Abstract
Context: Previous studies have shown that mean 24-h GH concentrations determine plasma IGF-I levels in patients with acromegaly. However, we have recently shown that continuous GH infusion, mimicking the interpulse GH levels, was significantly more effective than the pulsatile GH administration at increasing IGF-I concentrations.
Objective: The aim of the study was to ascertain relative roles of total GH output (24-h mean), GH pulses, and interpulse GH level in determining plasma IGF-I concentrations.
Design and Setting: We conducted a point-in-time observational inpatient study in the General Clinical Research Center at the University of Michigan.
Patients or Other Participants: Eighteen patients with acromegaly and 19 healthy control subjects participated in the study.
Intervention(s): We performed frequent (every 10 or 20 min) blood sampling over 24 h.
Main Outcome Measure(s): Before data collection, we hypothesized that interpulse nadir levels of GH would correlate with IGF-I levels in normal and acromegalic subjects.
Results: Mean and valley levels of GH correlated with serum IGF-I levels (r2 = 0.44 and 0.48, respectively) in normal and acromegalic patients in a log-linear fashion. The strongest correlation, however, was observed between the log of nadir GH and IGF-I concentrations (r2 = 0.77). GH pulse mass did not significantly correlate with IGF-I (r2 = 0.001).
Conclusions: Plasma IGF-I concentrations correlated with mean 24-h GH concentrations. This relationship is dependent exclusively on the basal GH levels. GH pulses do not determine plasma IGF-I concentrations.
Using populations of healthy subjects and patients with acromegaly and normal overall GH output, it is the interpulse GH level that is specifically responsible for the ambient IGF-1 concentrations.
GH exerts a multitude of effects, including the stimulation of IGF-I production and lipolysis and the regulation of various parameters of hepatic function, cell growth, differentiation, and survival (1,2,3,4,5,6). GH is secreted by the anterior pituitary in a sexually dimorphic and pulsatile fashion with a serum half-life on the order of minutes (7,8,9,10). Studies have shown that downstream intracellular processes are affected by the kinetic pattern of GH delivery to the target tissue (11). For example, cytochrome P-450 (CYP) expression in rats is differentially influenced by gender-specific GH patterns (12,13,14). Most importantly, tissue IGF-I mRNA expression in rats and their subsequent somatic growth are also differentially influenced by the mode of GH administration (continuous vs. pulsatile) (15). In humans, pulsatile or continuous GH delivery differentially affects various parameters of GH action (1,2,16), with continuous GH administration being the preferential pattern for the induction of plasma IGF-I level and muscle IGF-I mRNA concentrations (16). However, it is still uncertain whether endogenous GH pulsatility (as opposed to exogenous GH administration) plays a role in determining the IGF-I milieu.
Acromegaly is a disorder characterized by elevated levels of IGF-I, whose production is stimulated by GH. Mean 24-h GH correlates with IGF-I levels in a log-linear fashion (17). Some patients, however, have normal mean 24-h GH levels and elevated IGF-I (18), suggesting that an additional layer of complexity such as hormone kinetics may serve an important role. Acromegalic patients typically have elevated tonic GH levels (19,20,21), whereas GH-deficient adults show decreased, albeit detectable nadir GH levels (22). Some studies suggested that both GH burst amplitude and the interpulse (nadir) levels play a role in determining plasma IGF-I, but the conclusions differed between irradiated and nonirradiated groups (21). The inclusion of patients with clearly elevated GH levels in other studies also complicated the analysis (19,20); because most of the GH was detected as a nonpulsatile component, both total and interpulse GH levels were elevated, and the differentiation between the relative role of the total GH output and the discrete parameters of GH pulsatility became difficult.
We have recently described a subset of acromegalic patients with normal overall GH levels yet with characteristically elevated IGF-I (18). These patients provided an ideal group for analysis because the total and discrete (pulsatile/nonpulsatile) GH levels were clearly separated and there was no intervening effect of radiotherapy. We analyzed frequent daylong GH profiles and respective IGF-I levels from normal subjects and from this subset of patients to examine kinetic characteristics of GH delivery to the periphery that promote IGF-I production. We hypothesized that interpulse GH concentrations would be a statistical determinant of IGF-I levels across both groups, in agreement with our earlier data on the high efficacy of continuous delivery of GH in normal individuals in inducing plasma and tissue IGF-I (16).
Patients and Methods
Patient data samples were retrospectively collected from prior research protocols approved by the University of Michigan Institutional Review Board and General Clinical Research Center (GCRC) Advisory Committee. Written informed consent was obtained from all subjects before their participation in protocol procedures.
