Abstract
Context: Ten to 30% of patients with papillary thyroid cancer (PTC) develop recurrent disease and may benefit from innovative adjuvant therapies. Immune-based therapies are under investigation to treat many types of cancer. The role of the immune system in PTC is poorly understood.
Objective: We investigated whether tumor-associated lymphocytes (TAL), in the absence of background thyroiditis (LT), contribute to disease severity. We hypothesized that the type of lymphocytes associated with PTC would correlate with parameters of disease.
Design: This retrospective study analyzed archived PTC samples for the presence of TAL and/or LT. A group of patients with TAL was evaluated for lymphocyte subsets by immunohistofluorescence.
Patients and Setting: One hundred PTC patients were analyzed for LT and TAL, and 10 PTC patients with TAL were assessed for lymphocyte subsets at University of Colorado Hospital.
Main Outcome: We assessed correlations between disease and the presence of TAL, LT, and lymphocyte subset frequency.
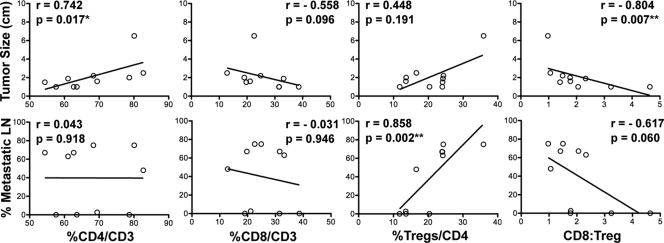
Results: Patients with TAL exhibited higher disease stage and increased incidence of invasion and lymph node metastasis compared with patients without lymphocytes or with LT. CD4+ T cell frequency correlated with tumor size (r = 0.742; P = 0.017). FoxP3+ regulatory T cell (Treg) frequency correlated with lymph node metastases (r = 0.858; P = 0.002), and CD8 to Treg ratio correlated inversely with tumor size (r = −0.804; P = 0.007).
Conclusions: TAL and high Treg frequency in primary thyroid tumors correlates with more aggressive disease. Future prospective studies may identify Treg frequency as a predictive factor in PTC, and the suppressive effects of Treg should be considered in the design of immune-based therapies.
FoxP3+ regulatory T lymphocytes are found in association with papillary thyroid cancer and may contribute to disease progression.
Thyroid carcinoma is the most common endocrine malignancy. The incidence of thyroid cancers increased more than 2-fold between 1973 and 2002, and this was attributed entirely to an increase of papillary thyroid cancer (PTC) (1). The 5-yr survival rate for patients with thyroid cancer is 97%; however, prognosis worsens with age, such that the survival rate of patients 65 and older is reduced to 87% (2). Although the prognostic significance of lymph node (LN) metastases in PTC is somewhat controversial, a recent study found that patients with evidence of nodal metastases had a higher risk of mortality (3). In patients 45 yr of age or older, LN involvement increases the risk of both recurrence and mortality (3,4). Despite the overall success of current therapies, 10–30% of patients develop recurrence and/or metastases (5). Novel adjuvant therapies could reduce recurrence rates and the need for additional surgery.
Lymphocytes are frequently found within and surrounding primary thyroid tumors (6,7). Previous studies suggest that the presence of a local inflammatory response predicts a more favorable prognosis for patients with PTC (3,8,9). Although tumor size and LN metastases did not correlate with the presence of lymphocytes, extrathyroidal invasion was significantly reduced in patients that showed evidence of lymphocytic infiltration (LI) (8). Patients with LN involvement or invasive tumors but no LI had a slightly higher rate of recurrence (8). Similarly, in a recent retrospective study, thyroid cancer patients with LI were found to have a more favorable rate of survival (3). In PTC patients 21 yr or younger, an increased number of proliferating lymphocytes correlated with improved disease-free survival (9). T cells, B cells, and NK cells were found near or within these tumors (10); however, additional studies are necessary to understand the role of specific lymphocyte subsets in PTC.
CD4+ T cells are central to the successful orchestration of the immune response. Naive CD4+ T cells differentiate into one of at least four functionally distinct fates (i.e. Th1, Th2, Th17, and Tregs) depending upon the presence of key cytokines and the expression of specific transcription factors (11). Regulatory T cells (Tregs) are commonly enriched within primary tumors, draining LN, and peripheral blood of cancer patients (12,13,14,15,16,17). An increased frequency of Tregs have been associated with poor prognosis in many cancers, including ovarian, breast, and lymphoma (18,19,20,21,22). In general, Tregs are identified as CD4+CD25+CTLA-4+FoxP3+ T lymphocytes. FoxP3+ Tregs have been classified into two categories based on their origin and may exert their suppressive function via distinct mechanisms (23). CD25hiFoxP3+ cells are commonly identified as natural Tregs (nTreg), which originate in the thymus. FoxP3+ expression may be induced in peripheral naive CD4+CD25− T cells under suboptimal activation conditions and in the presence of TGFβ (23,24,25). Both nTreg and inducible Tregs (iTreg) are thought to contribute to tumor-specific T cell tolerance (26). Direct targeting of Tregs via CD25- or CTLA-4-specific therapies has lead to improved tumor immunity and, in some cases, clinical benefit (19,27,28).
