Abstract
Context: Lipoprotein-associated phospholipase A2 (Lp-PLA2) is bound predominately to low-density lipoprotein and has been implicated as a risk factor for coronary artery disease (CAD).
Objective: We investigated the association between Lp-PLA2 and CAD in a biethnic African-American and Caucasian population.
Design: Lp-PLA2 mass, activity, and index, an integrated measure of mass and activity, and other cardiovascular risk factors were determined in 224 African-Americans and 336 Caucasians undergoing coronary angiography.
Main Outcome Measures: We assessed the distribution of Lp-PLA2 levels and determined the predictive role of Lp-PLA2 as a risk factor for CAD.
Results: Levels of Lp-PLA2 mass and activity were higher among Caucasians compared with African-Americans (293 ± 75 vs. 232 ± 76 ng/ml, P < 0.001 for mass and 173 ± 41 vs. 141 ± 39 nmol/min/ml, P < 0.001 for activity, respectively). However, Lp-PLA2 index was similar in the two groups (0.61 ± 0.17 vs. 0.64 ± 0.19, P = NS). In both ethnic groups, Lp-PLA2 activity and index was significantly higher among subjects with CAD. African-American subjects with CAD had significantly higher Lp-PLA2 index than corresponding Caucasian subjects (0.69 ± 0.20 vs. 0.63 ± 0.18, P = 0.028). In multivariate regression analyses, after adjusting for other risk factors, Lp-PLA2 index was independently (odds ratio 6.7, P = 0.047) associated with CAD in African-Americans but not Caucasians.
Conclusions: Lp-PLA2 activity and index was associated with presence of CAD among African-Americans and Caucasians undergoing coronary angiography. The findings suggest an independent impact of vascular inflammation among African-Americans as contributory to CAD risk and underscore the importance of Lp-PLA2 as a cardiovascular risk factor.
The importance of Lp-PLA2 as a cardiovascular risk factor and the role of vascular inflammation in coronary artery disease development are discussed.
Over the past decade, the role of inflammation in the pathogenesis of cardiovascular disease (CVD) has been well established (1). Inflammatory processes contribute significantly to the initiation, progression, and rupture of atherosclerotic plaques (1). Recently considerable attention has been paid to lipoprotein-associated phospholipase A2 (Lp-PLA2) as an emerging marker of cardiovascular risk (2,3,4,5,6,7,8). Lp-PLA2 is a highly specific marker for vascular inflammation (2,3,7) and appears to capture a different inflammatory burden than C-reactive protein. Recent observational studies carried out in both primary and secondary prevention settings found that elevated Lp-PLA2 levels are associated with an increased risk for CVD (9,10). Furthermore, Lp-PLA2 has been recommended as an adjunct to traditional risk assessment and as a diagnostic test for vascular inflammation to better identify patients at high risk (11,12).
Emerging data suggest that metabolic and inflammatory factors impacting on CVD risk differ across ethnic groups (13,14). The present study was undertaken to evaluate the association between Lp-PLA2 mass and activity with coronary artery disease (CAD) in African-Americans and Caucasians undergoing coronary angiography.
Subjects and Methods
Subjects
Subjects were recruited from a patient population scheduled for diagnostic coronary arteriography at either Harlem Hospital Center (New York, NY) or the Mary Imogene Bassett Hospital (Cooperstown, NY). The study design including exclusion and inclusion criteria has been described previously (15,16). Briefly, a total of 648 patients, self-identified as Caucasians (n = 344), African-American (n = 232) or other (n = 72) were enrolled. The present report is based on findings in 560 subjects (336 Caucasians, 224 African-Americans); 16 subjects were excluded due to incomplete data. The study was approved by the Institutional Review Boards at Harlem Hospital, the Mary Imogene Bassett Hospital, Columbia University College of Physicians and Surgeons, and University of California, Davis, and informed consent was obtained from all subjects.
Clinical and biochemical assessment
Blood pressure was measured with a random-zero mercury sphygmomanometer. Waist circumference was calculated as the average of two measurements taken after inspiration and expiration at the midpoint between the lowest rib and iliac crest. Participants were asked to fast for 12 h, and blood samples were drawn approximately 2–4 h before the catheterization procedure. Serum and plasma samples were separated and stored at −80 C before analysis. Concentrations of triglycerides (Sigma Diagnostics, St. Louis, MO), total and high-density lipoprotein (HDL) cholesterol, and glucose (Roche, Sommerville, NJ) were determined using standard enzymatic procedures (17,18). HDL cholesterol levels were measured after precipitation of apolipoproteins (apo) B-containing lipoproteins with dextran sulfate (19). ApoA-I and apoB were measured by rate immunonephelometry (Array; Beckman, Brea, CA) (20). Homeostasis model assessment-insulin resistance (HOMA-IR) was calculated using the updated model available from the Oxford Centre for Endocrinology and Diabetes (21). Lp-PLA2 mass was assayed using a microplate-based ELISA (diaDexus, Inc., South San Francisco, CA) (22). Lp-PLA2 activity was measured with a colorimetric activity method (diaDexus) (23,24). All assays were performed at a central laboratory at diaDexus, with laboratory personnel blinded to all clinical data. Lp-PLA2 index (nanomoles per minute per nanogram), an integrated measure of mass and activity, expressing enzymatic properties, was calculated as activity per mass (25).
