Abstract
Context: The human growth hormone/chorionic somatomammotropin (hGH/CSH) locus at 17q22-24, consisting of one pituitary-expressed postnatal (GH1) and four placenta-expressed genes (GH2, CSH1, CSH2, and CSHL1), is implicated in regulation of postnatal and intrauterine growth. A positive correlation has been reported between the offspring’s birth weight and serum placental GH (coded by GH2) and placental lactogen (coded by CSH1, CSH2) levels in pregnant women.
Objective: The objective of the study was the investigation of the hypothesis that the mRNA expression profile of placental hGH/CSH genes contributes to the determination of birth weight.
Design and Subjects: We developed a sensitive, fluorescent-labeled semiquantitative RT-PCR assay coupled with gene-specific restriction analysis, capable of distinguishing alternative splice-products of individual placental hGH/CSH genes and quantification of their relative expression levels. The detailed profile of alternative transcripts of GH2, CSH1, CSH2, and CSHL1 genes in placenta from uncomplicated term pregnancies of the REPROMETA sample collection was addressed in association with the birth weight of newborns, grouped as appropriate for gestational age (AGA; n = 23), small for gestational age (SGA; n = 15), and large for gestational age (LGA; n = 34).
Results: The majority of pregnancies with SGA newborn showed down-regulation of the entire hGH/CSH cluster in placenta, whereas in the case of LGA, the expression of CSH1-1, CSH2-1, and CSHL1-4 mRNA transcripts in placenta was significantly increased compared with AGA newborns (P < 0.0001, P = 0.009, P = 0.002, respectively).
Conclusion: The expression profile of placental hGH/CSH genes in placenta is altered in pregnancies accompanied by SGA and LGA compared with AGA newborns, and thus, it may directly affect the circulating fetal and maternal placental GH and placental lactogen levels.
The expression of human GH/CSH mRNA transcripts is significantly altered in placentas from pregnancies with small- and large-for-gestational-age babies compared to normal-sized babies.
The human (h) GH/CSH locus, located on chromosome 17q22-24 (1), is involved in regulating postnatal and intrauterine growth (2). It contains five highly homologous and structurally similar genes: a single ancestral GH1 gene for pituitary GH and four placenta-expressed paralogs (CSHL1, CSH1, GH2, CSH2) (Fig. 1A) (3,4). GH2 codes for placental GH (PGH), whereas the CSH1 and CSH2 genes encode jointly a single protein chorionic somatomammotropin (CSH), alternatively named placental lactogen (PL). CSHL1 was originally considered as a pseudogene (5), although evidence for its expression in placenta (6,7,8) has been reported. The roles of GH in child’s growth and adult intermediary metabolism are well known. The expression of GH2, CSH1, CSH2, and CSHL1 is coordinately induced during fetal development in the syncytiotrophoblasts of the placenta, and their products may play an essential role in directing fetal and maternal metabolism (2,9).
Figure 1.
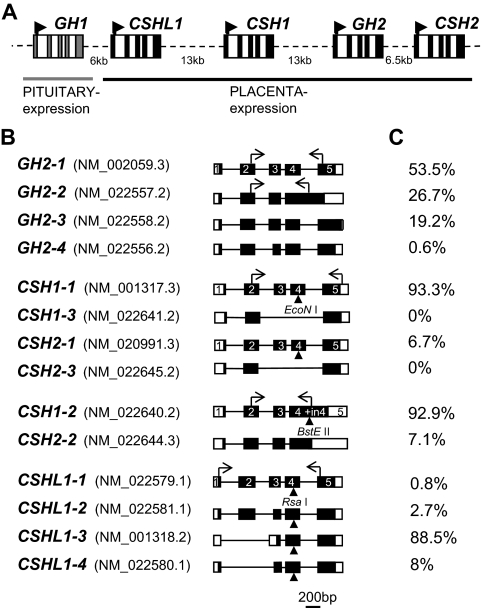
Organization of human GH/CSH genes in chromosome 17q22-24 and alternatively spliced transcripts of placental genes. A, Schematic representation of the hGH/CSH gene cluster. Rectangles represent each of the five genes (five exons highlighted by colored boxes), and dashed lines denote intergenic regions. The arrowheads indicate the start and direction of transcription. B, Alternatively spliced transcripts of GH2, CSH1, CSH2, and CSHL1 are drawn to approximate scale and are combined according to assay design into four groups. Exons are shown as boxes and introns as lines. The coding region is denoted by black-filled area. Exon numbers are marked according to the main transcripts of GH2 and CSH1. The horizontal arrows indicate the binding sites for the RT-PCR primers. The vertical arrowheads indicate the gene-specific restriction enzyme recognition sites for EcoNI (for CSH1 and CSH2), BstEII (for CSH1-2/CSH2-2), and RsaI (for CSHL1). The National Center for Biotechnology Information GenBank accession number for each alternative mRNA transcript is indicated in parentheses. C, The relative abundance of each gene alternative mRNA transcripts analyzed by semiquantitative RT-PCR and gene-specific restriction detected on an ABI 3130XL genetic analyzer (see examples on Fig. 2).
Despite structural similarity, the four placental hGH/CSH cluster genes exhibit substantial heterogeneity in their diversity patterns (10), expression levels and usage of alternative splicing pathways, giving rise to three or four protein isoforms (Fig. 1, A and B) (6). The predominant splicing pattern shared by GH2, CSH1, CSH2, and GH1 transcripts involves ligation of five common exons (Fig. 1B). These mRNAs encode secreted proteins of about 200 amino acids and with 85% or greater peptide sequence identity (6). The longest alternative mRNA transcripts of GH2, CSH1, and CSH2 (GH2-2, CSH1-2, CSH2-2) retain intron 4 and therefore encode a new putative protein with a different C terminus (Fig. 1B) (6,11). The GH2-3 transcript results from the usage of an alternative donor splice-site within exon 4 causing a 4-bp deletion and consequently a frame shift. The GH2-4 transcript skips the regular acceptor splice site and uses an alternative site 45 bp downstream within exon 3 (12). Alternative splicing of CSHL1 differs from other hGH/CSH genes due to loss of the traditional donor splice site in intron 2. Thus, majority of CSHL1 transcripts miss exon 2 encoding the signal peptide and therefore are unable to express secreted protein (7).