In this study, we investigated whether the type of immune response generated to PTC correlates with disease severity. Our data revealed that patients with tumor-associated LI presented with more aggressive disease when compared with patients with concurrent thyroiditis or no LI. Analysis of specific lymphocyte subsets revealed, for the first time, that Tregs are consistently found within and surrounding thyroid tumors, and their frequency correlates with disease severity. These data suggest that Treg frequency may be a useful diagnostic marker in determining PTC severity and treatment regimen.
Materials and Methods
PTC patients, PTC staging, and disease parameters
PTC patients seen at the University of Colorado Hospital between 2002 and 2007 were selected randomly for this study. Patient sample analysis was retrospective and was performed after Internal Review Board approval. Although sections of archived primary thyroid tumors were revaluated to assess LI, previous pathology reports provided data on tumor type, tumor size, invasion, and LN metastases. This group included 56 conventional PTC and 44 PTC variants. The variants include 37 follicular, four mixed follicular and papillary, one mixed follicular and insular, one mixed follicular and tall cell, and one solid variant. Standard American Joint Committee on Cancer (29) tumor-node-metastases scoring was used for PTC staging. Patients were categorized as positive or negative for invasion, LN metastases, and autoantibody production as follows. Tumors considered positive for invasion showed disruption of capsule barrier and/or extrathyroidal extension. Positive LN were determined by pathological examination of clinically suspicious nodes. Percent LN metastases was calculated as the number of patients with evidence of positive LN at the time of surgery divided by the total number of patients in each group. Serum thyroglobulin and thyroid peroxidase autoantibody levels for patients at the University of Colorado Hospital were determined using a sequential immunoenzymatic sandwich assays (Access immunoassay; Beckman Coulter, Fullerton, CA). Laboratory results were reviewed in each patient’s medical record, and values above the respective reference range were reported as positive. The percentage of patients positive for autoantibody was determined as the number of patients positive for at least one autoantibody (thyroglobulin or thyroid peroxidase) divided by the total number of patients tested in each group.
LI scoring
Archived hematoxylin- and eosin-stained thyroid tissue sections from 100 PTC patients were independently reviewed for the presence of background lymphocytic thyroiditis or tumor-associated lymphocytes (TAL) in the absence of thyroiditis. The tumor, the surrounding tissue immediately adjacent to the tumor, and normal thyroid tissue were reviewed for the presence of lymphocytes. Specimens containing lymphocytic inflammation within the normal thyroid tissue were designated as background lymphocytic thyroiditis. These tissues had at least one and often multiple dense lymphoid aggregates in the surrounding normal thyroid parenchyma. Tumor-associated LI, in the absence of concurrent lymphocytic thyroiditis, was defined as consisting of single cells, lymphocytic aggregates (≥10 lymphocytes in close proximity), or both, and the relationship of each aggregate to the tumor (i.e. intratumoral or peritumoral) was documented. Peritumoral tumor-associated inflammation was considered to be lymphocytes in the immediately adjacent fibrous tissue or tumor capsule. The extent of LI was scored from 1–5, with a score of 5 indicating extensive infiltration: 1, single lymphocytes in the peritumoral region; 2, lymphocyte aggregates in the peritumoral region; 3, intratumoral single lymphocytes with or without lymphocytes in the peritumoral region; 4, fewer than 10 intratumoral aggregates with or without peritumoral lymphocytes; and 5, 10 or more intratumoral aggregates with or without peritumoral lymphocytes. Assessment of tissue lymphocytes was independent of knowledge of disease status.
Antibodies
Antibodies specific for human CD3 (SP7), CD4 (4B12), CD8 (4B11), CD20 (L26), CD25 (4C9), and CD16 (2H7) were purchased from Vector Laboratories (Burlingame, CA). Anti-FoxP3 (PCH101) was purchased from eBioscience (San Diego, CA). Goat antimouse IgG1-AF647, antimouse IgG2a-AF488, antimouse IgG2a-AF546, antimouse IgG2b-AF488, antirabbit-AF546, and streptavidin-AF594 were purchased from Invitrogen (Molecular Probes, Eugene, OR). Goat antirat-biotin was purchased from Jackson ImmunoResearch (West Grove, PA). Anti-TGFβ (1D11; R&D Systems, Minneapolis, MN) and anti-vascular endothelial growth factor (anti-VEGF) (A20, rabbit; Santa Cruz Biotechnology, Santa Cruz, CA) were detected by standard immunohistochemistry methods.
Immunohistofluorescence and immunohistochemistry
Lymphocyte subsets and tumor-mediated cytokine production were assessed in a subset of 10 PTC patients with TAL using standard immunohistofluorescence or immunohistochemistry methods. Tissue blocks were cut into 4-μm sections for analysis. After dewaxing in xylene, tissues were hydrated in decreasing concentrations of ethanol. Antigen retrieval was performed using the Decloaking Chamber (Biocare Medical, Concord, CA) in 10 mm sodium citrate buffer (pH 6.0, 0.5% Tween 20) at 120 C for 5 min. Samples were subsequently cooled for 30 min and blocked with 10% goat serum for 2 h at room temperature, followed by a standard avidin/biotin block (0.002%) for endogenous biotin.