Coronary angiography
The coronary angiograms were read by two experienced readers blinded to patient identity, the clinical diagnosis, and laboratory results. The readers recorded the location and extent of luminal narrowing for 15 segments of the major coronary arteries (26). In the present study, patients were classified as having CAD if a stenosis of 50% or greater was found in at least one of the segments. Patients without CAD were defined as having less than 50% stenosis in all of the segments. A composite cardiovascular score (0–75) was calculated based on determination of presence of stenosis on a scale of 0–5 of the 15 predetermined coronary artery segments.
Statistics
Analysis of data was done with SPSS statistical analysis software (SPSS Inc., Chicago, IL). Results were expressed as means ± sd. Levels of triglycerides, insulin, HOMA-IR, Lp-PLA2 mass and activity, and the cardiovascular score were logarithmically transformed to achieve normal distributions. Proportions were compared between groups using χ2 analysis and Fisher exact test where appropriate. Group means were compared using Student’s t test. One-way ANOVA was used to compare mean levels of independent variables across Lp-PLA2 mass, activity, and index tertile groups, and post hoc analyses were performed by Tukey’ honestly significant difference test. Univariate relationship between Lp-PLA2 mass, activity, and index and other anthropometric and biochemical variables were described by the Pearson correlation coefficients. Stepwise multiple logistic regression analysis was applied to predict the variables that independently and significantly contributed to the dependent variable, the presence of cardiovascular disease. All analyses were two tailed, and P < 0.05 was considered statistically significant.
Results
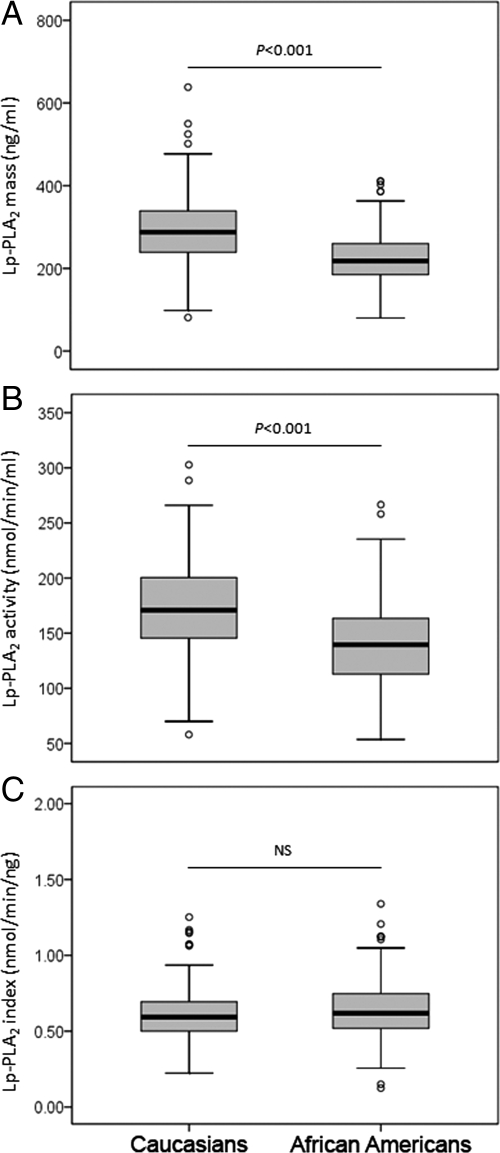
Compared with Caucasians, African-Americans were younger and less obese and had higher diastolic blood pressure (Supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). There was no difference in the levels of total and low-density lipoprotein (LDL) cholesterol, apoB, or composite cardiovascular score between the two ethnic groups. African-Americans had significantly higher levels of HDL cholesterol and apoA-I and lower levels of triglyceride, glucose, insulin, and HOMA-IR compared with Caucasians. The overall prevalence of smoking, alcohol consumption and postmenopausal women were 23 vs. 48%; 43 vs. 27% and 79 vs. 66% for Caucasians and African-Americans, respectively. The distribution of Lp-PLA2 mass and activity levels differed between Caucasians and African-Americans (Fig. 1). Compared with African-Americans, Caucasians had significantly higher median and mean levels of both Lp-PLA2 mass and activity. However, as seen in Fig. 1C, Lp-PLA2 index was similar in the two groups.
Figure 1.
Distribution of Lp-PLA2 mass (A), activity (B), and index (C) across ethnicity. Results were based on 336 Caucasians and 224 African-Americans. NS, Not significant. Data represent median and interquartile range. ○, Outliers. P values were calculated using Student’s t test analysis, and values for Lp-PLA2 mass and activity were logarithmically transformed before analysis. Nontransformed values are shown in the graphs.
