Abstract
Context: Kisspeptin, encoded by the KISS1 gene, is a key stimulatory factor of GnRH secretion and puberty onset. Inactivating mutations of its receptor (KISS1R) cause isolated hypogonadotropic hypogonadism (IHH). A unique KISS1R-activating mutation was described in central precocious puberty (CPP).
Objective: Our objective was to investigate KISS1 mutations in patients with idiopathic CPP and normosmic IHH.
Patients: Eighty-three children with CPP (77 girls) and 61 patients with IHH (40 men) were studied. The control group consisted of 200 individuals with normal pubertal development.
Methods: The promoter region and the three exons of KISS1 were amplified and sequenced. Cells expressing KISS1R were stimulated with synthetic human wild-type or mutant kisspeptin-54 (kp54), and inositol phosphate accumulation was measured. In a second set of experiments, kp54 was preincubated in human serum before stimulation of the cells.
Results: Two novel KISS1 missense mutations, p.P74S and p.H90D, were identified in three unrelated children with idiopathic CPP. Both mutations were absent in 400 control alleles. The p.P74S mutation was identified in the heterozygous state in a boy who developed CPP at 1 yr of age. The p.H90D mutation was identified in the homozygous state in two unrelated girls with CPP. In vitro studies revealed that the capacity of the P74S and H90D mutants to stimulate IP production was similar to the wild type. After preincubation of wild-type and mutant kp54 in human serum, the capacity to stimulate signal transduction was significantly greater for P74S compared with the wild type, suggesting that the p.P74S variant is more stable. Only polymorphisms were found in the IHH group.
Conclusion: Two KISS1 mutations were identified in unrelated patients with idiopathic CPP. The p.P74S variant was associated with higher kisspeptin resistance to degradation in comparison with the wild type, suggesting a role for this mutation in the precocious puberty phenotype.
A mutation in the KISS1 gene (P74S), identified in a boy with central precocious puberty, results in higher kisspeptin resistance to degradation in vitro.
Human puberty is triggered by the activation of the hypothalamic-pituitary-gonadal axis. The resurgence of pulsatile GnRH release at the time of puberty is controlled by a complex network of regulatory signals (1).
A number of genes have been associated with isolated hypogonadotropic hypogonadism (IHH), whereas central precocious puberty (CPP) is rarely related to genetic defects (2,3,4,5). CPP has remarkable female gender predominance, and the vast majority of cases are idiopathic (1). Interestingly, a 27.5% prevalence of familial cases has been reported, suggesting a genetic origin (6,7). Segregation analysis suggested autosomal dominant transmission with incomplete, sex-dependent penetrance (6).
Kisspeptin, encoded by the KISS1 gene, is a key gatekeeper of human puberty (8). Loss-of-function mutations of the kisspeptin receptor (GPR54 or KISS1R) cause normosmic IHH (9,10). Recently, an activating mutation of KISS1R was identified in a girl with CPP, implicating the kisspeptin system in the pathogenesis of sexual precocity (5). Kisspeptin is a powerful stimulus for GnRH-induced gonadotropin secretion, and intermittent kisspeptin administration to immature animals was able to induce precocious activation of the gonadotropic axis and pubertal development (8).
Given the importance of kisspeptin in the regulation of puberty onset, alterations in the KISS1 gene might contribute to the pathogenesis of GnRH-dependent disorders. We investigated the presence of KISS1 mutations in patients with idiopathic CPP and normosmic IHH.
Patients and Methods
Patients
The protocol was approved by the Ethical Committee of Hospital das Clinicas. Written informed consent was obtained from all patients or parents. We studied 83 children with CPP (77 girls and six boys) and 61 individuals with normosmic IHH (40 males and 21 females). The control population consisted of 200 Brazilian adults with normal pubertal development.
All CPP patients initiated puberty before 8 yr in girls and 9 yr in boys and had pubertal basal and/or GnRH-stimulated LH levels, advanced bone age (Greulich and Pyle method), and normal central nervous system (CNS) magnetic resonance imaging (MRI). Pubertal onset ranged from 0.3–7.7 yr (mean, 5.2 ± 2 yr). Seventeen patients (15.3%) from 12 families had familial CPP.
All patients with IHH had partial or complete lack of pubertal development after 18 yr, prepubertal or low testosterone or estradiol levels for age, and low or normal basal gonadotropin levels but otherwise normal pituitary function, normal CNS MRI scans, and normal olfactory tests (Smell Identification Test, Philadelphia, PA, or Alcohol Sniff Test, San Diego, CA). Ten patients (16.4%) had familial IHH (for hormone assay details, see the Supplemental Methods published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org).
