPT. We were able to adjust for differences in demographic and cardiovascular risk factors between cases and controls and had adequate power to detect small differences (<0.4 sd) between groups. Whereas it is possible that our study failed to reveal differences below this magnitude, we suspect that such differences would not be clinically meaningful.
In conclusion, in contrast to data from cohorts with more severe PHPT (higher serum calcium and PTH and perhaps lower vitamin D levels), we did not find increased LVMI, more frequent valvular calcification, or evidence of diastolic dysfunction in patients with biochemically mild PHPT. The data do, however, suggest a possible relationship between vitamin D deficiency and increased LVMI in PHPT and that diastolic dysfunction may occur only in those with higher calcium and PTH levels. Additionally, recent data suggest that mild PHPT does affect other aspects of the cardiovascular system. Therefore, further studies looking comprehensively at multiple facets of the cardiovascular system and examining the independent roles of PTH, calcium, and vitamin D in the pathogenesis of cardiovascular disease will be invaluable in fully characterizing the effect of mild PHPT on cardiovascular health.
Footnotes
This work was supported by National Institutes of Health Grants R01 DK066329, K24 DK074457, and UL1 RR024156.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 12, 2010
Abbreviations: BMI, Body mass index; e′, peak early; E/A, ratio of early to late phase of the mitral inflow pattern; IMT, intima-media thickness; LV, left ventricular; LVH, LV hypertrophy; LVM, LV mass; LVMI, LVM index; MAC, mitral annular calcification; NOMAS, Northern Manhattan Study; 25OHD, 25-hydroxyvitamin D; PHPT, primary hyperparathyroidism.
References
- Palmér M, Adami HO, Bergström R, Akerström G, Ljunghall S 1987 Mortality after surgery for primary hyperparathyroidism: a follow-up of 441 patients operated on from 1956 to 1979. Surgery 102:1–7 [PubMed] [Google Scholar]
- Hedbäck G, Tisell LE, Bengtsson BA, Hedman I, Odén A 1990 Premature death in patients operated on for primary hyperparathyroidism. World J Surg 14:829–835; discussion 836 [DOI] [PubMed] [Google Scholar]
- Hedbäck G, Odén A, Tisell LE 1991 The influence of surgery on the risk of death in patients with primary hyperparathyroidism. World J Surg 15:399–405; discussion 406–407 [DOI] [PubMed] [Google Scholar]
- Hedbäck G, Odén A 1999 Survival of patients operated on for primary hyperparathyroidism. Surgery 125:240–241 [DOI] [PubMed] [Google Scholar]
- Hedbäck G, Odén A 1998 Increased risk of death from primary hyperparathyroidism—an update. Eur J Clin Invest 28:271–276 [DOI] [PubMed] [Google Scholar]
- Nilsson IL, Yin L, Lundgren E, Rastad J, Ekbom A 2002 Clinical presentation of primary hyperparathyroidism in Europe—nationwide cohort analysis on mortality from nonmalignant causes. J Bone Miner Res 17(Suppl 2):N68–N74 [PubMed] [Google Scholar]
- Øgard CG, Engholm G, Almdal TP, Vestergaard H 2004 Increased mortality in patients hospitalized with primary hyperparathyroidism during the period 1977–1993 in Denmark. World J Surg 28:108–111 [DOI] [PubMed] [Google Scholar]
- Wermers RA, Khosla S, Atkinson EJ, Grant CS, Hodgson SF, O'Fallon WM, Melton 3rd LJ 1998 Survival after the diagnosis of hyperparathyroidism: a population-based study. Am J Med 104:115–122 [DOI] [PubMed] [Google Scholar]
- Kardys I, Deckers JW, Stricker BH, Vletter WB, Hofman A, Witteman JC 2009 Echocardiographic parameters and all-cause mortality: the Rotterdam Study. Int J Cardiol 133:198–204 [DOI] [PubMed] [Google Scholar]
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP 1990 Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322:1561–1566 [DOI] [PubMed] [Google Scholar]
- Gardin JM, McClelland R, Kitzman D, Lima JA, Bommer W, Klopfenstein HS, Wong ND, Smith VE, Gottdiener J 2001 M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study). Am J Cardiol 87:1051–1057 [DOI] [PubMed] [Google Scholar]
- Almqvist EG, Bondeson AG, Bondeson L, Nissborg A, Smedgård P, Svensson SE 2002 Cardiac dysfunction in mild primary hyperparathyroidism assessed by radionuclide angiography and echocardiography before and after parathyroidectomy. Surgery 132:1126–1132; discussion 1132 [DOI] [PubMed] [Google Scholar]