Lymphocyte subsets were detected with antibodies specific for CD20 (1:200), CD16 (1:100), CD3 (1:200), CD8 (1:100), CD4 (1:100), FoxP3 (1:200), and CD25 (1:100) in two-color or three-color combinations. Primary antibodies were incubated overnight at 4 C and detected with fluorescently tagged secondary antibodies (1 h at room temperature). All secondary antibodies were used at a 1:800 dilution. FoxP3-specific antibody was detected with a biotinylated antirat secondary antibody followed by streptavidin-AF594 (1:400). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride (Sigma Chemical Co., St. Louis, MO). For immunohistochemistry, antibodies specific for human VEGF and TGFβ were detected using the R.T.U. Vectastain Universal Elite ABC kit and ImmPact diaminobenzidine peroxidase substrate (Vector) and counterstained with Mayer’s hematoxylin (Sigma). Tissue sections incubated with secondary antibody alone served as negative controls. Fluorescent images were obtained at ×10 and ×60 using the Spinning Disk IX81 microscope (Olympus, Center Valley, PA) and Slidebook software (Intelligent Imaging Innovations, Inc., Denver, CO). Immunohistochemistry images were obtained at ×20 using the Zeiss Axiovert 135 microscope and AxioVison software. Protocols were optimized using sections of LN for lymphocyte subsets or the mouse 4T1 breast carcinoma cell line, which is known to express VEGF and TGFβ.
Lymphocyte subset quantification
Lymphocyte aggregates were imaged at ×60 magnification. A minimum of four and a maximum of 10 aggregates were imaged. Tumor-associated CD20+, CD3+, CD4+CD3+, CD8+CD3+, and CD4+CD25±FoxP3+ lymphocytes were counted manually using ImageJ software (National Institutes of Health, Bethesda, MD). The number of cells counted for each lymphocyte subset ranged from 70–4800 depending on the frequency of the subset and the extent of LI in each sample. The CD8 to Treg ratio was determined by quantifying total CD8+CD3+ and CD4+FoxP3+ lymphocytes in the same fields of view. The CD20 to CD3 ratios were calculated from raw counts. T cell subset frequencies were determined from two separate stains, and the mean value was calculated. Relative subset frequencies were assessed by two independent investigators (J.D.F. and D.L.F.), and the mean of two analyses is presented.
Statistical analysis
Investigators were blinded to pathological information at the time of analysis. Patient groups were compared using the Mann-Whitney t test, χ2, or Fisher’s exact test. The frequencies of lymphocyte subsets were assessed for correlation with parameters of disease by calculating the Spearman correlation coefficient (r). Analysis was performed using GraphPad Prism 5.0b software. P values <0.05 were considered statistically significant.
Results
Tumor-associated LI and disease severity in PTC patients
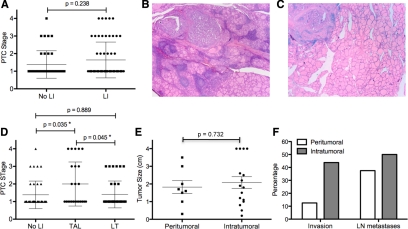
Although previous studies have assessed the incidence of LI in PTC, most of these analyses did not distinguish background lymphocytic thyroiditis from TAL. We analyzed thyroid specimens taken from 100 patients with PTC patients to determine the frequency of patients that exhibit TAL in the absence of background thyroiditis. Each sample was reassessed for LI and, if present, was characterized as TAL with or without the presence of thyroiditis. Sixty-one percent of tissue specimens showed evidence of LI. As shown in Fig. 1A, the presence of generalized LI had no effect on disease stage. To determine the effects of TAL independent of background thyroiditis, patients were subdivided into three groups: 39% of samples showed no evidence of LI (group I), 37% exhibited background thyroiditis (group III, Fig. 1B), and 24% revealed TAL in the absence of thyroiditis (group II, Fig. 1C). Although disease severity was similar in patients without LI and those with background thyroiditis, patients with TAL had significantly higher-stage disease (Fig. 1B). Higher-risk disease (stages 3 and 4) was more common in patients with TAL (33%) compared with those who had no LI (13%) or background thyroiditis (16%). Moreover, patients with TAL exhibited a higher incidence of invasive tumors and LN metastases compared with the other two groups (Table 1). Of note, more aggressive tumor subtypes did not account for the observed correlation between TAL and disease severity (data not shown). Forty-four percent of these tumors were PTC variants, the majority of which were follicular variants (41 of 44). PTC variants were more frequent in groups I (54%) and II (49%) compared with group I (21%). Three patients with tall cell, insular, or solid tumor variants were assigned to groups I, II, and III, respectively. These tumors were neither invasive nor metastatic.