Genetic analysis
Genomic DNA was extracted from peripheral leukocytes and the promoter region and the three exons of the KISS1 gene (GenBank accession no. NM 002256.3) were amplified by PCR (for details, see Supplemental Methods).
Studies of KISS1R signaling
Kisspeptin-54 (kp54) wild type (kp54-wt) and mutant (kp54-P74S and kp54-H90D) was chemically synthesized (Tufts University Peptide Synthesis Core Facility, Boston, MA). A stable KISS1R-expressing Chinese hamster ovary cell line (CHO-KISS1R) was stimulated with increasing concentrations of kp54-wt, kp54-P74S, or kp54-H90D for 2 h, and total inositol phosphate (IP) accumulation was measured.
In some experiments, kp54-wt, kp54-P74S, or kp54-H90D was incubated with 50% human serum (Atlanta Biologicals, Lawrenceville, GA) for 2 h at 37 C before CHO-KISS1R cell stimulation to assess the stability of the wild-type and mutant kp54 (for details, see Supplemental Methods).
Results
Genetic analysis
CPP
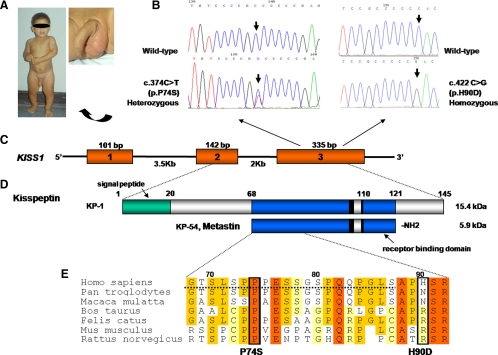
One boy with sporadic idiopathic CPP had a heterozygous c.C369T transition in exon 3 of the KISS1 gene, resulting in substitution of a proline by a serine at position 74 of kisspeptin-1 (1-145) (p.P74S) (Fig. 1).
Figure 1.
A, The propositus (P74S) at age 1.6 yr and detail of the genitalia showing enlarged penis and testes for age and Tanner stage III pubic hair. B, Nucleotide sequence of KISS1 gene showing the wild type and the two variants identified in patients with CPP. Both variants are located in exon 3 of the KISS1 gene, schematically represented in C. D, Precursor peptide kisspeptin-1 (KP-1) encoded by exons 2 and 3 of KISS1 and processed to generate the mature 54-amino-acid peptide, kp54 (KP-54) or metastin. The two variants are located in the amino-terminal portion of kp54, outside of the receptor-binding domain. E shows detailed kisspeptin amino acid sequence alignment among species, showing that proline in position 74 is highly conserved among mammals, whereas histidine in position 90 is not conserved. The p.P74S variant is within a PEST sequence [sequence rich in the aminoacids proline (P), glutamic acid (E), serine (S) and threonine (T)] (dotted line). The following were sequence sources: Homo sapiens (GenBank NP_002247.2), Pan troglodytes (GenBank XP_514123.1), Macaca mulatta (GenBank XP_001098284.1), Bos taurus (GenBank XP_872566.1), Felis catus (ENSFCAP00000013771) Rattus norvegicus (GenBank NP_859043.1), and Mus musculus (GenBank NP_839991.1).
The proband was referred at 17 months of age with a history of pubertal development since age 12 months. He was born at term after an uneventful pregnancy. He had no neurological symptoms, and exposure to sex steroids was denied. Physical examination showed penis size of 8.0 × 2.0 cm, testicular sizes of 2.3 × 1.5 cm (right) and 2.6 × 1.2 cm (left) with no palpable nodules or masses, and Tanner stage III pubic hair. His height was 85 cm (0.5 sd), with advanced bone age (3 yr). Hormonal evaluation revealed basal FSH level (RIA), of 8.3 U/liter, basal LH level (RIA) of 11.5 U/liter, LH after acute GnRH stimulation (RIA) of 47.2 U/liter, and basal testosterone level (RIA) of 600 ng/dl (normal prepubertal level, <30 ng/dl) (Supplemental Table 1). Androgen precursors and thyroid function were normal. CNS MRI was normal on two separate occasions. He was treated with a GnRH analog (depot leuprolide acetate, 3.75 mg/month) for 9 yr with adequate control. Treatment was withdrawn at a bone age of 12.5 yr, and the boy reached a final height of 165.6 cm (−1.4 sd) at a chronological age of 16 yr (target height, 165 cm). Long-term follow-up confirmed absence of any other associated clinical or neurological conditions. He was a single child, and only his mother and maternal grandmother, who had menarche at appropriate ages, were available for molecular studies. Both were carriers of the same variant in the heterozygous state.