The association of Lp-PLA2 mass, activity, and index with other cardiovascular risk factors across ethnicity is show in Table 1. Lp-PLA2 mass was positively associated with LDL cholesterol and triglyceride levels and the cardiovascular composite score among Caucasians but not among African-Americans. Among the latter group, Lp-PLA2 mass was negatively associated with glucose, insulin, and insulin resistance and positively with apoA-I levels. A somewhat different pattern was seen for Lp-PLA2 activity. Among Caucasians, Lp-PLA2 activity levels were significantly associated with lipoprotein levels, insulin resistance, apoB, apoA-I, and composite score. Among African-Americans, significant and strong associations were seen for lipoprotein levels, apoB, and composite score but not glucose and insulin resistance. For both ethnic groups, Lp-PLA2 index levels were significantly associated with all variables, except for glucose and insulin among Caucasians.
Table 1.
Correlations of Lp-PLA2 mass, activity, and index with other variables across ethnicity
| Lp-PLA2 mass
|
Lp-PLA2 activity
|
Lp-PLA2 index
|
||||
|---|---|---|---|---|---|---|
| r | P value | r | P value | r | P value | |
| Caucasians | ||||||
| Total cholesterol (mg/dl) | 0.083 | NS | 0.286 | <0.001 | 0.158 | 0.004 |
| LDL cholesterol (mg/dl) | 0.163 | 0.003 | 0.390 | <0.001 | 0.158 | 0.005 |
| HDL cholesterol (mg/dl) | 0.024 | NS | −0.358 | <0.001 | −0.343 | <0.001 |
| Triglyceride (mg/dl) | −0.145 | 0.008 | 0.127 | 0.021 | 0.266 | <0.001 |
| Glucose (mg/dl) | −0.093 | NS | 0.010 | NS | 0.091 | NS |
| Insulin (μU/ml) | 0.006 | NS | 0.120 | 0.031 | 0.108 | NS |
| HOMA-IR | −0.009 | NS | 0.144 | 0.010 | 0.144 | 0.010 |
| ApoA-I (mg/dl) | 0.068 | NS | −0.272 | <0.001 | −0.310 | <0.001 |
| ApoB (mg/dl) | 0.096 | NS | 0.428 | <0.001 | 0.285 | <0.001 |
| Composite score | 0.114 | 0.040 | 0.241 | <0.001 | 0.126 | 0.023 |
| African-Americans | ||||||
| Total cholesterol (mg/dl) | −0.042 | NS | 0.417 | <0.001 | 0.381 | <0.001 |
| LDL cholesterol (mg/dl) | −0.075 | NS | 0.488 | <0.001 | 0.456 | <0.001 |
| HDL cholesterol (mg/dl) | 0.137 | NS | −0.197 | 0.006 | −0.268 | <0.001 |
| Triglyceride (mg/dl) | −0.125 | NS | 0.164 | 0.022 | 0.264 | <0.001 |
| Glucose (mg/dl) | −0.221 | 0.002 | 0.036 | NS | 0.222 | 0.001 |
| Insulin (μU/ml) | −0.207 | 0.004 | 0.023 | NS | 0.183 | 0.006 |
| HOMA-IR | −0.207 | 0.004 | 0.025 | NS | 0.179 | 0.008 |
| ApoA-I (mg/dl) | 0.158 | 0.028 | −0.119 | NS | −0.241 | <0.001 |
| ApoB (mg/dl) | −0.135 | NS | 0.495 | <0.001 | 0.557 | <0.001 |
| Composite score | −0.107 | NS | 0.169 | 0.020 | 0.201 | 0.003 |
NS, Not significant.
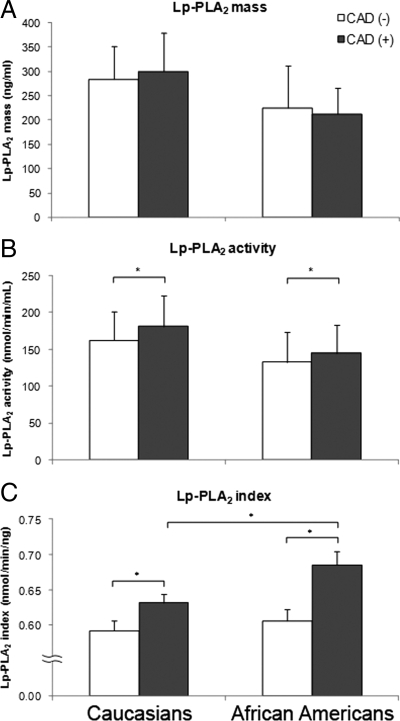
We next analyzed the correlation of Lp-PLA2 with the presence of CAD. Whereas we did not observe any association between Lp-PLA2 mass levels and CAD status in either ethnic group (Fig. 2A), subjects with CAD had significantly higher levels of Lp-PLA2 activity compared with those without CAD across ethnicity (Fig. 2B). Notably, as seen in Fig. 2C, Lp-PLA2 index was significantly increased among subjects with CAD among both African-Americans and Caucasians. Whereas the Lp-PLA2 index was similar in the two groups for subjects without CAD, African-American subjects with CAD had significantly higher Lp-PLA2 index than corresponding Caucasian subjects (0.69 ± 0.20 vs. 0.63 ± 0.18, P = 0.028). Furthermore, the difference in Lp-PLA2 index between subjects with or without CAD was 2-fold higher among African-Americans compared with Caucasians (0.08 ± 0.03 vs. 0.04 ± 0.02, respectively).