Figure 1.
Tumor-associated LI and disease severity. Hematoxylin and eoxin stains of thyroid tissue sections from 100 PTC patients were reanalyzed for LI. A, PTC stage was compared between patients with or without LI; B and C, representative hematoxylin and eosin images are shown from patient with lymphocytic thyroiditis (B) or tumor-associated lymphocytes (C); D, samples were grouped based on the absence of lymphocytes (No LI), the presence of TAL in the absence of thyroiditis, or the presence of lymphocytes with background thyroiditis (LT), and disease stage was compared for each group; E and F, 24 patients with TAL were grouped based on lymphocyte localization and compared for tumor size (E) and the percentage of patients with invasive tumors (F, P = 0.189) or LN metastases (F, P = 0.679).
Table 1.
LI and disease parameters
| LI
|
P value (I vs. II) | P value (I vs. III) | P value (II vs. III) | |||
|---|---|---|---|---|---|---|
| Absent, n = 39 (group I) | Tumor-associated, n = 24 (group II) | Thyroiditis, n = 37 (group III) | ||||
| Age, yr [mean ± sem (range)] | 50.2 ± 2.47 (23–82) | 54.0 ± 2.50 (27–81) | 49.4 ± 2.05 (24–69) | 0.430 | 0.916 | 0.364 |
| Gender, n (males/females) | 10/29 | 11/13 | 4/33 | 0.099 | 0.096 | 0.002b |
| Tumor size, cm [mean ± sem (range)] | 1.93 ± 0.35 (0.2–12.0) | 2.00 ± 0.25 (0.2–4.0) | 1.25 ± 0.17 (0.1–4.0) | 0.200 | 0.204 | 0.012a |
| Lymph node metastasis, n (%) | 3/39 (7.69) | 11/24 (45.8) | 4/37 (10.8) | <0.001 | 0.649 | 0.003b |
| Tumor invasion, n (%) | 5/39 (12.8) | 8/24 (33.3) | 4/37 (8.11) | 0.051 | 0.786 | 0.031a |
| Autoantibody positive, n (%) | 4/27 (14.8) | 0/16 (0.0) | 12/19 (63.2) | 0.106 | 0.042a | 0.004b |
A pathological evaluation of LN metastases (pN0 or pN1) was performed on 20, 16, and 26 patients in groups I, II, and III, respectively. Statistical significance was calculated by the Mann-Whitney t test or χ2.
P ≤ 0.05.
P ≤ 0.01.
To determine whether the extent of TAL was associated with disease severity, we scored each patient sample for both relative lymphocyte frequency and peritumoral and/or intratumoral localization (data not shown). 50% of tumors displayed extensive LI, with multiple, intratumoral aggregates (grade 4, n = 7; grade 5, n = 5). In the remaining tumors, LI was confined to the peritumoral regions or was evident as diffuse intratumoral lymphocytes (grade 1, n = 1; grade 2, n = 7; grade 3, n = 4). No significant correlation was observed between LI grade and disease stage (r = 0.349; P = 0.094). When patients were separated based on the presence of only peritumoral LI (grades 1 and 2) vs. the presence of intratumoral lymphocytes (grades 3–5), no statistically significant correlations with tumor size (Fig. 1E), invasiveness, or LN metastases (Fig. 1F) were observed. However, invasive tumors were more common in patients with intratumoral LI (seven of 16) compared with those with peritumoral LI (one of eight, Fig. 1F).
Lymphocyte subset frequency in PTC patients with TAL
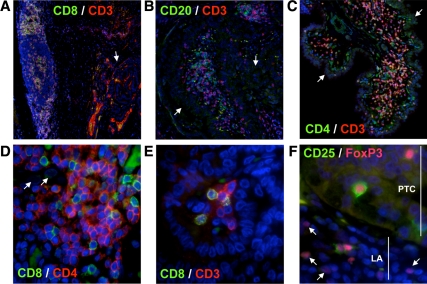
To define the types of lymphocytes found in association with PTC, we analyzed primary tumors from 10 patients with evident TAL by immunohistofluorescence. These patients exhibited extensive LI (grade 4 or 5, Table 2), with lymphocytes present as peritumoral (Fig. 2A) and intratumoral (Fig. 2, B and C) aggregates and as single cells or multicellular foci (Fig. 2D). We identified CD20+ B cells, CD4+CD3+ T cells, and CD8+CD3+ T cells both within and surrounding thyroid tumors. To determine the relative frequencies of lymphocyte subsets, we imaged lymphocyte aggregates at high magnification (Fig. 2E) and manually counted each cell type within these aggregates. CD16+ NK cells were consistently found in low frequency and were not quantified (data not shown). As shown in Table 2, the ratio of B cells to T cells varied significantly between patients. CD4+ T cells consistently represented the majority (54–83%) of the CD3+ population, whereas CD8+ T cells accounted for 13–39% of CD3+ cells (Table 2). Comparison of relative lymphocyte subset frequency with individual disease parameters revealed that patients with relatively high levels of tumor-associated CD4+ T cells presented with larger tumors (Fig. 3, panel 1). Although CD8+ T cell frequency showed a modest inverse correlation with tumor size, this association was not statistically significant (Fig. 3, panel 2).