A second variant (c.417 C→G) in exon 3 was identified in the homozygous state in two unrelated girls with sporadic CPP, leading to the substitution of a histidine by an aspartic acid at position 90 of kisspeptin-1 (p.H90D) (Fig. 1).
The affected girls had pubertal onset at 6 and 5.5 yr of age, respectively. Both had advanced bone age, pubertal levels of estradiol and basal LH, and a normal CNS MRI scan (Supplemental Table 1). They were treated with GnRH analog with adequate control. The parents denied consanguinity. The mother of the first case had menarche at age 10 yr and was heterozygous for the p.H90D variant, and her father was unavailable for molecular analysis. The parents of the second case were not studied.
Both mutations were located in the amino-terminal region of the mature 54-amino-acid peptide, kp54, and were absent in 400 control alleles.
Normosmic IHH
No mutation was identified in the coding region of KISS1 in the IHH group.
Single-nucleotide polymorphisms (SNPs)
Twelve SNPs within the promoter and coding regions of KISS1 were identified with similar frequencies in the studied groups (Supplemental Table 2). The g.−936delC and c.583-584insT were novel variants.
Functional analysis of p.P74S and p.H90D variants
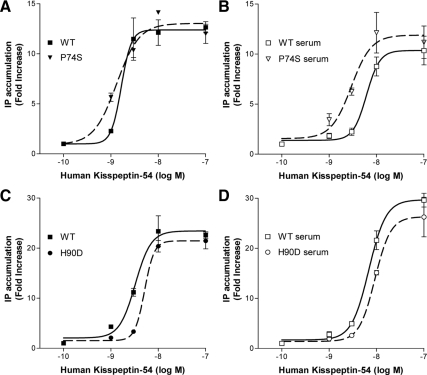
CHO-KISS1R cells were stimulated with increasing concentrations (10−10 to 10−7 m) of synthetic wild-type or mutant kp54 (kp54-wt or kp54-P74S) for 2 h. Maximal levels of IP accumulation were achieved with 10−7 m of both kp54-wt and kp54-P74S. There was no significant difference between kp54-wt and kp54-P74S in the dose-response curve, maximal response, or EC50 values (EC50 kp54-wt, 1.6 × 10−9 m; kp54-P74S, 1.3 × 10−9 m) (Fig. 2A).
Figure 2.
Stimulation of IP production by wild-type or mutant kisspeptin in CHO-KISS1R cells. A, Total IP accumulation was measured after stimulation for 2 h with 10−10 to 10−7 m wild-type human kp54 (WT) or mutant P74S kp54. B, The same concentrations of wild-type and mutant P74S kp54 were also preincubated in 50% human serum for 2 h before using them to stimulate CHO-KISS1R cells. C and D, The effects of wild-type and mutant H90D kp54 on total IP accumulation were similarly assessed without (C) or with (D) serum preincubation. Data points represent mean ± se for triplicate samples from representative experiments. Dose-response curves for both kisspeptins were done in the same experiments so that conditions were identical.
After incubation in 50% human serum for 2 h, the dose-response curves of both kp54-wt and kp54-P74S shifted rightward, suggesting a decrease in the potency of the kisspeptin (EC50 kp54-wt, 6.1 × 10−9 m; kp54-P74S, 2.9 × 10−9 m). The kp54-P74S curve had less of a rightward shift than kp54-wt, suggesting that the P74S variant may be more resistant to degradation in human serum (Fig. 2B).
Similar studies were performed to compare kp54-H90D and kp54-wt signaling. The kp54-H90D dose-response curve was similar to the kp54-wt curve, indicating a comparable response (EC50 kp54-wt, 3.1 × 10−9 m; kp54-H90D, 5.0 × 10−9 m) (Fig. 2C). Preincubation in human serum shifted the dose-response curves of both kisspeptins rightward to a similar extent, suggesting similar degradation rates (EC50 kp54-wt, 6.6 × 10−9 m; kp54-H90D, 9.0 × 10−9 m) (Fig. 2D).