Thirty-seven adults (26 males, 11 females) were studied. Their ages ranged from 20 to 74 yr. Eighteen “low GH” acromegalic patients (13 males, five females; average age, 47 ± 3.0 yr; range, 28–72) and 19 normal controls (13 males, six females; average age, 34.5 ± 4.0 yr; range, 20–74) were included in the analysis. The normal GH profiles were collected from healthy adults. All “low GH” acromegalics had a mean 24-h GH level below 4.2 μg/liter, i.e. below the highest mean 24-h GH (4.3 μg/liter) found by us previously in a group of normal adults using the same Nichols immunoluminometric assay GH assay (Nichols Institute Diagnostics, Nijmegen, The Netherlands) (18). Diagnosis of acromegaly was based on standard clinical and laboratory assessments confirmed by analysis of resected tumor tissue. Exclusion criteria for normal and acromegalic patients included the presence of diabetes, renal or hepatic impairment, or treatment with dopamine agonists, GH receptor antagonist, somatostatin analogs, raloxifene, estrogen, and any medication potentially affecting GH secretion within 3 months preceding the time of data collection.
Serum GH profiles and concurrent IGF-I levels were collected from all participants over 24 h in the University of Michigan GCRC. Blood sampling for GH was performed at a frequency of every 10 or 20 min, depending on the subject. Participants consumed a standard hospital diet consisting of three meals and a bedtime snack during sample collection.
Serum GH and IGF-I concentrations were measured by chemiluminescent assays (Nichols Institute Diagnostics, San Juan Capistrano, CA). All samples were assayed in duplicate. All GH and IGF-I measurements for each patient were performed in the same assay throughout the duration of the study. GH assay sensitivity in our experience was 0.01 μg/liter. Age-adjusted normal IGF-I values were referenced from a previously published large multicenter study by Brabant et al. (23) employing the same assay used in this study. IGF-I z-scores were calculated by reverse transformation (factor of 0.4) of our sample values and the reference values provided by Brabant et al. (23). Z-score calculation necessitated that age-specific sd reference values be averaged because the reference data existed in a slightly non-Gaussian distribution.
GH profile parameters were analyzed by the CLUSTER program (24) using a t-statistic of 2 and a cluster size of 2 × 2 for pulse recognition. Based upon the sensitivity of our assay, minimal pulse/peak amplitude was set at 0.03 μg/liter. Mean 24-h GH levels were obtained by averaging all values in corresponding profiles. Mean pulse amplitude was calculated from the values of the maximal concentrations attained within each pulse. Mean pulse mass/area was represented by the average area under the curve of a pulse between two flanking valleys in a profile. GH valley concentrations were defined as GH concentrations flanked by two CLUSTER-identified pulses. Mean nadirs/troughs were defined as the average of the lowest 5% of values from each serum profile (n = 7 for the every 10-min sampling; n = 4 for the every 20-min sampling) (21).
Statistical analysis was performed by Excel 2007 (Microsoft Corporation, Redmond, WA) and GraphPad software (GraphPad Software, Inc., La Jolla, CA). Data groups were compared with the Student’s unpaired t test where appropriate. P values <0.05 were considered significant. All values are shown as mean ± se. Relationships between profile parameters and IGF-I values were analyzed by regression analysis.
Results
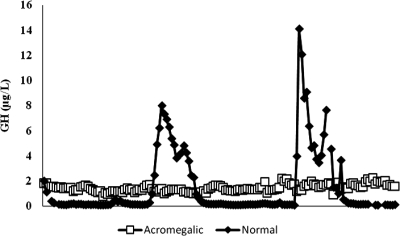
Eighteen acromegalic subjects (13 males, five females) had an average age of 47 ± 3.0 yr, and 19 normal subjects (13 males, six females) averaged 34.5 ± 4.0 yr of age. The acromegalic and normal patient groups had similar proportions of men and women, although the acromegalic patients had a higher average age. Representative profiles from a normal subject and an active acromegalic patient are shown in Fig. 1.
Figure 1.
Representative GH profiles from a normal subject (mean GH = 1.4 μg/liter; IGF-I = 322 μg/liter) and low GH acromegalic subject (mean GH = 1.4 μg/liter; IGF-I = 552 μg/liter).