Table 2.
Disease parameters and lymphocyte subset frequency for 10 PTC patients with TAL
| Patient | Gender | Age (yr) | Stage | Tumor size (cm) | Invasion | LN metastases | LI grade | CD20:CD3 | % CD4/CD3 | % CD8/CD3 | % FoxP3/CD4 | CD8:FoxP3 | % CD25neg/FoxP3 | VEGF | TGFβ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 38 | T1, N0, M0 | 1 | − | 0/3 | 4 | 0.39 | 63.6 | 31.5 | 11.8 | 4.64 | 50.1 | + | + |
| 2 | F | 62 | T4a, N1, M0 | 1.6 | ++ | 1/38 (2.6%) | 4 | 1.78 | 69.4 | 21.1 | 13.6 | 1.78 | 55.3 | + | − |
| 3 | M | 42 | T1, N0, M0 | 2 | − | 0/12 | 4 | 0.20 | 78.7 | 19.0 | 13.6 | 1.77 | 85.7 | + | − |
| 4 | F | 35 | T3a, N1b, M0 | 2.5 | + | 13/27 (48%) | 5 | 0.68 | 82.7 | 12.8 | 16.6 | 1.06 | 66.7 | + | − |
| 5 | F | 29 | T1, N0, M0 | 1 | − | 0/1 | 4 | 0.03 | 57.6 | 38.6 | 20.3 | 3.24 | 45.9 | + | − |
| 6 | F | 80 | T1, N1, M0 | 1 | − | 2/3 (67%) | 5 | 0.07 | 62.7 | 31.6 | 24.0 | 2.06 | 76.4 | + | − |
| 7 | F | 31 | T1, N1, M0 | 1.5 | − | 6/9 (67%) | 5 | 0.52 | 54.4 | 19.7 | 24.1 | 1.41 | 59.2 | + | + |
| 8 | M | 38 | T1, N1, M0 | 1.9 | − | 5/8 (63%) | 5 | 0.57 | 61.1 | 33.1 | 24.2 | 2.33 | 73.3 | + | + |
| 9 | M | 31 | T2, N1, M0 | 2.2 | − | 3/4 (75%) | 4 | 0.06 | 68.4 | 24.9 | 24.4 | 1.50 | 50.0 | + | − |
| 10 | M | 66 | T4a, N1, M0 | 6.5 | ++ | 6/8 (75%) | 5 | 1.09 | 80.1 | 22.6 | 35.9 | 0.97 | 80.3 | + | − |
LN metastases are displayed as the number of pathologically involved nodes/total number of nodes examined. −, No invasion; +, minimal extrathyroidal invasion; ++, extensive extrathyroidal invasion; F, female; M, male.
Figure 2.
Lymphocyte subset analysis in PTC patients with TAL. Archived thyroid tissues from 10 PTC patients with evidence of TAL were stained for key lymphocyte markers using standard immunohistofluorescence techniques. A–C, Both peritumoral (A) and intratumoral (B and C) lymphocyte aggregates are shown at ×10 magnification; D, lymphocyte aggregates were imaged at ×60 for quantification; E, lymphocytes were also found as single cells or multicellular clusters within the tumor. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride (blue). The nuclei of PTC cells are characterized by their grooved appearance and were easily distinguished from infiltrating lymphocytes (E). CD25+ and CD25−CD4+FoxP3+ Tregs were identified within lymphoid aggregates (LA) and within the tumor (PTC, F). Arrows designate thyroid tumor cells (A–D) and CD25− Tregs (F). Nonspecific, noncellular staining was due to antibody interactions with residual follicular colloid proteins.
Figure 3.
Correlation analysis of subset frequency and disease severity. Lymphocyte aggregates in 10 PTC samples were imaged at ×60 magnification. CD4+, CD8+, and CD4+FoxP3+ subsets were quantified, and relative frequencies were analyzed for correlation with tumor size and LN metastases. Spearman correlation coefficient (r) and P values are shown. The correlation between CD8 to Treg ratio and tumor size remains statistically significant when patient 10 is omitted (r = −0.729; P = 0.031).