Discussion
We identified a heterozygous KISS1 mutation, p.P74S, in a Brazilian boy with sporadic CPP. The absence of this variant in the control Brazilian population as well as in European and Asiatic populations suggests that the p.P74S is a rare variant (11,12).
In this boy, the very early initiation of precocious puberty in the absence of CNS lesions and high levels of basal LH and testosterone are suggestive of a genetic cause for CPP. The majority of boys with CPP, especially younger than 4 yr, have an underlying CNS abnormality, such as hypothalamic hamartomas (13,14). Comparative analysis of the amino acid sequence of kisspeptin showed that proline in position 74 is highly conserved among species (Fig. 1). Proline is a unique amino acid, in which the nitrogen atom is incorporated into a ring, causing a sharp transition in the direction of the polypeptide chain (15). Therefore, the loss of a proline may cause a major disruption of the protein structure (15,16). The p.P74S mutation is located in the amino-terminal region of the kisspeptin, within a PEST sequence, a region rich in the aminoacids proline (P), glutamic acid (E), serine (S) and threonine (T), associated with rapid degradation of proteins (16,17). Potential activating mechanisms of this mutation might involve increased protein bioavailability due to increased stability and decreased degradation.
Initial in vitro evaluation revealed no significant differences between kp54-wt and kp54-P74S capacity to stimulate KISS1R-mediated signaling, indicating that kp54-P74S does not affect either the binding affinity or the ability of the ligand to activate KISS1R signaling. However, after preincubation in human serum to more closely mimic in vivo conditions, the kp54-wt dose-response curve was shifted to the right to a greater extent than the kp54-P74S curve. These findings suggest that the p.P74S kisspeptin is more resistant to degradation. Taken together, these data support a potential role for the p.P74S mutation in the CPP phenotype.
In accordance with the functional analysis results, increased receptor affinity or signaling potency would be unlikely, because the p.P74S mutation is not located in the binding domain. Furthermore, increased receptor affinity could lead to receptor desensitization, because kisspeptin secretion is most likely pulsatile, and prolonged stimulation of the KISS1R may induce receptor desensitization (5,18). Conversely, an increased availability of bioactive kisspeptin could lead to higher amplitude pulses, augmenting receptor activation.
The proband’s mother and maternal grandmother were carriers of the same variant, although they had no history of precocious puberty, suggesting a sex-dependent phenotype and/or incomplete penetrance. Interestingly, in rodents, there is a notable sexual dimorphism in kisspeptin expression in the anteroventral periventricular nucleus. The number of neurons expressing kisspeptin in the anteroventral periventricular nucleus is over 10-fold higher in the female (19). Taking this into consideration, KISS1 mutations might lead to different degrees of functional alteration in the male and female hypothalamus.
The p.H90D variant was identified in the homozygous state in two unrelated girls who developed puberty before 6 yr of age. This variant changes a nonconserved amino acid in the amino-terminal region of kisspeptin-1. Although the p.H90D variant was not present in the Brazilian control population, it has been identified in the heterozygous state in a few normal individuals as well as in an IHH case in American and French populations, respectively, suggesting that it can be considered a rare SNP (20). The p.H90D variant did not show increased activity or resistance to degradation in vitro. Nevertheless, the importance of this kisspeptin variant in the homozygous state cannot be completely discarded in the precocious puberty phenotype.
In conclusion, we have identified a KISS1 mutation associated with male CPP. This mutation resulted in higher kisspeptin resistance to degradation in vitro, suggesting greater kisspeptin bioavailability and a potential role in the precocious puberty phenotype in this boy.
Supplementary Material
Footnotes
This work was supported by FAPESP Grants 05/55745-4 and 06/52583-6, by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Grants 300209/2008-8 (to A.C.L.) and 301339/2008-9 (to B.B.M.), by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH), through cooperative agreement U54 HD28138 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (to U.B.K.), and by the Harvard K12 HD051959 Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) Program supported by the NIH Office of Research in Women’s Health (S.D.C.B.) and NIH R21 HD059015 (S.D.C.B.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 17, 2010
Abbreviations: CNS, Central nervous system; CPP, central precocious puberty; IHH, isolated hypogonadotropic hypogonadism; IP, inositol phosphate; KISS1R, kisspeptin receptor; kp54, kisspeptin-54; kp54-wt, kp54 wild type; MRI, magnetic resonance imaging; SNP, single-nucleotide polymorphism.