Figure 2.
Lp-PLA2 mass (A), activity (B), and index (C) levels in subjects with and without CAD across ethnicity. Data are means ± sd. *, P < 0.05. P values were calculated using Student’s t test analysis, and values for Lp-PLA2 mass and activity were logarithmically transformed before analysis. Nontransformed values are shown in the graphs. Results were based on n = 140 CAD(−), n = 187 CAD(+) Caucasians, and n = 119 CAD(−), n = 99 CAD(+) African-Americans.
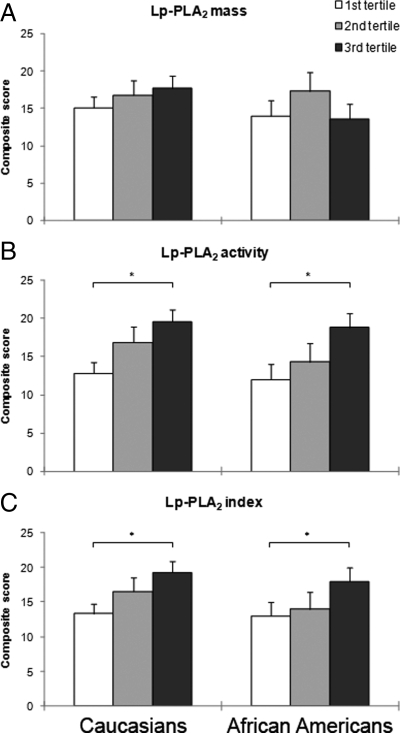
The association of Lp-PLA2 mass, activity, and index with the composite cardiovascular score, an integrated measure of the degree of stenosis, is shown in Fig. 3. No differences in composite score were seen among Caucasians and African-Americans across Lp-PLA2 mass tertiles (Fig. 3A). However, for both Caucasians and African-Americans, the composite score was significantly higher among subjects in the third tertile compared with those in the first tertile of Lp-PLA2 activity (Fig. 3B). Also for Lp-PLA2 index, the degree of stenosis was higher in the top tertile vs. the bottom tertile, irrespective of ethnicity.
Figure 3.
Cardiovascular composite score across Lp-PLA2 mass (A), activity (B), and index (C) tertiles in Caucasian and African-American subjects. Data are means ± sd. *, P < 0.05. P values were calculated using one-way ANOVA test, and post hoc analyses were performed by Tukey’ honestly significant difference test. Values for Lp-PLA2 mass and activity were logarithmically transformed before analysis. Nontransformed values are shown in the graphs.
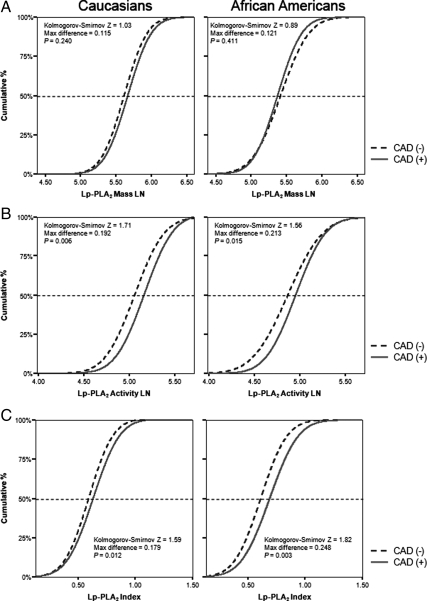
We next analyzed the cumulative distribution of Lp-PLA2 mass, activity, and index levels in subjects with and without CAD. As seen in Fig. 4A, there was no difference of the cumulative distribution of Lp-PLA2 mass between CAD and non-CAD subjects in both ethnic groups. Furthermore, differences in Lp-PLA2 activity levels between CAD and non-CAD subjects were similar for Caucasians and African-Americans (Fig. 4B). Thus, the maximum difference of the cumulative Lp-PLA2 activity distribution between CAD and non-CAD subjects in Caucasians and African-Americans were similar between two groups (maximum difference 0.19, P = 0.006 vs. maximum difference 0.21, P = 0.015). Similarly, Lp-PLA2 index levels were significantly higher in subjects with CAD compared with non-CAD subjects (Fig. 4C). However, as seen in Fig. 4C, the maximum difference of the cumulative Lp-PLA2 index distribution between CAD and non-CAD was substantially higher among African-American subjects (maximum difference 0.25, P = 0.003) compared with that in Caucasian subjects (maximum difference 0.18, P = 0.012), in agreement with the findings in Fig. 2.
Figure 4.
Cumulative distribution of Lp-PLA2 mass (A), activity (B), and index (C) in Caucasians and African-Americans with and without CAD.