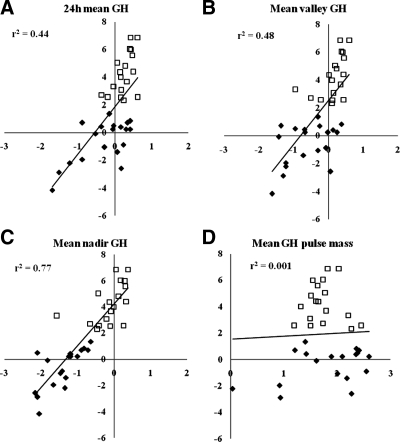
Figure 2A shows mean 24-h GH levels in the two groups and respective IGF-I z-scores. Daily mean GH was higher in acromegalic patients (2.0 ± 0.4 μg/liter) than normal individuals (1.0 ± 0.2 μg/liter; P = 0.003), but in the majority the values clearly overlapped. Plasma IGF-I levels were likewise higher in the acromegalic patients vs. normals (573 ± 49 and 193 ± 23 μg/liter, respectively; P < 0.0001). Mean GH did have a log-linear relationship with IGF-I z-scores across the entire spectrum of participants (r2 = 0.44). As expected, there was substantial overlap among GH levels between patients and normal subjects. Nearly half of the acromegalic subjects had mean GH levels less than 1.5 μg/liter.
Figure 2.
Log-linear correlations between GH and IGF-I. Acromegaly, □; normal controls, ♦. y-axis = IGF-I z-score; x-axis = Log GH (μg/liter), except for panel D where x-axis = Log GH (μg/liter × min). A, 24-h mean GH; B, mean valley GH; C, mean nadir GH; D, mean GH pulse mass.
Mean valley GH concentrations differed significantly between untreated acromegalics and the control subjects (1.7 ± 0.3 and 0.57 ± 0.1 μg/liter, respectively; P = 0.0001) (Fig. 2B). There was a strong log-linear correlation between this parameter and serum IGF-I z-scores (r2 = 0.48), but there was still some overlap between the two groups.
The relationship between profile nadirs and IGF-I is illustrated in Fig. 2C. GH nadir levels were markedly higher in acromegalic patients with virtually no overlap compared with normal subjects (1.0 ± 0.2 and 0.06 ± 0.01 μg/liter, respectively; P < 0.0001). There was a strong log-linear correlation between GH nadirs and IGF-I z-scores (r2 = 0.77). This pattern was maintained, albeit less robustly, when normal subjects and acromegalic patients were analyzed separately (r2 = 0.51 and 0.25, respectively).
In contrast, pulse mass did not correlate with subjects’ IGF-I z-scores (r2 = 0.001) (Fig. 2D). Mean pulse mass was lower in patients with active acromegaly than in normal subjects (74 ± 15 and 131 ± 28 μg/liter × min, respectively; P = 0.06). Mean pulse amplitudes were similar in acromegalic patients vs. normal subjects (2.8 ± 0.4 and 2.8 ± 0.5 μg/liter, respectively). Pulse amplitude did not appear to influence IGF-I z-scores (r2 = 0.11).
When males and females were analyzed separately, we obtained similar results overall. Lower participant numbers, particularly females (n = 11), did limit the strength of the statistical analysis. Twenty-four-hour mean GH did correlate with IGF-I in males (r2 = 0.52) but only weakly in females (r2 = 0.2). In males and females, GH nadirs had a strong log-linear relationship to IGF-I z-scores (r2 = 0.84 and 0.49, respectively). Mean valley GH showed a similar relationship in males (r2 = 0.55) but correlated weakly in females (r2 = 0.24). GH pulse mass/area did not correlate with IGF-I z-scores in males (r2 = 0.01) or females (r2 = 0.12).
Discussion
In this study, we showed that valley/nadir GH levels, but not GH secreted within pulses, strongly correlate with plasma IGF-I in a log-linear relationship. These data are fully consistent with the known effects of GH infusion in humans (16).
Serum hormone kinetics has increasingly been recognized as a significant factor that differentially promotes downstream effects in various target tissues. Kinetic- specific responses could potentially offer an additional method to regulate numerous hormone effects and represent one mechanism for gender-related variances. GH is secreted in a sexually dimorphic pattern. This has been observed in humans and other species (7,8,9,12,13,14,25,26,27). In humans, women tend to have more uniform pulses and a less pronounced nocturnal surge than men (7). Gender-specific GH patterns have been shown to affect processes such as cytochrome P-450 metabolism and apolipoprotein levels (12,13,14,28,29). Experiments have shown that intracellular pathways involved in GH signaling, such as signal transducer and activator of transcription 5 tyrosine phosphorylation, respond differently to distinct patterns of GH stimulation (11). Our paper expands on this concept of kinetic-related GH effects with regard to IGF-I.