The rise in circulating concentrations of PGH and PL protein in maternal serum during gestation is well established using specific monoclonal antibodies (reviewed in Refs. 2 and 13). Reduced levels of both PGH and PL have been reported in women with fetal intrauterine growth retardation (IUGR)/small-for-gestational-age (SGA) pregnancies (14,15,16,17).
Unlike the abundance of studies on pituitary-expressed GH1, the data about the expression profiles and use of alternative splicing pathways of the human placenta-expressed GH2, CSH1, CSH2, and CSHL1 genes is scarce. Detailed studies on the hGH/CSH cluster have been hindered by high-sequence identity among the genes and extensive usage of alternative splicing pathways resulting in multiple mRNA transcripts.
We addressed the detailed profile of alternative mRNA transcripts of GH2, CSH1, CSH2, and CSHL1 genes in placenta from uncomplicated term pregnancies in association with the birth weight of newborns, grouped as appropriate for gestational age (AGA), SGA, and large for gestational age (LGA). We developed a sensitive, fluorescent-labeled semiquantitative RT-PCR assay coupled with gene-specific restriction analysis, which proved to be capable of distinguishing alternative splice-products of individual placental hGH/CSH genes and quantification of their relative expression levels. Compared with normal-weight babies, significantly altered mRNA expression levels were detected in placentas from pregnancies with SGA and LGA newborns.
Materials and Methods
Experimental subjects
The study was approved by the Ethics Review Committee of Human Research of the University of Tartu (Estonia; permissions 146/18, 27.02.2006; 150/33, 18.06.2006; 158/80, 26.03.2007). A written informed consent to participate in the study was obtained from every family.
The study group was recruited at the Women’s Clinic of Tartu University Hospital (2006–2008) in the framework of the REPROMETA study sample collection and comprised 72 newborns born from uncomplicated, singleton pregnancies at gestational wk 38–42. All subjects were of white European ancestry and living in Estonia. Information regarding mother’s diseases, age, smoking, parity, parturition, childbirth history, and somatometric data was obtained from medical records during the course of pregnancy and after birth. Additionally, information regarding father’s diseases, age, height, and weight were documented. Fetal outcome data collected at delivery included weeks of gestation, gender, birth weight, crown-heel length, and head and abdominal circumference. According to birth weight adjusted to sex and gestational age, newborns were divided into three groups: AGA (birth weight between 10th and 90th centile), SGA (<10th centile), and LGA (>90th centile). The weight centiles were calculated on the basis of data from Estonian Medical Birth Registry (18). Cases with documented fetal anomalies, chromosomal abnormalities, preeclampsia, and gestational diabetes and families with history of inherited diseases were excluded. The basic clinical characteristics of the study population are summarized in Table 1.
Table 1.
Parental and offspring characteristics of the family trios in the study
| Study group relative to birth weight
|
|||
|---|---|---|---|
| AGA (n = 23) | SGA (n = 15) | LGA (n = 34) | |
| Mother | |||
| Age (yr) | 27 (20; 38) | 26 (18; 34) | 28.5 (17; 40) |
| Height (cm) | 168 (163; 177) | 168 (160; 177) | 168 (156; 182) |
| BMI before pregnancy (kg/m2) | 22.3 (17.4; 37.7) | 20.7 (16.5; 42.8) | 25 (20.5; 35)a |
| Gestational weight gain (kg) | 15.8 (8; 39.3) | 11 (2.5; 22)a | 19 (9; 33) |
| Women with parity 2 or more, n | 4 | 2 | 7 |
| Women smoking during pregnancy, n | 0 | 5a | 0 |
| Father | |||
| Age (yr) | 31.5 (23; 54) | 33 (23; 42) | 28 (18; 44) |
| Height (cm) | 180 (172; 191) | 178.5 (174; 184) | 180 (168; 197) |
| BMI (kg/m2) | 26 (20; 29.5) | 25.8 (17.7; 28.7) | 26.4 (19.6; 37) |
| Offspring | |||
| Gestational age at birth (d) | 285 (275; 295) | 277 (266; 289)a | 285.5 (267; 293) |
| Birth weight (g)b | 3960 (3340; 4470) | 2586 (2194; 2811) | 4717 (4364; 5228) |
| Birth length (cm)b | 51 (49; 55) | 47 (45; 51) | 54 (51; 57) |
| Head circumference (cm)b | 35 (33; 37) | 32.5 (29; 35) | 37 (35.5; 39) |
| Abdominal circumference (cm)b | 35 (32; 38) | 31 (29; 34) | 38 (36; 40) |
| Placental weightb | 650 (460; 880) | 455 (390; 600) | 777.5 (550; 1050) |
| Boys/girls, n | 14/9 | 7/8 | 23/11 |
Data are given as medians with ranges, except where indicated differently.
P < 0.05 vs. other two groups, Wilcoxon rank-sum test.
P < 0.05 among all three groups, Wilcoxon rank-sum test.
Tissue collection, RNA extraction, and reverse transcription
Full-thickness blocks of 2–3 cm were taken from middle region of placenta within 2 h after cesarean section or vaginal delivery, placed immediately into RNAlater (Ambion Inc., Austin, TX) and kept at −20 C until RNA isolation. All samples have been collected by the same medical personnel.