We next tested whether the higher difference in Lp-PLA2 index between subjects with or without CAD among African-Americans compared with Caucasians might be confounded by a difference in the degree of stenosis, i.e. the composite cardiovascular score. Arguing against this hypothesis, we did not observe any differences in composite score between two ethnic groups among subjects with CAD, e.g. African-Americans with CAD had a similar degree of stenosis compared with Caucasians with CAD (28.6 ± 13.7 vs. 26.6 ± 13.3, P = NS).
Finally, we performed a stepwise multiple logistic regression analysis to identify variables that independently and significantly contributed to the presence of CAD. In univariate analysis, increased levels of Lp-PLA2 activity was associated with higher odds ratio for CAD in both Caucasians [odds ratio (OR) 5.29, P < 0.001] and African-Americans (OR 2.96, P = 0.028) (Table 2, model 1A). However, in a multivariate logistic regression model (model 2A) after adjusting for other risk factors, Lp-PLA2 activity was not independently associated with CAD.
Table 2.
Multiple stepwise logistic regression analysis relating Lp-PLA2 activity and index to the presence of CAD across ethnicity
| Models | Caucasians
|
African-Americans
|
||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| A. Lp-PLA2 activity | ||||
| Model 1A: Lp-PLA2 activity only | ||||
| Lp-PLA2 activity | 5.3 (2.15–13.0) | <0.001 | 2.9 (1.12–7.78) | 0.028 |
| Model 2A: other CAD risk factors | ||||
| Lp-PLA2 activity | 2.1 (0.59–7.35) | NS | 1.8 (0.50–6.50) | NS |
| LDL cholesterol | 1.0 (1.00–1.01) | NS | 1.0 (0.99–1.01) | NS |
| Gender | 3.0 (1.58–5.68) | <0.001 | 0.8 (0.43–1.55) | NS |
| Age | 1.1 (1.05–1.12) | <0.001 | 1.1 (1.05–1.14) | <0.001 |
| Hypertension | 0.4 (0.22–0.78) | 0.006 | 1.5 (0.72–3.23) | NS |
| BMI | 0.9 (0.93–1.03) | NS | 0.9 (0.91–1.01) | NS |
| Triglyceride | 2.0 (1.02–3.91) | 0.044 | 3.1 (1.37–6.81) | 0.006 |
| Smoking | 3.4 (1.62–7.26) | 0.001 | 1.4 (0.72–2.62) | NS |
| B. Lp-PLA2 index | ||||
| Model 1B: Lp-PLA2 index only | ||||
| Lp-PLA2 index | 1.5 (1.15–1.99) | 0.003 | 1.6 (1.13–2.21) | 0.007 |
| Model 2B: other CAD risk factors | ||||
| Lp-PLA2 index | 2.4 (0.39–14.2) | NS | 6.7 (1.02–44.5) | 0.047 |
| LDL cholesterol | 1.0 (1.01–1.02) | 0.019 | 1.0 (0.99–1.01) | NS |
| Gender | 3.2 (1.79–5.84) | <0.001 | 0.8 (0.42–1.53) | NS |
| Age | 1.1 (1.06–1.13) | <0.001 | 1.1 (1.05–1.14) | <0.001 |
| Hypertension | 2.0 (1.18–3.42) | 0.010 | 0.8 (0.41–1.69) | NS |
| BMI | 0.9 (0.94–1.04) | NS | 0.9 (0.91–1.01) | NS |
| Triglyceride | 1.9 (0.97–3.67) | NS | 2.6 (1.14–5.82) | 0.023 |
| Smoking | 2.9 (1.41–5.99) | 0.004 | 1.4 (0.77–2.79) | NS |
CI, Confidence interval; BMI, body mass index; NS, not significant.
We next performed similar logistic regression analyses for Lp-PLA2 index. In univariate analysis, increased index was significantly associated with higher OR for CAD in both ethnic groups (model 1B). In a multivariate model, the Lp-PLA2 index was independently (OR 6.7, P = 0.047) associated with CAD after adjustment for other risk factors in African-Americans (model 2B). We did not observe any independent association of Lp-PLA2 index with CAD among Caucasians.
Discussion
In the present study, we investigated the association between Lp-PLA2 and CAD in African-Americans and Caucasians undergoing coronary angiography. We report that Lp-PLA2 activity and index were associated with presence of CAD among African-Americans and Caucasians undergoing coronary angiography. We noted several important differences between African-Americans and Caucasians with respect to Lp-PLA2. First, among subjects with CAD, Lp-PLA2 activity levels were higher among African-Americans. Second, the difference in Lp-PLA2 activity levels between CAD and non-CAD subjects was higher among African-Americans. Furthermore, the Lp-PLA2 index, a measure of enzymatic properties, was independently associated with the extent of CAD among African-Americans. Our results underscore the importance of Lp-PLA2 as a cardiovascular risk factor and suggest that vascular inflammation may be of particular importance as risk factor among African-Americans.