GH is related to IGF-I levels in a log-linear fashion, yet elevated IGF-I is occasionally observed in patients with low GH levels (17,18). Previous investigations have arrived at disparate conclusions regarding GH kinetics and IGF-I. Data from acromegalic patient groups show a strong correlation between nonpulsatile GH secretion and IGF-I (19,20). Complementary in vitro experiments show that minimally elevated continuous levels of GH can increase IGF-I above normal values (1,16). Our data are congruent with these studies. The high valley/nadir levels of GH levels in acromegalic profiles, analogous to elevated tonic GH administration, strongly and selectively correlated with IGF-I concentrations.
Other studies have proposed that pulsatility is significant for IGF-I production. Hindmarsh et al. (30) used 83 normal elderly males and females. Their analysis concluded that IGF-I levels were related to peak GH concentrations, albeit weakly (r2 = 0.13 in males, r2 = 0.25 in females). On close observation, however, the female subjects had a higher mean peak GH than males, yet they also had lower average IGF-I levels. Scatterplots from the study appeared to show a wide variation of IGF-I levels for a given peak GH concentration. Peacey et al. (21) found a correlation between GH pulse amplitude and IGF-I only in previously irradiated patients with acromegaly, but not in those cured by surgery alone. Reutens et al. (22) reached a similar conclusion in a group composed of healthy and severely GH-deficient subjects, but the latter had both pulsatile and nonpulsatile GH components markedly reduced.
The limitations of these studies highlight particular strengths of our study population. The range of IGF-I values, when studied in groups of normal and/or GH-deficient individuals, is necessarily relatively small, spanning an approximately 100 μg/liter range in females and a 150 μg/liter range in males (30). Our study compared healthy young subjects and newly diagnosed, untreated acromegalic patients and looked at IGF-I concentrations spanning more than 1000 μg/liter. The inclusion of acromegalic patients with “normal” mean 24-h GH concentrations isolated the discrete parameters of GH pulsatility from the globally increased GH output. Finally, as opposed to some earlier studies (31), GH and IGF-I in this study were measured by the same assays, eliminating the confounding factor of interassay variability.
This choice of model provided an opportunity to isolate a sole kinetic variable that correlated with IGF-I. We show here that mean valley GH concentrations and, even stronger, true nadir GH concentrations are the only factors that correlate with IGF-I levels, whereas GH secreted within pulses is unlikely to be involved in IGF-I regulation. Our data suggest that a minimum mean nadir cutoff of 0.20 μg/liter or a minimum mean valley level above 0.68 μg/liter will likely result in an IGF-I z-score above 2, i.e. into the acromegalic range. The average mean nadir and mean valley GH among normal subjects were 0.06 ± 0.01 and 0.57 ± 0.15 μg/liter, respectively.
The relationship between mean nadir GH and IGF-I (and lack of correlation with pulsatile GH) remained present when subjects were analyzed separately according to gender or grouped into normal subjects and acromegalic patients. Lower subject numbers, particularly with females, did limit statistical interpretation of other variables, as noted in the Results. Additional study subjects could not be recruited, however, because our study’s assays are no longer commercially available. This limitation also impacted the study population. Specifically, normal subjects had a lower mean age than acromegalic patients, although the difference was less than 1 sd.
We did not perform secretion analysis on our data by methods such as deconvolution analysis or approximate entropy as was done in other studies (21,22). We sought to investigate peripheral tissue response to different circulating GH patterns. Ultimately, GH serum profiles are manifestations of specific secretion patterns that are separated by time and space from their origin in the pituitary. Tissue response is dictated by the temporal-spatial milieu of regulatory factors. Therapeutic endpoints of GH treatment might therefore be affected by the kinetics of administration. Analysis of serum hormone profiles and target tissue response potentially illuminates strategies for exogenous replacement and supplementation for specific therapeutic goals.
In sum, our results suggest that interpulse nadir levels of GH are the determining factor of IGF-I production in normal and acromegalic adults. In contrast to certain other effects of GH, pulses do not appear to have a significant correlation with IGF-I levels. Our data are fully consistent with previously published experiments using exogenous GH administration and observations in typical acromegalics (16,19,20). This study helps elucidate the mechanism(s) whereby acromegalic patients with low or normal mean 24-h GH levels can generate elevated IGF-I and GH-deficient patients may still produce detectable IGF-I (18,21,22). Our data reinforce observations that kinetic characteristics of GH delivery to the periphery have implications on downstream effects and may potentially have applications for exogenous GH replacement strategies.