Total RNA from 200–230 mg of tissue was homogenized and extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Isolated total RNA was purified using NucleoSpin II isolation kit (Macherey-Nagel, Düren, Germany) and subsequently quantified by NanoDrop1000 spectrophotometer (Thermo Scientific, Wilmington, DE). One microgram of total RNA was reverse transcribed to cDNA using first-strand cDNA synthesis kit (Fermentas, Vilnius Lithuania) in accordance with manufacturer’s instructions.
Assay design
The National Center for Biotechnology Information web server (Bethesda, MD) was searched to retrieve sequence information of all predicted alternative mRNA transcripts for the placenta-expressed hGH/CSH genes: GH2, CSH1, CSH2, and CSHL1. This information is summarized in Fig. 1. Primers were designed based on hGH/CSH locus sequence NG_001334.1 using the Primer3 software (http://frodo.wi.mit.edu/primer3) (19), taking into account the information about alternative splicing and polymorphic positions in the coding region (10). Due to high similarity in DNA sequence of hGH/CSH genes (91–97%), additional gene-specific restriction digestion to guarantee specificity of RT-PCR products was used. The 5′-end of one gene specific primer in each RT-PCR was labeled with an ABI-compatible fluorescent dye (6-FAM, HEX; Metabion International AG, Martinsried, Germany). Primer sequences for RT-PCR, expected amplicon and restriction product sizes after gene-specific enzyme digestion are listed in Table 2. The RT-PCR conditions are given in the supplemental data, published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org.
Table 2.
Primer sequences and gene-specific restriction enzyme digestion for detection
| Gene/primer | Primer sequence (5′–3′) | Amplicons (bp) | Enzyme | Restriction products (bp) |
|---|---|---|---|---|
| GH2 | ||||
| ex2-F | CGCCTGTACCAGCTGGCATAa | |||
| ex5-R | ATTGAAGATCTGCCCAGTCC | 320, 360, 364 | na | |
| in4-R | TTTCTCTCCCCAGTCCCTGG | 390 | na | |
| CSH1/CSH2 | ||||
| ex2-F | CTCCAAGCCCATCGCGCGb | |||
| ex5-R | GCACTGGAGTGGCACCTTa | 601, 606, 316, 321 | EcoNI | 304, 309, 316, 321 |
| in4-R | TTTCTCTCCCCCAGCCCTC | 401 | BstEII | 358, 401 |
| CSHL1 | ||||
| ex1-F | ACAGCTCACCTAGCGGCAATa | |||
| ex5-R | AGGGTCTGCCCAGTCAGGT | 277, 350, 457, 526 | RsaI | 147, 220, 326, 395 |
| GAPDH | ||||
| F-ML | CCATGGAGAAGGCTGGGG | |||
| R-ML | CCAAGTTGTCATGGATGACCc | 196 | na |
The ML primers were originally published elsewhere (40). ex, Exon; in, intron; F, forward primer; R, reverse primer; na, not applicable.
Primer labeled at 5′-end with 6-FAM.
Primer labeled at 5′-end with 6-FAM when used with CSH1/CSH2 in4-R primer.
Primer labeled at 5′-end with HEX.
Capillary electrophoresis
After amplification (GH2, reference gene GAPDH) and further restriction analyses (CSH1, CSH2, CSHL1), the equal amount of reference gene and gene-specific product was combined with Hi-Di formamide (Applied Biosystems Inc., Foster City, CA) including 0.2 μl internal size standard (MegaBACE ET400-R size standard; GE Healthcare, Pittsburgh, PA) and resolved on an Applied Biosystems 3130XL genetic analyzer. The samples were injected at 1.2 kV for 23 sec, run at 15 kV for 20 min at 60 C. Data were detected using filter set D and analyzed with GeneMapper version 4.0 or Peak Scanner version 1.0 software (Applied Biosystems). The resolution of this method allows separation of PCR products differing in length by as little as 1 bp (Applied Biosystems).
Data analysis
All samples were amplified at least in triplicate using two different cDNAs made from the same individual. The relative expression level of each gene was calculated as follows: the value of the peak area (in relative fluorescent units) of each target gene was divided with the value of peak area of the reference gene (GAPDH) from the same lane of the electrophoresis. Intraassay coefficient of variation was 9–11%.
The statistical analyses were performed using the statistical package SPSS version 17.0 (SPSS Inc., Chicago, IL) and R 2.9.0, a free software environment for statistical computing and graphics (http://www.r-project.org/). Descriptive statistics are given as median values and ranges. The study population was grouped according to birth weight adjusted to sex and gestational age of the newborns (see above). Nonparametric Wilcoxon rank-sum test was used to compare continuous phenotypic variables of women with AGA newborns to mothers with SGA and LGA newborns. Differences in relative gene expression between groups were assessed by analysis of covariance (ANCOVA) using Bonferroni correction and gestational age as well as mother’s prepregnancy body mass index (BMI), weight gain, and smoking during pregnancy as covariates. Square root (CSHL1) and log (GH2, CSH1, CSH2) transformations were applied to all quantitative data to improve the approximation of normal distribution. Linear regression analysis was used to assess the correlation between expressions of different alternative gene specific mRNA transcripts. P < 0.05 was considered as significant.
Results
Characteristics of the study group
The study sample consisted of 72 women with singleton uncomplicated term (gestational wk 38–42) pregnancies, resulting in the birth AGA (n = 23), SGA (n = 15), or LGA (n = 34) newborns (Table 1).