- Dalberg K, Brodin LA, Juhlin-Dannfelt A, Farnebo LO 1996 Cardiac function in primary hyperparathyroidism before and after operation. An echocardiographic study. Eur J Surg 162:171–176 [PubMed] [Google Scholar]
- Näppi S, Saha H, Virtanen V, Limnell V, Sand J, Salmi J, Pasternack A 2000 Left ventricular structure and function in primary hyperparathyroidism before and after parathyroidectomy. Cardiology 93:229–233 [DOI] [PubMed] [Google Scholar]
- Piovesan A, Molineri N, Casasso F, Emmolo I, Ugliengo G, Cesario F, Borretta G 1999 Left ventricular hypertrophy in primary hyperparathyroidism. Effects of successful parathyroidectomy. Clin Endocrinol (Oxf) 50:321–328 [DOI] [PubMed] [Google Scholar]
- Stefenelli T, Abela C, Frank H, Koller-Strametz J, Globits S, Bergler-Klein J, Niederle B 1997 Cardiac abnormalities in patients with primary hyperparathyroidism: implications for follow-up. J Clin Endocrinol Metab 82:106–112 [DOI] [PubMed] [Google Scholar]
- Stefenelli T, Globits S, Bergler-Klein J, Woloszczuk W, Längle F, Niederle B 1993 [Cardiac changes in patients with hypercalcemia]. Wien Klin Wochenschr 105:339–341 [PubMed] [Google Scholar]
- Barletta G, De Feo ML, Del Bene R, Lazzeri C, Vecchiarino S, La Villa G, Brandi ML, Franchi F 2000 Cardiovascular effects of parathyroid hormone: a study in healthy subjects and normotensive patients with mild primary hyperparathyroidism. J Clin Endocrinol Metab 85:1815–1821 [DOI] [PubMed] [Google Scholar]
- Nuzzo V, Tauchmanovà L, Fonderico F, Trotta R, Fittipaldi MR, Fontana D, Rossi R, Lombardi G, Trimarco B, Lupoli G 2002 Increased intima-media thickness of the carotid artery wall, normal blood pressure profile and normal left ventricular mass in subjects with primary hyperparathyroidism. Eur J Endocrinol 147:453–459 [DOI] [PubMed] [Google Scholar]
- Nilsson IL, Aberg J, Rastad J, Lind L 2000 Left ventricular systolic and diastolic function and exercise testing in primary hyperparathyroidism—effects of parathyroidectomy. Surgery 128:895–902 [DOI] [PubMed] [Google Scholar]
- Mogelvang R, Sogaard P, Pedersen SA, Olsen NT, Marott JL, Schnohr P, Goetze JP, Jensen JS 2009 Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation 119:2679– 2685 [DOI] [PubMed] [Google Scholar]
- Baykan M, Erem C, Erdogan T, Ersoz HO, Gedikli O, Korkmaz L, Kucukosmanoglu M, Haclhasanoglu A, Kaplan S, Celik S 2007 Assessment of left ventricular diastolic function and the Tei index by tissue Doppler imaging in patients with primary hyperparathyroidism. Clin Endocrinol (Oxf) 66:483–488 [DOI] [PubMed] [Google Scholar]
- Ogino K, Burkhoff D, Bilezikian JP 1995 The hemodynamic basis for the cardiac effects of parathyroid hormone (PTH) and PTH-related protein. Endocrinology 136:3024–3030 [DOI] [PubMed] [Google Scholar]
- Schlüter KD, Piper HM 1992 Trophic effects of catecholamines and parathyroid hormone on adult ventricular cardiomyocytes. Am J Physiol 263:H1739–H1746 [DOI] [PubMed] [Google Scholar]
- Kamensky G, Lisy L, Polak E, Piknova E, Plevova N 2001 Mitral annular calcifications and aortic plaques as predictors of increased cardiovascular mortality. J Cardiol 37(Suppl 1):21–26 [PubMed] [Google Scholar]
- Kohsaka S, Jin Z, Rundek T, Boden-Albala B, Homma S, Sacco RL, Di Tullio MR 2008 Impact of mitral annular calcification on cardiovascular events in a multiethnic community: the Northern Manhattan Study. JACC Cardiovasc Imaging 1:617–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Längle F, Abela C, Koller-Strametz J, Mittelböck M, Bergler-Klein J, Stefenelli T, Woloszczuk W, Niederle B 1994 Primary hyperparathyroidism and the heart: cardiac abnormalities correlated to clinical and biochemical data. World J Surg 18:619–624 [DOI] [PubMed] [Google Scholar]
- Stefenelli T, Mayr H, Bergler-Klein J, Globits S, Woloszczuk W, Niederle B 1993 Primary hyperparathyroidism: incidence of cardiac abnormalities and partial reversibility after successful parathyroidectomy. Am J Med 95:197–202 [DOI] [PubMed] [Google Scholar]
- Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, Paik MC, Hauser WA 1998 Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol 147:259–268 [DOI] [PubMed] [Google Scholar]