FoxP3+ Treg cell frequency in PTC
As described above, CD4+ T lymphocytes constituted the majority of tumor-associated T cells in PTC patients and were associated with larger tumors. To further define the types of CD4+ T cells present in PTC, we analyzed the same 10 patient samples for the presence of CD4+FoxP3+ Tregs. Of interest, Tregs were found in all tumor samples, and their relative frequency was variable, ranging from 12–36% of CD4+ T cells (Table 2). Both CD25+ and CD25− Tregs were present in PTC, and CD25− cells constituted 50% or greater of CD4+FoxP3+ Tregs in nine of the 10 patients (Table 2). Correlation analysis revealed a strong association between percent Tregs/CD4 and LN metastasis (Fig. 3, panel 3). As shown in Table 2, patients 6–10 developed extensive LN metastases, and Tregs constituted greater than 20% of total CD4+ T cells. Patient 4 presented with LN metastases but only moderate levels of Tregs (17%). In contrast, patient 5 exhibited 20% Tregs and showed no evidence of LN metastases (Table 2). Direct comparison of Tregs and CD8+ T cell frequencies revealed that patient 4 had nearly equivalent frequencies of CD8+ T cells and Tregs, whereas CD8+ T cells outnumbered Tregs by 3-fold in patient 5 (Table 2). As shown in Fig. 3, a trend was observed between a low CD8 to Treg ratio and increased incidence of LN metastases, and the CD8 to Tregs ratio showed a strong inverse correlation with tumor size (panel 4).
Expression of VEGF and TGFβ by PTC
Numerous studies have documented the potent immunosuppressive capacity of tumors. Immune modulation can be achieved through a number of mechanisms, including the production of immune-suppressive cytokines. Previous studies have shown that papillary thyroid tumors are capable of expressing TGFβ and VEGF (30,31). Although TGFβ is important in driving Treg differentiation (24), VEGF acts indirectly to inhibit dendritic cell maturation leading to inefficient activation of naive T cells and Treg induction (32). To determine whether these cytokines contribute to the high frequency of Tregs in PTC, we analyzed the same 10 patient samples for cytokine expression via immunohistochemistry. As shown in Table 2, all tumors expressed high levels of VEGF, and three of the 10 samples expressed TGFβ. Cytokine expression did not correlate with FoxP3+ Treg frequency, as all tumors expressed VEGF, and Treg levels were variable among both TGFβ-positive and TGFβ-negative tumors (Table 2).
Discussion
TAL have been linked to disease severity in many types of cancer. We show, for the first time, that the presence of lymphocytes within and/or surrounding papillary thyroid tumors correlates with more severe disease. Our findings contrast previous correlations between LI and reduced invasion, reduced disease recurrence, and increased survival (3,8). These studies did not distinguish between TAL and background lymphocytic thyroiditis or relied on existing clinical pathology reports without independent review. Of note, we found no significant difference in disease stage between patients with or without LI when autoimmune and tumor-associated LI were grouped together. Thus, the milder phenotype of PTC patients with concurrent thyroiditis (i.e. reduced tumor size, invasion, and metastasis) mitigated the more aggressive phenotype of patients with TAL. Our analyses suggest, not surprisingly, that autoimmune and tumor-specific immune responses are distinct and should be assessed separately.
A detailed analysis of the immune cells responding to PTC revealed that lymphocyte subsets differentially predict disease severity in patients with PTC. CD4+ T cells constituted the majority of tumor-associated T cells and increased CD4+ T cell frequency correlated directly with tumor size. CD4+FoxP3+ Tregs were consistently present within lymphocytic aggregates, and their frequency showed the strongest correlation with disease severity. The accumulation of Tregs in PTC may explain the observed negative association between the presence of LI and disease severity. Tregs not only inhibit the ensuing tumor immune response but also encourage tumor progression through TGFβ-induced expression of potent angiogenic factors, such as VEGF, within the tumor environment (33,34). Thus, CD4+ T cell polarization likely plays an important role in immune regulation of PTC. We predict that PTC patients with more severe disease may generate a less productive antitumor CD4+ T cell response (i.e. Th2 and Treg), whereas patients capable of mounting a protective Th1 or Th17 response may exhibit less severe disease even in the presence of suppressive Tregs.
TAL were present in nearly one quarter of the patients in the current study. The immune response in the majority (83%) of these patients was extensive, with multiple, large lymphocytic aggregates within and/or surrounding the tumor. Despite this aggressive response, the tumor had successfully evaded destruction. Our studies identify Treg recruitment and induction as one potential mechanism of immune evasion. Although TGFβ and/or VEGF expression within the tumor did not correlate with CD25−FoxP3+ iTreg frequencies in our small patient subset, these cytokines likely contribute to an overall immune-suppressive environment that fosters FoxP3 expression and Treg induction. In line with this theory, although 14.8% of patients with no evident LI produced thyroid-specific antibodies, none of the patients with TAL expressed autoantibodies (Table 1). Thus, PTC-mediated immune suppression may extend to the humoral response. Additional studies are necessary to determine whether the tumor environment supports the generation of myeloid-derived suppressor cells and/or modulates dendritic cell maturation. Both CD68+ and CD11b+ macrophages have been identified in human thyroid cancers and RP3 murine tumors (35,36), and increasing frequencies of CD68+ macrophages were found in more aggressive dedifferentiated and anaplastic thyroid cancers (36). CD1a+ immature dendritic cells are also present in PTC; however, in contrast to macrophages, dendritic cell frequency was reduced in poorly differentiated and anaplastic thyroid cancers (37). Although the function of these cells has not yet been addressed, our data suggest that the tumor environment in PTC is highly immune suppressive, with multiple mechanisms at work.