As determined by the study design, birth weight, crown-heel length, and head and abdominal circumference of newborns were higher in the LGA group and lower in case of SGA compared with the normal-sized babies (P < 0.05). The study groups differed significantly also in placental weights (P < 0.05), which were closely correlated with birth weight (r = 0.80). In addition, women who delivered an LGA newborn tend to have a greater prepregnancy BMI and women delivering a SGA newborn showed a tendency to gain the least weight during gestation compared with other groups (P < 0.05). Gestational age at delivery was significantly different between the SGA and LGA groups. Besides, five of 15 women in the SGA group continued smoking during pregnancy. To avoid these confounding effects, maternal prepregnancy BMI, weight gain, and smoking during pregnancy along with gestational age were included as covariates in statistical analyses addressing the association between expression profile of hGH/CSH genes in placenta and birth weight.
Expression profile of alternative mRNA transcripts of the GH2, CSH1, CSH2, and CSHL1 genes in normal term placenta
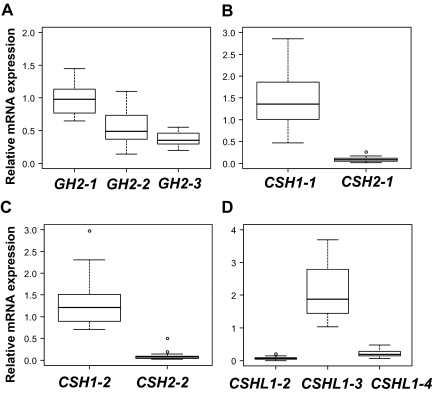
First, the detailed expression profile of individual placental hGH/CSH cluster genes was assessed using 23 term placenta samples from the uncomplicated pregnancies, resulting in the birth of AGA babies. For all hGH/CSH genes and their alternatively spliced transcripts, a wide range of expression level was detected in normal term placenta among studied individuals (Fig. 3).
Figure 3.
Relative expression level of major alternative mRNA transcripts of GH2, CSH1, CSH2, and CSHL1 genes in term placentas from pregnancies resulting in the birth of AGA newborns (n = 23). The relative expression is given as ratio to the reference gene GAPDH. The boxes represent the 25th and 75th percentiles. The median is denoted as the line that bisects the boxes. The whiskers are lines extending from each end of the box covering the extent of the data on 1.5 X interquartile range. Circles represent the outlier values. A, GH2 mRNA transcripts. B, CSH1-1 and CSH2-1. C, CSH1-2 and CSH2-1. D, CSHL1 mRNA transcripts.
In term placentas three major (>10% of total) and one minor transcript of placental GH gene GH2 was detected. In accordance with the earlier studies (20), the major transcript was GH2-1, which formed more than half (53.5%) of the GH2 mRNA pool (Figs. 1, B and C, and 3A). The contribution of other transcripts to total GH2-derived mRNA was measured 26.7% for GH2-2, 19.2% for GH2-3, and 0.6% for GH2-4 (Figs. 1, B and C, and 3A). The expression of GH2-1 and GH2-3 transcripts was highly correlated (r = 0.97), whereas lower correlation was measured among other GH2 transcripts (r = 0.75), especially GH2-2 (r = 0.50).
The majority of the chorionic somatomammotropin encoding mRNA transcripts (>90%) in term placenta were derived from CSH1 (Fig. 1C). The major transcript CSH1-1 containing all five coding exons was on average 15-fold more abundant than the respective transcript from CSH2 (CSH1-2) (Fig. 3B). The ratio of minor transcripts retaining intron 4 CSH1-2 to CSH2-2 was 14:1 (Fig. 3C). The shortest transcripts of CSH genes, CSH1-3, and CSH2-3 were not detected.
Compared with the other hGH/CSH genes, the major CSHL1 transcript CSHL1-3 (88.5% of CSHL1 mRNA pool) lacks a signal peptide coding exon 2 (Fig. 1C, 3). Unlike all other hGH/CSH transcripts, CSHL1-3 mRNA might be using an alternative AUG site in the exon 3 (Fig. 1B). The average relative expression levels of alternative CSHL1 transcripts in term placenta were detected 2.3, 0.2, 0.07, and 0.02 for CSHL1-3, CSHL1-4, CSHL1-2, and CSHL1-1, respectively (Figs. 1C and 3D). The two transcripts containing all five exons, CSHL1-1 and CSHL1-2 contributed to the CSHL1 mRNA pool at low level (<5% together) compared with both exon 2-lacking transcripts (Fig. 3D).
Significantly lower expression of GH2 in placentas of low-weight babies
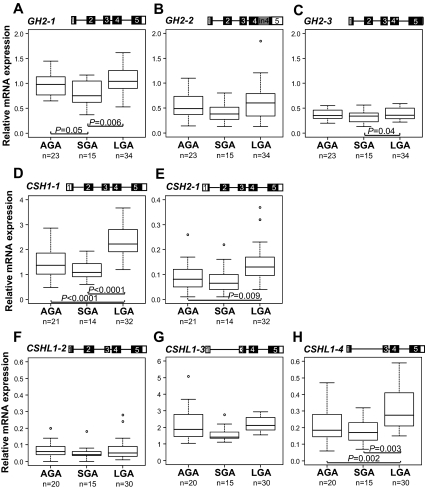
The expression patterns of the three major transcripts of GH2 gene (GH2-1, GH2-2, GH2-3) in placenta were correlated with birth weight (Fig. 4, A–C). When placenta samples were grouped based on the birth weight of newborns as AGA, SGA, or LGA cases, the differences in GH2-1 mRNA quantity were statistically significant among the subsamples [ANCOVA: F (2, 62) = 5.2301, P = 0.008]. The expression of GH2-2 transcript showed a similar trend [ANCOVA: F (2, 62) = 2.431, P = 0.096]. Compared with normal-sized babies, the expression of GH2-1 was about 1.1-fold lower in the SGA (P = 0.05) and about 1.3-fold higher in the LGA group (P = 0.006, Fig. 4A). Consistently, statistically different mean expression levels among the study groups were detected also for GH2-3 splice variant [ANCOVA: F (2, 62) = 3.290, P = 0.044; SGA vs. LGA P = 0.04, Fig. 4C].