- White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL 2005 Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 111:1327–1331 [DOI] [PubMed] [Google Scholar]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ 2005 Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18:1440–1463 [DOI] [PubMed] [Google Scholar]
- Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N 1986 Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 57:450–458 [DOI] [PubMed] [Google Scholar]
- Simpson RU, Hershey SH, Nibbelink KA 2007 Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol 103:521–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP 2002 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110:229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, O'Keefe JH, Bell D, Hensrud DD, Holick MF 2008 Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol 52:1949–1956 [DOI] [PubMed] [Google Scholar]
- Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC 2005 Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab 288:E125–E132 [DOI] [PubMed] [Google Scholar]
- Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS 2008 Vitamin D deficiency and risk of cardiovascular disease. Circulation 117:503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa A, Mallamaci F, Benedetto F, Pisano A, Tripepi G, Thadhani R, Zoccali C 2010 Vitamin D receptor (VDR) gene polymorphism is associated with left ventricular (LV) mass and predicts LVH progression in end stage renal disease (ESRD) patients. J Bone Miner Res 25(2):313–319 [DOI] [PubMed] [Google Scholar]
- Silverberg SJ, Shane E, Dempster DW, Bilezikian JP 1999 The effects of vitamin D insufficiency in patients with primary hyperparathyroidism. Am J Med 107:561–567 [DOI] [PubMed] [Google Scholar]
- Rao DS, Honasoge M, Divine GW, Phillips ER, Lee MW, Ansari MR, Talpos GB, Parfitt AM 2000 Effect of vitamin D nutrition on parathyroid adenoma weight: pathogenetic and clinical implications. J Clin Endocrinol Metab 85:1054–1058 [DOI] [PubMed] [Google Scholar]
- Ozbey N, Erbil Y, Ademoğlu E, Ozarmağan S, Barbaros U, Bozbora A 2006 Correlations between vitamin D status and biochemical/clinical and pathological parameters in primary hyperparathyroidism. World J Surg 30:321–326 [DOI] [PubMed] [Google Scholar]
- Beyer TD, Chen EL, Nilubol N, Prinz RA, Solorzano CC 2007 Short-term outcomes of parathyroidectomy in patients with or without 25-hydroxy vitamin D insufficiency. J Surg Res 143:145–150 [DOI] [PubMed] [Google Scholar]
- Morgan JP 1991 Abnormal intracellular modulation of calcium as a major cause of cardiac contractile dysfunction. N Engl J Med 325:625–632 [DOI] [PubMed] [Google Scholar]
- Walker MD, Fleischer J, Rundek T, McMahon DJ, Homma S, Sacco R, Silverberg SJ 2009 Carotid vascular abnormalities in primary hyperparathyroidism. J Clin Endocrinol Metab 94:3849–3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis JP, von Muhlen D, Michos ED, Miller 3rd ER, Appel LJ, Araneta MR, Barrett-Connor E 2009 Serum vitamin D, parathyroid hormone levels, and carotid atherosclerosis. Atherosclerosis 207:585–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty FW 1981 Primary hyperparathyroidism. Changing clinical spectrum, prevalence of hypertension, and discriminant analysis of laboratory tests. Arch Intern Med 141:1761–1766 [DOI] [PubMed] [Google Scholar]
- Luboshitzky R, Chertok-Schaham Y, Lavi I, Ishay A 2009 Cardiovascular risk factors in primary hyperparathyroidism. J Endocrinol Invest 32:317–321 [DOI] [PubMed] [Google Scholar]
- Bolland MJ, Grey AB, Gamble GD, Reid IR 2005 Association between primary hyperparathyroidism and increased body weight: a meta-analysis. J Clin Endocrinol Metab 90:1525–1530 [DOI] [PubMed] [Google Scholar]
- Nainby-Luxmoore JC, Langford HG, Nelson NC, Watson RL, Barnes TY 1982 A case-comparison study of hypertension and hyperparathyroidism. J Clin Endocrinol Metab 55:303–306 [DOI] [PubMed] [Google Scholar]
- Hagstrom E, Lundgren E, Lithell H, Berglund L, Ljunghall S, Hellman P, Rastad J 2002 Normalized dyslipidaemia after parathyroidectomy in mild primary hyperparathyroidism: population-based study over five years. Clin Endocrinol (Oxf) 56:253–260 [DOI] [PubMed] [Google Scholar]
- Cardenas MG, Vigil KJ, Talpos GB, Lee MW, Peterson E, Rao DS 2008 Prevalence of type 2 diabetes mellitus in patients with primary hyperparathyroidism. Endocr Pract 14:69–75 [DOI] [PubMed] [Google Scholar]