Elevated Lp-PLA2 mass has been an independent predictor of CAD in some but not all studies (2,4,5,6,23,27). Beyond Lp-PLA2 mass, associations between Lp-PLA2 activity (8,23,27,28,29,30,31) and risk of CAD have been reported (Supplemental Table 2). In the prospective population-based Rotterdam study, Lp-PLA2 activity was an independent predictor of CHD and ischemic stroke (28). Iribarren et al. (27) studied the association between Lp-PLA2 (mass and activity) and coronary artery calcification in young black and white adults in the Coronary Artery Risk Development in Young Adults study. Although Lp-PLA2 mass and activity were significantly higher in cases than controls, the significant association between Lp-PLA2 activity and coronary artery calcification diminished after adjustment for lipids. The authors speculated that a stronger correlation between Lp-PLA2 enzymatic activity and LDL cholesterol (r = 0.52) than between enzymatic mass, and LDL cholesterol (r = 0.39) contributed to this attenuation. In agreement with Coronary Artery Risk Development in Young Adults (27) and other studies (4,8), we observed significantly higher Lp-PLA2 activity levels in subjects with CAD irrespective of ethnicity. Furthermore, in both Caucasians and African-Americans, the composite cardiovascular score was significantly higher among subjects in the third tertile of Lp-PLA2 activity compared with those in the first tertile. Because Lp-PLA2 activity levels were predictive of CAD for both ethnic groups, we performed multiple logistic regression analyses to determine whether Lp-PLA2 activity would independently contribute to the presence of CAD beyond traditional risk factors. In univariate analysis, we observed a significant association of the Lp-PLA2 activity levels with the presence of CAD in both Caucasians and African-Americans. However, when taking other risk factors into account, Lp-PLA2 activity levels were no longer associated with the presence of CAD.
We observed ethnic differences in Lp-PLA2 mass and activity because Caucasians had significantly higher levels of both compared with African-Americans. Although the majority of studies on Lp-PLA2 have been undertaken in Caucasian populations (2,4,6,28), studies among other race/ethnic groups have recently been reported (27,32,33,34). Notably, our data on Lp-PLA2 mass and activity in Caucasians and African-Americans are in agreement with the findings from the Dallas Heart Study (32). However, the use of Lp-PLA2 index allowed us to assess quantitative and qualitative properties of Lp-PLA2 with regard to both disease status and ethnicity. Our results indicate clear differences with regard to these parameters; Lp-PLA2 index was similar across ethnicity but differed across disease status. Thus, our results support the notion of an increased number of Lp-PLA2 molecules, i.e. increased molarity, among Caucasians compared with African-Americans and argue against any qualitative differences in Lp-PLA2 molecular properties across ethnicity. A second important observation was that Lp-PLA2 index was higher in subjects with CAD compared with subjects without CAD, irrespective of ethnicity. This finding suggests a qualitative impact on Lp-PLA2 properties as a consequence of CAD. Furthermore, the difference in Lp-PLA2 index between CAD and non-CAD subjects was higher in African-Americans compared with Caucasians. Importantly, the higher delta between CAD and non-CAD subjects for African-Americans was not simply due to a parallel differences in the degree of stenosis, i.e. cardiovascular score, compared with Caucasians. As Lp-PLA2 has been indicated as a marker of vascular inflammation, our results indicate that the degree of vascular inflammation in subjects with CAD may be more pronounced among African-Americans. It has been reported that the association and distribution of Lp-PLA2 activity and index with apoB-containing lipoproteins is differ across lipoprotein subfractions (25). Thus, the higher Lp-PLA2 index observed among African-Americans with CAD might be associated with a different lipoprotein distribution in these subjects.
We acknowledge some of the limitations of this study. Subjects in our study were recruited from patients scheduled for coronary angiography and are likely more typical of a high-risk patient group than the healthy population at large. Furthermore, we defined and assessed CAD on the basis of the presence of stenotic lesions. The clinical and laboratory parameters were in agreement with differences generally observed between healthy African-American and Caucasian populations from other studies. Furthermore, the cross-sectional study design does not allow us to evaluate whether Lp-PLA2 is causative in the development of CAD. Although our findings support the notion of vascular inflammation as contributory to CAD risk, additional studies are needed to verify these results in other populations.
In conclusion, Lp-PLA2 activity and index was associated with presence of CAD among African-Americans and Caucasians undergoing coronary angiography. In the former group, Lp-PLA2 index was an independent predictor of CAD. The results underscore the importance of Lp-PLA2 as a cardiovascular risk factor and the role of vascular inflammation in coronary artery disease development.
Supplementary Material
Acknowledgments
We thank diaDexus, Inc. for assistance with Lp-PLA2 measurements.
Footnotes
This work was supported by Grants 49735 (to T.A.P., principal investigator) and 62705 (to L.B., principal investigator) from the National Heart, Lung, and Blood Institute. This work was supported in part by the University of California, Davis, Clinical and Translational Science Center (RR 024146). E.A. is a recipient of an American Heart Association Postdoctoral Fellowship (0725125Y).
Disclosure Summary: E.A., Z.O., and B.E. have nothing to disclose. T.A.P. received consulting fees from Bayer, Cardex, Coca-Cola, Forbes Medi-Tech, and Merck and lecture fees from Abbott, Bayer, KOS Pharmaceuticals, Merck, and Merck/Schering Plough. L.B. received consulting fees from Merck, Novartis, and Merck/Schering Plough and has equity interest in Pfizer.