Figure 4.
Comparison of relative expression levels of major alternatively spliced mRNA transcripts of GH2 (A–C), CSH1 (D), CSH2 (E), and CSHL1 (F–H) among term placentas from pregnancies resulting in the birth of AGA, SGA, and LGA newborns. The corresponding transcript representing the presented data are shown on the top of each panel. An asterisk denotes the 4-bp deletion in exon 4 of GH2-3. Relative expression of each transcript is given as ratio to the reference gene GAPDH. The boxes represent the 25th and 75th percentiles. The median is denoted as the line that bisects the boxes. The whiskers are lines extending from each end of the box covering the extent of the data on 1.5 × interquartile range. Circles represent the outlier values. Plotted values are presented without covariate adjustment. Statistical differences between study groups were assessed by ANCOVA using Bonferroni correction. Statistical tests were adjusted for gestational age as well as mother’s prepregnancy BMI, weight gain, and smoking during pregnancy. P values reflecting significant differences (P < 0.05) are shown.
Increased expression of CSH transcripts is characteristic to macrosomic newborns
The abundance of CSH1-1 and CSH2-1 mRNA transcripts, the major products of the chorionic somatomammotropin CSH1 and CSH2 genes (>90% of mRNA pool, Fig. 1C), determines the level of circulating PL hormone. When placentas from AGA, LGA, and SGA groups were compared, significant differential expression of these transcripts was detected [ANCOVA: CSH1-1 F (2, 58) = 15.744, P < 0.0001; CSH2-1 F (2, 58) = 5.541, P = 0.006]. Placental samples from pregnancies with LGA newborns had an average approximately 1.6-fold higher expression levels of CSH1-1 (P < 0.0001, Fig. 4D) and CSH2-1 (P = 0.009, Fig. 4E) compared with the normal-size newborns. In case of SGA, the expression of CSH1-1 was approximately 1.9-fold (P < 0.0001, Fig. 4D) and CSH2-1 approximately 1.6-fold lower compared with LGA group (P = 0.084, Fig. 4E).
Male newborns have been shown to be taller and heavier then female babies at birth (21). In the current study, the LGA group contained proportionally more placental samples from pregnancies, resulting in the birth of a boy. To exclude the possibility that the observed higher expression of CSH1-1 in LGA group reflected the bias, additional analyses were conducted in study groups stratified by sex. No correlation was detected between CSH1-1 transcript abundance and gender of the baby. Relative expression was higher in the LGA group for the placentas resulting in the birth of a girl and AGA/SGA groups in the birth of a boy (Supplemental Fig. 1).
The expression of CSHL1 follows trends of the hormone-coding genes in the hGH/CSH cluster
The expression patterns of three detected CSHL1 transcripts (CSHL1-2, CSHL1-3, and CSHL1-4) in placentas from AGA, SGA, and LGA pregnancies were mutually correlated and reflected birth weight (Fig. 4, F–H). The highest transcript levels were measured in the LGA and the lowest in the SGA group. Differences among study groups reached statistical significance for the CSHL1-4 transcript [ANCOVA: F (2, 55) = 9.494, P < 0.0001]. The CSHL1-4 was on average 1.6-fold and 1.8-fold higher expressed in placental samples from pregnancies with LGA newborns compared with AGA (P = 0.003) and SGA (P = 0.002) babies, respectively (Fig. 4H).
Expression of placental hGH/CSH genes in correlation with placental weight and newborn gender
The expression of the alternative transcripts of hGH/CSH genes was not correlated with placental weight within AGA, SGA, and LGA subgroups (r < 0.5). Although no statistically significant difference was detected in gene expression between placentas from female and male newborns, a trend for higher expression of GH2 was observed in placenta tissue obtained from women giving the birth to a baby girl. This is in accordance with the earlier studies on PGH levels in maternal bloodstream (16,22).
Discussion
We performed a systematic study on the quantitative expression profiles of GH2, CSH1, CSH2, and CSHL1 genes and their alternative mRNA transcripts in substantial number of placental samples of uncomplicated term pregnancies. Surprisingly, for the GH2 gene, almost half of the produced mRNA represents transcripts with yet-unknown function and encoding for predicted alternative proteins (Fig. 1C). The GH2-2 and GH2-3 splice products have a frame shift, resulting in a unique C terminus. The GH2-2 putative protein has been predicted to contain membrane-spanning domain (11), and its expression is restricted to a small fraction of syncytiotrophoblasts (23). Our study indicated that the majority of the transcript pool (>90%) encoding PL arises from the CSH1 gene and average one 15th of the CSH mRNA is transcribed from CSH2. Earlier reports have determined the approximate ratio of relative expression of CSH1-1 to CSH2-1 to be 5:1 (6) and 6:1 (4) at term. Almost 3-fold difference could be explained by a more sensitive detection method and larger sample size in this study. As a novel finding, we detected CSHL1-3 as the most abundant CSHL1 mRNA (88.5%), which uses an alternative AUG site in exon 3 and encodes a predicted protein product of 128 amino acids. A previous report (7) showed that CSHL1-3 [alias hCS-L(1-3N-4-5)] was initiated at the primary AUG in exon 1 and encoded a putative protein of only 18 amino acids. The abundance of alternative splicing variants of hGH/CSH genes might be explained by three alternative scenarios: 1) these splice variants are in fact translated and present as functional proteins in the human placenta; 2) they are part of regulatory RNA machinery; and 3) they represent junk RNA resulting from still ongoing optimization of splicing regulation of young placental hGH/CSH genes.