Acknowledgments
We thank Kathy Symons for her technical assistance with the assays and the University of Michigan General Clinical Research Center staff for their nursing support.
Footnotes
This work was supported by National Institutes of Health Grant RO-1 DK071955 (to A.L.B.).
Disclosure Summary: The authors have nothing to declare.
First Published Online February 26, 2010
References
- Jaffe CA, Turgeon DK, Lown K, Demott-Friberg R, Watkins PB 2002 Growth hormone secretion pattern is an independent regulator of growth hormone actions in humans. Am J Physiol Endocrinol Metab 283:E1008–E1015 [DOI] [PubMed] [Google Scholar]
- Cersosimo E, Danou F, Persson M, Miles JM 1996 Effects of pulsatile delivery of basal growth hormone on lipolysis in humans. Am J Physiol 271:E123–E126 [DOI] [PubMed] [Google Scholar]
- Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Shalet SM, Vance ML, Stephens PA 2006 Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 91:1621–1634 [DOI] [PubMed] [Google Scholar]
- Liddle C, Goodwin BJ, George J, Tapner M, Farrell GC 1998 Separate and interactive regulation of cytochrome P450 3A4 by triiodothyronine, dexamethasone, and growth hormone in cultured hepatocytes. J Clin Endocrinol Metab 83:2411–2416 [DOI] [PubMed] [Google Scholar]
- Cheung NW, Liddle C, Coverdale S, Lou JC, Boyages SC 1996 Growth hormone treatment increases cytochrome P450-mediated antipyrine clearance in man. J Clin Endocrinol Metab 81:1999–2001 [DOI] [PubMed] [Google Scholar]
- Delafontaine P, Song YH, Li Y 2004 Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol 24:435–444 [DOI] [PubMed] [Google Scholar]
- Jaffe CA, Ocampo-Lim B, Guo W, Krueger K, Sugahara I, DeMott-Friberg R, Bermann M, Barkan AL 1998 Regulatory mechanisms of growth hormone secretion are sexually dimorphic. J Clin Invest 102:153–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD 1996 Gender differences in secretory activity of the human somatotropic (growth hormone) axis. Eur J Endocrinol 134:287–295 [DOI] [PubMed] [Google Scholar]
- Winer LM, Shaw MA, Baumann G 1990 Basal plasma growth hormone levels in man: new evidence for rhythmicity of growth hormone secretion. J Clin Endocrinol Metab 70:1678–1686 [DOI] [PubMed] [Google Scholar]
- Pincus SM 2000 Irregularity and asynchrony in biologic network signals. Methods Enzymol 321:149–182 [DOI] [PubMed] [Google Scholar]
- Gebert CA, Park SH, Waxman DJ 1997 Regulation of signal transducer and activator of transcription (STAT) 5b activation by the temporal pattern of growth hormone stimulation. Mol Endocrinol 11:400–414 [DOI] [PubMed] [Google Scholar]
- Choi HK, Waxman DJ 1999 Growth hormone, but not prolactin, maintains low-level activation of STAT5a and STAT5b in female rat liver. Endocrinology 140:5126–5135 [DOI] [PubMed] [Google Scholar]
- Waxman DJ, Pampori NA, Ram PA, Agrawal AK, Shapiro BH 1991 Interpulse interval in circulating growth hormone patterns regulates sexually dimorphic expression of hepatic cytochrome P450. Proc Natl Acad Sci USA 88:6868–6872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ, Ram PA, Pampori NA, Shapiro BH 1995 Growth hormone regulation of male-specific rat liver P450s 2A2 and 3A2: induction by intermittent growth hormone pulses in male but not female rats rendered growth hormone deficient by neonatal monosodium glutamate. Mol Pharmacol 48:790–797 [PubMed] [Google Scholar]
- Isgaard J, Carlsson L, Isaksson OG, Jansson JO 1988 Pulsatile intravenous growth hormone (GH) infusion to hypophysectomized rats increases insulin-like growth factor I messenger ribonucleic acid in skeletal tissues more effectively than continuous GH infusion. Endocrinology 123:2605–2610 [DOI] [PubMed] [Google Scholar]