First Published Online March 1, 2010
Abbreviations: apo, Apolipoprotein; CAD, coronary artery disease; CVD, cardiovascular disease; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment-insulin resistance; LDL, low-density lipoprotein; Lp-PLA2, lipoprotein-associated phospholipase A2; OR, odds ratio.
References
- Ross R 1999 Atherosclerosis—an inflammatory disease. N Engl J Med 340:115–126 [DOI] [PubMed] [Google Scholar]
- Packard CJ, O'Reilly DS, Caslake MJ, McMahon AD, Ford I, Cooney J, Macphee CH, Suckling KE, Krishna M, Wilkinson FE, Rumley A, Lowe GD 2000 Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. N Engl J Med 343:1148–1155 [DOI] [PubMed] [Google Scholar]
- Caslake MJ, Packard CJ 2003 Lipoprotein-associated phospholipase A2 (platelet-activating factor acetylhydrolase) and cardiovascular disease. Curr Opin Lipidol 14:347–352 [DOI] [PubMed] [Google Scholar]
- Koenig W, Khuseyinova N, Löwel H, Trischler G, Meisinger C 2004 Lipoprotein-associated phospholipase A2 adds to risk prediction of incident coronary events by C-reactive protein in apparently healthy middle-aged men from the general population: results from the 14-year follow-up of a large cohort from southern Germany. Circulation 110:1903–1908 [DOI] [PubMed] [Google Scholar]
- Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, Sharrett AR 2004 Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation 109:837–842 [DOI] [PubMed] [Google Scholar]
- Blake GJ, Dada N, Fox JC, Manson JE, Ridker PM 2001 A prospective evaluation of lipoprotein-associated phospholipase A(2) levels and the risk of future cardiovascular events in women. J Am Coll Cardiol 38:1302–1306 [DOI] [PubMed] [Google Scholar]
- Lavi S, McConnell JP, Rihal CS, Prasad A, Mathew V, Lerman LO, Lerman A 2007 Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation: association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation 115:2715–2721 [DOI] [PubMed] [Google Scholar]
- Blankenberg S, Stengel D, Rupprecht HJ, Bickel C, Meyer J, Cambien F, Tiret L, Ninio E 2003 Plasma PAF-acetylhydrolase in patients with coronary artery disease: results of a cross-sectional analysis. J Lipid Res 44:1381–1386 [DOI] [PubMed] [Google Scholar]
- Ballantyne C, Cushman M, Psaty B, Furberg C, Khaw KT, Sandhu M, Oldgren J, Rossi GP, Maiolino G, Cesari M, Lenzini L, James SK, Rimm E, Collins R, Anderson J, Koenig W, Brenner H, Rothenbacher D, Berglund G, Persson M, Berger P, Brilakis E, McConnell JP, Koenig W, Sacco R, et al. 2007 Collaborative meta-analysis of individual participant data from observational studies of Lp-PLA2 and cardiovascular diseases. Eur J Cardiovasc Prev Rehabil 14:3–11 [DOI] [PubMed] [Google Scholar]
- Garza CA, Montori VM, McConnell JP, Somers VK, Kullo IJ, Lopez-Jimenez F 2007 Association between lipoprotein-associated phospholipase A2 and cardiovascular disease: a systematic review. Mayo Clin Proc 82:159–165 [DOI] [PubMed] [Google Scholar]
- Davidson MH, Corson MA, Alberts MJ, Anderson JL, Gorelick PB, Jones PH, Lerman A, McConnell JP, Weintraub HS 2008 Consensus panel recommendation for incorporating lipoprotein-associated phospholipase A2 testing into cardiovascular disease risk assessment guidelines. Am J Cardiol 101:51F–57F [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon 3rd RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith Jr SC, Taubert K, Tracy RP, Vinicor F 2003 Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107:499–511 [DOI] [PubMed] [Google Scholar]
- 2002 Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106:3143–3421 [PubMed] [Google Scholar]
- Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L 2004 Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 364:937–952 [DOI] [PubMed] [Google Scholar]
- Paultre F, Pearson TA, Weil HF, Tuck CH, Myerson M, Rubin J, Francis CK, Marx HF, Philbin EF, Reed RG, Berglund L 2000 High levels of Lp (a) with a small apo (a) isoform are associated with coronary artery disease in African American and white men. Arterioscler Thromb Vasc Biol 20:2619–2624 [DOI] [PubMed] [Google Scholar]
- Anuurad E, Rubin J, Lu G, Pearson TA, Holleran S, Ramakrishnan R, Berglund L 2006 Protective effect of apolipoprotein E2 on coronary artery disease in African Americans is mediated through lipoprotein cholesterol. J Lipid Res 47:2475–2481 [DOI] [PubMed] [Google Scholar]
- McGowan MW, Artiss JD, Strandbergh DR, Zak B 1983 A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin Chem 29:538–542 [PubMed] [Google Scholar]