References
- Palmert MR, Boepple PA 2001 Variation in the timing of puberty: clinical spectrum and genetic investigation. J Clin Endocrinol Metab 86:2364–2368 [DOI] [PubMed] [Google Scholar]
- Bianco SD, Kaiser UB 2009 The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat Rev Endocrinol 5:569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouligand J, Ghervan C, Tello JA, Brailly-Tabard S, Salenave S, Chanson P, Lombès M, Millar RP, Guiochon-Mantel A, Young J 2009 Isolated familial hypogonadotropic hypogonadism and a GNRH1 mutation. N Engl J Med 360:2742–2748 [DOI] [PubMed] [Google Scholar]
- Chan YM, de Guillebon A, Lang-Muritano M, Plummer L, Cerrato F, Tsiaras S, Gaspert A, Lavoie HB, Wu CH, Crowley Jr WF, Amory JK, Pitteloud N, Seminara SB 2009 GNRH1 mutations in patients with idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA 106:11703–11708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles MG, Bianco SD, Brito VN, Trarbach EB, Kuohung W, Xu S, Seminara SB, Mendonca BB, Kaiser UB, Latronico AC 2008 A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med 358:709–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries L, Kauschansky A, Shohat M, Phillip M 2004 Familial central precocious puberty suggests autosomal dominant inheritance. J Clin Endocrinol Metab 89:1794–1800 [DOI] [PubMed] [Google Scholar]
- Brito VN, Mendonca BB, Guilhoto LM, Freitas KC, Arnhold IJ, Latronico AC 2006 Allelic variants of the γ-aminobutyric acid-A receptor α1-subunit gene (GABRA1) are not associated with idiopathic gonadotropin-dependent precocious puberty in girls with and without electroencephalographic abnormalities. J Clin Endocrinol Metab 91:2432–2436 [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, García-Galiano D, Tena-Sempere M 2007 Neuroendocrine factors in the initiation of puberty: the emergent role of kisspeptin. Rev Endocr Metab Disord 8:11–20 [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E 2003 Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno Jr JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley Jr WF, Aparicio SA, Colledge WH 2003 The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- Semple RK, Achermann JC, Ellery J, Farooqi IS, Karet FE, Stanhope RG, O'Rahilly S, Aparicio SA 2005 Two novel missense mutations in G protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab 90:1849–1855 [DOI] [PubMed] [Google Scholar]
- Luan X, Zhou Y, Wang W, Yu H, Li P, Gan X, Wei D, Xiao J 2007 Association study of the polymorphisms in the KISS1 gene with central precocious puberty in Chinese girls. Eur J Endocrinol 157:113–118 [DOI] [PubMed] [Google Scholar]
- Pescovitz OH, Comite F, Hench K, Barnes K, McNemar A, Foster C, Kenigsberg D, Loriaux DL, Cutler Jr GB 1986 The NIH experience with precocious puberty: diagnostic subgroups and response to short-term luteinizing hormone releasing hormone analogue therapy. J Pediatr 108:47–54 [DOI] [PubMed] [Google Scholar]
- Partsch CJ, Heger S, Sippell WG 2002 Management and outcome of central precocious puberty. Clin Endocrinol (Oxf) 56:129–148 [DOI] [PubMed] [Google Scholar]
- MacArthur MW, Thornton JM 1991 Influence of proline residues on protein conformation. J Mol Biol 218:397–412 [DOI] [PubMed] [Google Scholar]
- Dice JF 1987 Molecular determinants of protein half-lives in eukaryotic cells. FASEB J 1:349–357 [DOI] [PubMed] [Google Scholar]
- Harms JF, Welch DR, Miele ME 2003 KISS1 metastasis suppression and emergent pathways. Clin Exp Metastasis 20:11–18 [DOI] [PubMed] [Google Scholar]
- Seminara SB, Dipietro MJ, Ramaswamy S, Crowley Jr WF, Plant TM 2006 Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology 147:2122–2126 [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE 2006 Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houang M, Roze C, Kaci FM, Yves LB, Carel JC, Leger J, de Roux N 2009 KiSS 1 polymorphisms analysis in a cohort of patients with disorder in the timing of pubertal onset shows association between 3′ UTR polymorphisms and central precocious puberty. Abstract in Hormone Research, Proceedings of the LWPES/ESPE 8th Joint Meeting, 72:49 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.