This is the first report showing that the majority of placentas from pregnancies resulting in the birth of SGA newborns express lower levels of all placental hGH/CSH genes when compared with the cases of AGA and LGA. In concordance, earlier studies reported a positive correlation between the levels of PGH and PL in the bloodstream of pregnant women and the offspring’s birth weight (14,15,16). Thus, we suggest that low maternal hormone levels might be primarily directed by the reduced expression of placental hGH/CSH genes. Contradictory conclusion has been reached in study (24) in which the up-regulation of GH2 mRNA in IUGR placentas in comparison with normal placentas was detected. Taking into account that SGA/IUGR may have several etiological backgrounds and comprises a heterogeneous group, selection criteria of SGA/IUGR may explain this inconsistency. Study group heterogeneity may also explain why all SGA placentas do not show down-regulation for the entire hGH/CSH cluster in the present report.
Placental samples from pregnancies with LGA newborns showed significantly higher amounts of CSH1-1, CSH2-1, and CSHL1-4 transcripts compared with pregnancies with normal-sized newborns. Consistently, it has been shown that elevated serum PL levels in uncomplicated pregnancies were associated with increased neonatal birth weight (25). It has been suggested that PL is contributing to fetal growth by stimulating a catabolic state in the mother in the second half of gestation via IGF-I and insulin (2,26). Thus, increase in circulating maternal nutrient levels results in an elevated nutrient supply to the fetus, which may lead to fetal macrosomia (LGA). This is supported by the correlation of maternal PL with birth weight and BMI (27). On the other hand, because PL is also present in the fetal circulation in relatively high concentrations, it may have direct growth-promoting actions on fetal tissues (reviewed in28). Recent studies suggest a role for autocrine/paracrine factors in the regulation of CSH1-1/CSH2-1 gene expression during pregnancy (reviewed in Ref. 28).
In present study, we show that placenta-expressed hGH/CSH genes are an important part of the genetic network controlling fetal growth. What could be the molecular mechanisms leading to the differential expression of these genes? First, changes in regulatory control regions may alter the expression. Activation of the entire gene cluster in placenta might be affected by polymorphisms or epimutations in locus control region (LCR) sequence responsible for the functional activation of all hGH/CSH in tissue-specific manner (29,30). So far, it has been shown that haplotypes of the LCR regulate tissue-specifically GH1 transcription in promoter haplotype-dependent manner (31). The expression of individual hGH/CSH gene might be altered by alternative allelic composition of the promotor or other regulatory regions (e.g. placental gene repeat units) affecting the efficacy of the binding of gene regulatory factors (autocrine/paracrine/transcription factors). Whether polymorphisms in LCR and/or other regulatory regions of placenta-expressed hGH/CSH genes have an effect on their expression remains to be elucidated. The second alternative explanation is that the number of cells expressing hGH/CSH genes in placenta is reduced in pregnancies with SGA. It has been demonstrated that in case of IUGR, the relative levels of GH2 mRNA per cell were comparable with those of normal placentas, but the mean number of cells expressing GH2 mRNA per area was significantly reduced compared with normal placentas (32). The hGH/CSH genes have shown to be expressed in syncytiotrophoblasts (33). The reduced number of syncytiotrophoblasts in placenta strongly implies dysfunction in the development and differentiation of the placenta, which ultimately deprives the fetus of the nutrients required for the optimal growth. Clear association between placental measures and fetal growth has been also established (34), adding more support to the idea of the impaired placental differentiation.
In summary, our study showed the abundance of alternative splice products of placental hGH/CSH genes and their altered expression in placenta in pregnancies terminating with SGA and LGA newborns compared with AGA newborns. In the case of SGA newborns, the expression of the entire hGH/CSH cluster tended to be down-regulated, whereas in cases of LGA, significantly increased transcript levels were detected for CSH1/CSH2 and CSHL1. This may reflect either polymorphic and epigenetic variation in regulatory regions of placenta-expressed hGH/CSH genes or compromised placental differentiation. The susceptibility to the SGA and LGA pregnancies may be mediated by different genetic mechanisms. In the long term, this may have a clinically prognostic implication, because both SGA and LGA newborns are shown to be at risk for adult-onset diseases (35). Reduced fetal growth has been associated with an increased incidence of cardiovascular diseases and type 2 diabetes in adulthood (35,36,37,38). Likewise, infants who are born LGA have a higher risk to develop metabolic syndrome (39).
Supplementary Material
Figure 2.
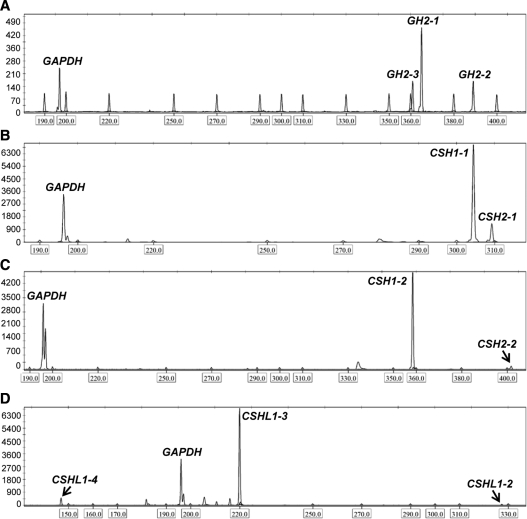
GeneMapper version 4.0 electropherogram showing the amplified DNA fragments of fluorescent-labeled (6-FAM, HEX) reference gene (GAPDH) and alternative mRNA transcripts of placental hGH/CSH genes in term placenta. The expression of the genes was assessed by semiquantitative RT-PCR (GAPDH, GH2) or RT-PCR combined with further gene-specific restriction analysis (CSH1, CSH2, and CSHL1), which were afterward resolved using ABI 3130XL genetic analyzer. The x-axis shows the size of the detected fragments in base pairs, and the y-axis represents the signal intensity in relative fluorescent units. Boxed numbers below the x-axis mark the peaks of the internal size standard. The peaks corresponding to the amplicons from the reference gene GAPDH (196 bp) are shown on all panels. The peaks representing the amplified DNA fragments of the major transcripts of the studied hGH/CSH genes are shown on subpanels GH2-1 (364 bp), GH2-2 (390 bp), GH2-3 (360 bp) (A); CSH1-1 (304 bp), CSH2-1 (309 bp) (B); CSH1-2 (358 bp), CSH2-2 (401 bp) (C); and CSHL1-2 (326 bp), CSHL1-3 (220 bp) and CSHL1-4 (147 bp) (D).