- Allain CC, Poon LS, Chan CS, Richmond W, Fu PC 1974 Enzymatic determination of total serum cholesterol. Clin Chem 20:470–475 [PubMed] [Google Scholar]
- Warnick GR, Benderson J, Albers JJ 1982 Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem 28:1379–1388 [PubMed] [Google Scholar]
- Maciejko JJ, Levinson SS, Markyvech L, Smith MP, Blevins RD 1987 New assay of apolipoproteins A-I and B by rate nephelometry evaluated. Clin Chem 33:2065–2069 [PubMed] [Google Scholar]
- Adler AI, Levy JC, Matthews DR, Stratton IM, Hines G, Holman RR 2005 Insulin sensitivity at diagnosis of type 2 diabetes is not associated with subsequent cardiovascular disease (UKPDS 67). Diabet Med 22:306–311 [DOI] [PubMed] [Google Scholar]
- Dada N, Kim NW, Wolfert RL 2002 Lp-PLA2: an emerging biomarker of coronary heart disease. Expert Rev Mol Diagn 2:17–22 [DOI] [PubMed] [Google Scholar]
- Koenig W, Twardella D, Brenner H, Rothenbacher D 2006 Lipoprotein-associated phospholipase A2 predicts future cardiovascular events in patients with coronary heart disease independently of traditional risk factors, markers of inflammation, renal function, and hemodynamic stress. Arterioscler Thromb Vasc Biol 26:1586–1593 [DOI] [PubMed] [Google Scholar]
- Kosaka T, Yamaguchi M, Soda Y, Kishimoto T, Tago A, Toyosato M, Mizuno K 2000 Spectrophotometric assay for serum platelet-activating factor acetylhydrolase activity. Clin Chim Acta 296:151–161 [DOI] [PubMed] [Google Scholar]
- Saougos VG, Tambaki AP, Kalogirou M, Kostapanos M, Gazi IF, Wolfert RL, Elisaf M, Tselepis AD 2007 Differential effect of hypolipidemic drugs on lipoprotein-associated phospholipase A2. Arterioscler Thromb Vasc Biol 27:2236–2243 [DOI] [PubMed] [Google Scholar]
- Miller M, Mead LA, Kwiterovich Jr PO, Pearson TA 1990 Dyslipidemias with desirable plasma total cholesterol levels and angiographically demonstrated coronary artery disease. Am J Cardiol 65:1–5 [DOI] [PubMed] [Google Scholar]
- Iribarren C, Gross MD, Darbinian JA, Jacobs Jr DR, Sidney S, Loria CM 2005 Association of lipoprotein-associated phospholipase A2 mass and activity with calcified coronary plaque in young adults: the CARDIA study. Arterioscler Thromb Vasc Biol 25:216–221 [DOI] [PubMed] [Google Scholar]
- Oei HH, van der Meer IM, Hofman A, Koudstaal PJ, Stijnen T, Breteler MM, Witteman JC 2005 Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam Study. Circulation 111:570–575 [DOI] [PubMed] [Google Scholar]
- Winkler K, Hoffmann MM, Winkelmann BR, Friedrich I, Schäfer G, Seelhorst U, Wellnitz B, Wieland H, Boehm BO, März W 2007 Lipoprotein-associated phospholipase A2 predicts 5-year cardiac mortality independently of established risk factors and adds prognostic information in patients with low and medium high-sensitivity C-reactive protein (the Ludwigshafen Risk and Cardiovascular Health Study). Clin Chem 53:1440–1447 [DOI] [PubMed] [Google Scholar]
- Furberg CD, Nelson JJ, Solomon C, Cushman M, Jenny NS, Psaty BM 2008 Distribution and correlates of lipoprotein-associated phospholipase A2 in an elderly cohort: the Cardiovascular Health Study. J Am Geriatr Soc 56:792–799 [DOI] [PubMed] [Google Scholar]
- Tsimikas S, Willeit J, Knoflach M, Mayr M, Egger G, Notdurfter M, Witztum JL, Wiedermann CJ, Xu Q, Kiechl S 2009 Lipoprotein-associated phospholipase A2 activity, ferritin levels, metabolic syndrome, and 10-year cardiovascular and non-cardiovascular mortality: results from the Bruneck study. Eur Heart J 30:107–115 [DOI] [PubMed] [Google Scholar]
- Brilakis ES, Khera A, McGuire DK, See R, Banerjee S, Murphy SA, de Lemos JA 2008 Influence of race and sex on lipoprotein-associated phospholipase A2 levels: observations from the Dallas Heart Study. Atherosclerosis 199:110–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Yoshida H, Ichihara S, Imaizumi T, Satoh K, Yokota M 2000 Correlations between plasma platelet-activating factor acetylhydrolase (PAF-AH) activity and PAF-AH genotype, age, and atherosclerosis in a Japanese population. Atherosclerosis 150:209–216 [DOI] [PubMed] [Google Scholar]
- El-Saed A, Sekikawa A, Zaky RW, Kadowaki T, Takamiya T, Okamura T, Edmundowicz D, Kita Y, Kuller LH, Ueshima H 2007 Association of lipoprotein-associated phospholipase A2 with coronary calcification among American and Japanese men. J Epidemiol 17:179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.