Although Tregs have been studied in other endocrine-related malignancies, we are the first to identify these cells in thyroid tumors. In prostate cancer, Tregs are enriched in both peripheral blood and tumor-infiltrating lymphocytes; however, Th17 cell frequency may be a more useful predictive factor (17,38,39). Treg frequency is increased in ovarian and breast cancer patients, correlates with higher tumor grade, and may be a valuable tool for assessing disease prognosis (14,15,20). Of interest, recent studies revealed that accumulation of Tregs in the sentinel LN of breast cancer patients correlates with the presence of metastases within these nodes (40). It remains to be determined whether Treg frequency in regional LN will correspond with the presence of local metastases, distant metastases, or recurrence in thyroid cancer patients.
In summary, we show, for the first time, that Tregs are present in PTC and may promote more aggressive disease. Furthermore, the relative frequency of Tregs in comparison with total CD4+ T cells and CD8+ T cells may be a useful tool in predicting disease severity in PTC patients. Future prospective, longitudinal studies are necessary to determine the predictive value of Tregs in thyroid cancer. Ten to 30% of PTC patients develop recurrent disease, most commonly in regional LN (5). Although many PTC patients respond well to current treatment strategies, patients with recurrent LN metastases and those resistant to radioactive iodine therapy may benefit from immune-based therapies. Thyroid cancers provide an ample source of tumor-specific antigens that could be manipulated in tumor vaccine strategies; however, the ability of PTC to suppress and evade the immune response suggests that standard tumor vaccine strategies would not likely succeed. The presence of Tregs and other barriers to immune function must be considered when designing such therapies.
Footnotes
This work was supported by American Thyroid Association THANC Grant 2009, American Cancer Society IRG 57-001-50, and National Institutes of Health National Center for Research Resources Colorado CTSI Grant UL1 RR025780 (J.D.F.) and Mary Rossick Kern and Jerome H. Kern Endowment in Endocrine Neoplasms Research (B.R.H.).
Disclosure Summary: We have no conflicts of interest to report.
First Published Online March 5, 2010
Abbreviations: iTreg, inducible Treg; LI, lymphocytic infiltration; LN, lymph node; nTreg, natural Treg; PTC, papillary thyroid cancer; TAL, tumor-associated lymphocytes; Treg, regulatory T cell; Tregs, regulatory T cells; VEGF, vascular endothelial growth factor.
References
- Davies L, Welch HG 2006 Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167 [DOI] [PubMed] [Google Scholar]
- National Cancer Institute, SEER cancer statistics review 1975–2005. Bethesda, MD: National Cancer Institute [Google Scholar]
- Lundgren CI, Hall P, Dickman PW, Zedenius J 2006 Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer 106:524–531 [DOI] [PubMed] [Google Scholar]
- Hughes CJ, Shaha AR, Shah JP, Loree TR 1996 Impact of lymph node metastasis in differentiated carcinoma of the thyroid: a matched-pair analysis. Head Neck 18:127–132 [DOI] [PubMed] [Google Scholar]
- Mazzaferri EL, Jhiang SM 1994 Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 97:418–428 [DOI] [PubMed] [Google Scholar]
- Clark OH, Greenspan FS, Dunphy JE 1980 Hashimoto’s thyroiditis and thyroid cancer: indications for operation. Am J Surg 140:65–71 [DOI] [PubMed] [Google Scholar]
- Hirabayashi RN, Lindsay S 1965 The relation of thyroid carcinoma and chronic thyroiditis. Surg Gynecol Obstet 121:243–252 [PubMed] [Google Scholar]
- Matsubayashi S, Kawai K, Matsumoto Y, Mukuta T, Morita T, Hirai K, Matsuzuka F, Kakudoh K, Kuma K, Tamai H 1995 The correlation between papillary thyroid carcinoma and lymphocytic infiltration in the thyroid gland. J Clin Endocrinol Metab 80:3421–3424 [DOI] [PubMed] [Google Scholar]
- Gupta S, Patel A, Folstad A, Fenton C, Dinauer CA, Tuttle RM, Conran R, Francis GL 2001 Infiltration of differentiated thyroid carcinoma by proliferating lymphocytes is associated with improved disease-free survival for children and young adults. J Clin Endocrinol Metab 86:1346–1354 [DOI] [PubMed] [Google Scholar]
- Modi J, Patel A, Terrell R, Tuttle RM, Francis GL 2003 Papillary thyroid carcinomas from young adults and children contain a mixture of lymphocytes. J Clin Endocrinol Metab 88:4418–4425 [DOI] [PubMed] [Google Scholar]
- Zhu J, Paul WE 2008 CD4 T cells: fates, functions, and faults. Blood 112:1557–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W 2004 Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10:942–949 [DOI] [PubMed] [Google Scholar]
- Javia LR, Rosenberg SA 2003 CD4+CD25+ suppressor lymphocytes in the circulation of patients immunized against melanoma antigens. J Immunother 26:85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC 2002 Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 169:2756–2761 [DOI] [PubMed] [Google Scholar]
- Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller- Holzner E, Deibl M, Gastl G, Gunsilius E, Marth C 2005 The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res 11:8326–8331 [DOI] [PubMed] [Google Scholar]
- Jandus C, Bioley G, Speiser DE, Romero P 2008 Selective accumulation of differentiated FOXP3+ CD4+ T cells in metastatic tumor lesions from melanoma patients compared to peripheral blood. Cancer Immunol Immunother 57:1795–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AM, Lundberg K, Ozenci V, Banham AH, Hellström M, Egevad L, Pisa P 2006 CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol 177:7398–7405 [DOI] [PubMed] [Google Scholar]
- Alvaro T, Lejeune M, Salvadó MT, Bosch R, García JF, Jaén J, Banham AH, Roncador G, Montalbán C, Piris MA 2005 Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res 11:1467–1473 [DOI] [PubMed] [Google Scholar]
- Barnett B, Kryczek I, Cheng P, Zou W, Curiel TJ 2005 Regulatory T cells in ovarian cancer: biology and therapeutic potential. Am J Reprod Immunol 54:369–377 [DOI] [PubMed] [Google Scholar]
- Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH 2006 Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 24:5373–5380 [DOI] [PubMed] [Google Scholar]
- Carreras J, Lopez-Guillermo A, Fox BC, Colomo L, Martinez A, Roncador G, Montserrat E, Campo E, Banham AH 2006 High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood 108:2957–2964 [DOI] [PubMed] [Google Scholar]
- Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I, Olive D, Perez S, Pasqual N, Faure C, Ray-Coquard I, Puisieux A, Caux C, Blay JY, Ménétrier-Caux C 2009 Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res 69:2000–2009 [DOI] [PubMed] [Google Scholar]
- Bluestone JA, Abbas AK 2003 Natural versus adaptive regulatory T cells. Nat Rev Immunol 3:253–257 [DOI] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM 2003 Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med 198:1875–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigouroux S, Yvon E, Biagi E, Brenner MK 2004 Antigen-induced regulatory T cells. Blood 104:26–33 [DOI] [PubMed] [Google Scholar]
- Zhou G, Levitsky HI 2007 Natural regulatory T cells and de novo-induced regulatory T cells contribute independently to tumor-specific tolerance. J Immunol 178:2155–2162 [DOI] [PubMed] [Google Scholar]
- Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J 2005 Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest 115:3623–3633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenger YM, Wolchok JD 2008 The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun 8:1 [PMC free article] [PubMed] [Google Scholar]
- American Joint Committee on Cancer 2002 AJCC cancer staging handbook: TNM classification of malignant tumors. 6th ed. New York: Springer-Verlag [Google Scholar]
- Soh EY, Duh QY, Sobhi SA, Young DM, Epstein HD, Wong MG, Garcia YK, Min YD, Grossman RF, Siperstein AE, Clark OH 1997 Vascular endothelial growth factor expression is higher in differentiated thyroid cancer than in normal or benign thyroid. J Clin Endocrinol Metab 82:3741–3747 [DOI] [PubMed] [Google Scholar]
- Vasko V, Espinosa AV, Scouten W, He H, Auer H, Liyanarachchi S, Larin A, Savchenko V, Francis GL, de la Chapelle A, Saji M, Ringel MD 2007 Gene expression and functional evidence of epithelial-to-mesenchymal transition in papillary thyroid carcinoma invasion. Proc Natl Acad Sci USA 104:2803–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich D 2004 Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol 4:941–952 [DOI] [PubMed] [Google Scholar]
- Cao X 2009 Regulatory T cells and immune tolerance to tumors. Immunol Res 10.1007/s12026-009-8124-7 [DOI] [PubMed] [Google Scholar]
- Kaminska B, Wesolowska A, Danilkiewicz M 2005 TGFβ signalling and its role in tumour pathogenesis. Acta Biochim Pol 52:329–337 [PubMed] [Google Scholar]
- Russell JP, Engiles JB, Rothstein JL 2004 Proinflammatory mediators and genetic background in oncogene mediated tumor progression. J Immunol 172:4059–4067 [DOI] [PubMed] [Google Scholar]
- Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA 2008 Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer 15:1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugolini C, Basolo F, Proietti A, Vitti P, Elisei R, Miccoli P, Toniolo A 2007 Lymphocyte and immature dendritic cell infiltrates in differentiated, poorly differentiated, and undifferentiated thyroid carcinoma. Thyroid 17:389–393 [DOI] [PubMed] [Google Scholar]
- Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG 2008 Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res 14:3254–3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokokawa J, Cereda V, Remondo C, Gulley JL, Arlen PM, Schlom J, Tsang KY 2008 Enhanced functionality of CD4+CD25high FoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin Cancer Res 14:1032–1040 [DOI] [PubMed] [Google Scholar]
- Mansfield AS, Heikkila PS, Vaara AT, von Smitten KA, Vakkila JM, Leidenius MH 2009 Simultaneous Foxp3 and IDO expression is associated with sentinel lymph node metastases in breast cancer. BMC Cancer 9:231 [DOI] [PMC free article] [PubMed] [Google Scholar]