- Surya S, Horowitz JF, Goldenberg N, Sakharova A, Harber M, Cornford AS, Symons K, Barkan AL 2009 The pattern of growth hormone delivery to peripheral tissues determines insulin-like growth factor-1 and lipolytic responses in obese subjects. J Clin Endocrinol Metab 94:2828–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly JM, Cotterill AM, Jemmott RC, Shears D, al-Othman S, Chard T, Wass JA 1991 Inter-relations between growth hormone, insulin, insulin-like growth factor-1 (IGF-1), IGF-binding protein-1 (IGFBP-1) and sex hormone-binding globulin in acromegaly. Clin Endocrinol (Oxf) 34:275–280 [DOI] [PubMed] [Google Scholar]
- Dimaraki EV, Jaffe CA, DeMott-Friberg R, Chandler WF, Barkan AL 2002 Acromegaly with apparently normal GH secretion: implications for diagnosis and follow-up. J Clin Endocrinol Metab 87:3537–3542 [DOI] [PubMed] [Google Scholar]
- Hartman ML, Veldhuis JD, Vance ML, Faria AC, Furlanetto RW, Thorner MO 1990 Somatotropin pulse frequency and basal concentrations are increased in acromegaly and are reduced by successful therapy. J Clin Endocrinol Metab 70:1375–1384 [DOI] [PubMed] [Google Scholar]
- Ho KY, Weissberger AJ 1994 Characterization of 24 hour growth hormone secretion in acromegaly: implications for diagnosis and therapy. Clin Endocrinol (Oxf) 41:75–83 [DOI] [PubMed] [Google Scholar]
- Peacey SR, Toogood AA, Veldhuis JD, Thorner MO, Shalet SM 2001 The relationship between 24-hour growth hormone secretion and insulin-like growth factor I in patients with successfully treated acromegaly: impact of surgery or radiotherapy. J Clin Endocrinol Metab 86:259–266 [DOI] [PubMed] [Google Scholar]
- Reutens AT, Veldhuis JD, Hoffman DM, Leung KC, Ho KK 1996 A highly sensitive growth hormone (GH) enzyme-linked immunosorbent assay uncovers increased contribution of a tonic mode of GH secretion in adults with organic GH deficiency. J Clin Endocrinol Metab 81:1591–1597 [DOI] [PubMed] [Google Scholar]
- Brabant G, von zur Mühlen A, Wüster C, Ranke MB, Kratzsch J, Kiess W, Ketelslegers JM, Wilhelmsen L, Hulthén L, Saller B, Mattsson A, Wilde J, Schemer R, Kann P 2003 Serum insulin-like growth factor I reference values for an automated chemiluminescence immunoassay system: results from a multicenter study. Horm Res 60:53–60 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Johnson ML 1986 Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol 250:E486–E493 [DOI] [PubMed] [Google Scholar]
- EdénS 1979 Age- and sex-related differences in episodic growth hormone secretion in the rat. Endocrinology 105:555–560 [DOI] [PubMed] [Google Scholar]
- Jansson JO, Ekberg S, Hoath SB, Beamer WG, Frohman LA 1988 Growth hormone enhances hepatic epidermal growth factor receptor concentration in mice. J Clin Invest 82:1871–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D, al-Shawi R, Bishop JO 1995 Sexual dimorphism and growth hormone induction of murine pheromone-binding proteins. J Mol Endocrinol 14:21–34 [DOI] [PubMed] [Google Scholar]
- Oscarsson J, Carlsson LM, Bick T, Lidell A, Olofsson SO, Edén S 1991 Evidence for the role of the secretory pattern of growth hormone in the regulation of serum concentrations of cholesterol and apolipoprotein E in rats. J Endocrinol 128:433–438 [DOI] [PubMed] [Google Scholar]
- Sjöberg A, Oscarsson J, Edén S, Olofsson SO 1994 Continuous but not intermittent administration of growth hormone to hypophysectomized rats increases apolipoprotein-E secretion from cultured hepatocytes. Endocrinology 134:790–798 [DOI] [PubMed] [Google Scholar]
- Hindmarsh PC, Dennison E, Pincus SM, Cooper C, Fall CH, Matthews DR, Pringle PJ, Brook CG 1999 A sexually dimorphic pattern of growth hormone secretion in the elderly. J Clin Endocrinol Metab 84:2679–2685 [DOI] [PubMed] [Google Scholar]
- Barkan AL, Beitins IZ, Kelch RP 1988 Plasma IGF-I/SmC in acromegaly: correlation with the degree of GH hypersecretion. J Clin Endocrinol Metab 67:69–73 [DOI] [PubMed] [Google Scholar]