Acknowledgments
We are very grateful to all the families who participated in the study. The personnel of the Department of Obstetrics and Gynecology, Women’s Clinic of Tartu University, are thanked for technical assistance.
Footnotes
This work was supported by Howard Hughes Medical Institute International Scholarship Grant 55005617 (to M. L.) and Estonian Science Foundation Grant 7471 (to M. L.). Additional support was provided by Wellcome Trust International Senior Research Fellowship 070191/Z/03/Z in Biomedical Science in Central Europe (to M.L.) and Estonian Ministry of Education and Science Core Grant 0182721s06.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 16, 2010
For editorial see page 2054
Abbreviations: AGA, Appropriate for gestational age; ANCOVA, analysis of covariance; BMI, body mass index; CSH, chorionic somatomammotropin; h, human; IUGR, intrauterine growth retardation; LCR, locus control region; LGA, large for gestational age; PGH, placental GH; PL, placental lactogen; SGA, small for gestational age.
References
- George DL, Phillips 3rd JA, Francke U, Seeburg PH 1981 The genes for growth hormone and chorionic somatomammotropin are on the long arm of human chromosome 17 in region q21 to qter. Hum Genet 57:138–141 [DOI] [PubMed] [Google Scholar]
- Handwerger S, Freemark M 2000 The roles of placental growth hormone and placental lactogen in the regulation of human fetal growth and development. J Pediatr Endocrinol Metab 13:343–356 [DOI] [PubMed] [Google Scholar]
- Barsh GS, Seeburg PH, Gelinas RE 1983 The human growth hormone gene family: structure and evolution of the chromosomal locus. Nucleic Acids Res 11:3939–3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EY, Liao YC, Smith DH, Barrera-Saldaña HA, Gelinas RE, Seeburg PH 1989 The human growth hormone locus: nucleotide sequence, biology, and evolution. Genomics 4:479–497 [DOI] [PubMed] [Google Scholar]
- Hirt H, Kimelman J, Birnbaum MJ, Chen EY, Seeburg PH, Eberhardt NL, Barta A 1987 The human growth hormone gene locus: structure, evolution, and allelic variations. DNA 6:59–70 [DOI] [PubMed] [Google Scholar]
- MacLeod JN, Lee AK, Liebhaber SA, Cooke NE 1992 Developmental control and alternative splicing of the placentally expressed transcripts from the human growth hormone gene cluster. J Biol Chem 267:14219–14226 [PubMed] [Google Scholar]
- Misra-Press A, Cooke NE, Liebhaber SA 1994 Complex alternative splicing partially inactivates the human chorionic somatomammotropin-like (hCS-L) gene. J Biol Chem 269:23220–23229 [PubMed] [Google Scholar]
- Hu L, Lytras A, Bock ME, Yuen CK, Dodd JG, Cattini PA 1999 Detection of placental growth hormone variant and chorionic somatomammotropin-L RNA expression in normal and diabetic pregnancy by reverse transcriptase-polymerase chain reaction. Mol Cell Endocrinol 157:131–142 [DOI] [PubMed] [Google Scholar]
- Lacroix MC, Guibourdenche J, Frendo JL, Muller F, Evain-Brion D 2002 Human placental growth hormone—a review. Placenta 23(Suppl A):S87–S94 [DOI] [PubMed] [Google Scholar]
- Sedman L, Padhukasahasram B, Kelgo P, Laan M 2008 Complex signatures of locus-specific selective pressures and gene conversion on human growth hormone/chorionic somatomammotropin genes. Hum Mutat 29:1181–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke NE, Ray J, Emery JG, Liebhaber SA 1988 Two distinct species of human growth hormone-variant mRNA in the human placenta predict the expression of novel growth hormone proteins. J Biol Chem 263:9001–9006 [PubMed] [Google Scholar]
- Boguszewski CL, Svensson PA, Jansson T, Clark R, Carlsson LM, Carlsson B 1998 Cloning of two novel growth hormone transcripts expressed in human placenta. J Clin Endocrinol Metab 83:2878–2885 [DOI] [PubMed] [Google Scholar]
- Fuglsang J, Ovesen P 2006 Aspects of placental growth hormone physiology. Growth Horm IGF Res 16:67–85 [DOI] [PubMed] [Google Scholar]
- McIntyre HD, Serek R, Crane DI, Veveris-Lowe T, Parry A, Johnson S, Leung KC, Ho KK, Bougoussa M, Hennen G, Igout A, Chan FY, Cowley D, Cotterill A, Barnard R 2000 Placental growth hormone (GH), GH-binding protein, and insulin-like growth factor axis in normal, growth-retarded, and diabetic pregnancies: correlations with fetal growth. J Clin Endocrinol Metab 85:1143–1150 [DOI] [PubMed] [Google Scholar]
- Mirlesse V, Frankenne F, Alsat E, Poncelet M, Hennen G, Evain-Brion D 1993 Placental growth hormone levels in normal pregnancy and in pregnancies with intrauterine growth retardation. Pediatr Res 34:439–442 [DOI] [PubMed] [Google Scholar]
- Chellakooty M, Vangsgaard K, Larsen T, Scheike T, Falck-Larsen J, Legarth J, Andersson AM, Main KM, Skakkebaek NE, Juul A 2004 A longitudinal study of intrauterine growth and the placental growth hormone (GH)-insulin-like growth factor I axis in maternal circulation: association between placental GH and fetal growth. J Clin Endocrinol Metab 89:384–391 [DOI] [PubMed] [Google Scholar]
- Spellacy WN, Buhi WC 1976 Glucagon, insulin and glucose levels in maternal and umbilical cord plasma with studies of placental transfer. Obstet Gynecol 47:291–294 [PubMed] [Google Scholar]
- Karro H, Rahu M, Gornoi K, Baburin A 1997 Sünnikaalu jaotumine raseduse kestuse järgi Eestis aastail 1992–1994. Eesti Arst 4:299–303 [Google Scholar]
- Rozen S, Skaletsky H 2000 Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386 [DOI] [PubMed] [Google Scholar]
- Cooke NE, Ray J, Watson MA, Estes PA, Kuo BA, Liebhaber SA 1988 Human growth hormone gene and the highly homologous growth hormone variant gene display different splicing patterns. J Clin Invest 82:270–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copper RL, Goldenberg RL, Cliver SP, DuBard MB, Hoffman HJ, Davis RO 1993 Anthropometric assessment of body size differences of full-term male and female infants. Obstet Gynecol 81:161–164 [PubMed] [Google Scholar]
- Chellakooty M, Skibsted L, Skouby SO, Andersson AM, Petersen JH, Main KM, Skakkebaek NE, Juul A 2002 Longitudinal study of serum placental GH in 455 normal pregnancies: correlation to gestational age, fetal gender, and weight. J Clin Endocrinol Metab 87:2734–2739 [DOI] [PubMed] [Google Scholar]
- Liebhaber SA, Urbanek M, Ray J, Tuan RS, Cooke NE 1989 Characterization and histologic localization of human growth hormone-variant gene expression in the placenta. J Clin Invest 83:1985–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh S, Satoskar P, Bhartiya D 2001 Expression of insulin-like growth factor-I and placental growth hormone mRNA in placentae: a comparison between normal and intrauterine growth retardation pregnancies. Mol Hum Reprod 7:287–292 [DOI] [PubMed] [Google Scholar]
- Henderson CE, Gatz M, LaRosa D, Divon MY 1995 Elevated human placental (HPL) levels in uncomplicated pregnancies are associated with increased neonatal birthweight. Am J Obstet Gynecol 172:293 [Google Scholar]
- Handwerger S 1991 Clinical counterpoint: the physiology of placental lactogen in human pregnancy. Endocr Rev 12:329–336 [DOI] [PubMed] [Google Scholar]
- Wolf HJ, Ebenbichler CF, Huter O, Bodner J, Lechleitner M, Föger B, Patsch JR, Desoye G 2000 Fetal leptin and insulin levels only correlate in large-for-gestational age infants. Eur J Endocrinol 142:623–629 [DOI] [PubMed] [Google Scholar]
- Handwerger S 2009 The growth hormone gene cluster: physiological actions and regulation during pregnancy. Growth Genet Horm 25:1–8 [Google Scholar]
- Ho Y, Liebhaber SA, Cooke NE 2004 Activation of the human GH gene cluster: roles for targeted chromatin modification. Trends Endocrinol Metab 15:40–45 [DOI] [PubMed] [Google Scholar]
- Kimura AP, Sizova D, Handwerger S, Cooke NE, Liebhaber SA 2007 Epigenetic activation of the human growth hormone gene cluster during placental cytotrophoblast differentiation. Mol Cell Biol 27:6555–6568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan M, Millar DS, Hedderich J, Lewis G, Newsway V, Mo N, Fryklund L, Procter AM, Krawczak M, Cooper DN 2003 Human growth hormone 1 (GH1) gene expression: complex haplotype-dependent influence of polymorphic variation in the proximal promoter and locus control region. Hum Mutat 21:408–423 [DOI] [PubMed] [Google Scholar]
- Chowen JA, Evain-Brion D, Pozo J, Alsat E, García-Segura LM, Argente J 1996 Decreased expression of placental growth hormone in intrauterine growth retardation. Pediatr Res 39:736–739 [DOI] [PubMed] [Google Scholar]
- Alsat E, Guibourdenche J, Luton D, Frankenne F, Evain-Brion D 1997 Human placental growth hormone. Am J Obstet Gynecol 177:1526–1534 [DOI] [PubMed] [Google Scholar]
- Sands J, Dobbing J 1985 Continuing growth and development of the third-trimester human placenta. Placenta 6:13–21 [DOI] [PubMed] [Google Scholar]
- Kunz LH, King JC 2007 Impact of maternal nutrition and metabolism on health of the offspring. Semin Fetal Neonatal Med 12:71–77 [DOI] [PubMed] [Google Scholar]
- Barker DJ 2005 The developmental origins of insulin resistance. Horm Res 64(Suppl 3):S2–S7 [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA 2004 Living with the past: evolution, development, and patterns of disease. Science 305:1733–1736 [DOI] [PubMed] [Google Scholar]
- Osmond C, Barker DJ 2000 Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ Health Perspect 108(Suppl 3):S545–S553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boney CM, Verma A, Tucker R, Vohr BR 2005 Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115:e290– e296 [DOI] [PubMed] [Google Scholar]
- Miller-Lindholm AK, LaBenz CJ, Ramey J, Bedows E, Ruddon RW 1997 Human chorionic gonadotropin-beta gene expression in first trimester placenta. Endocrinology 138:5459–5465 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.