Abstract
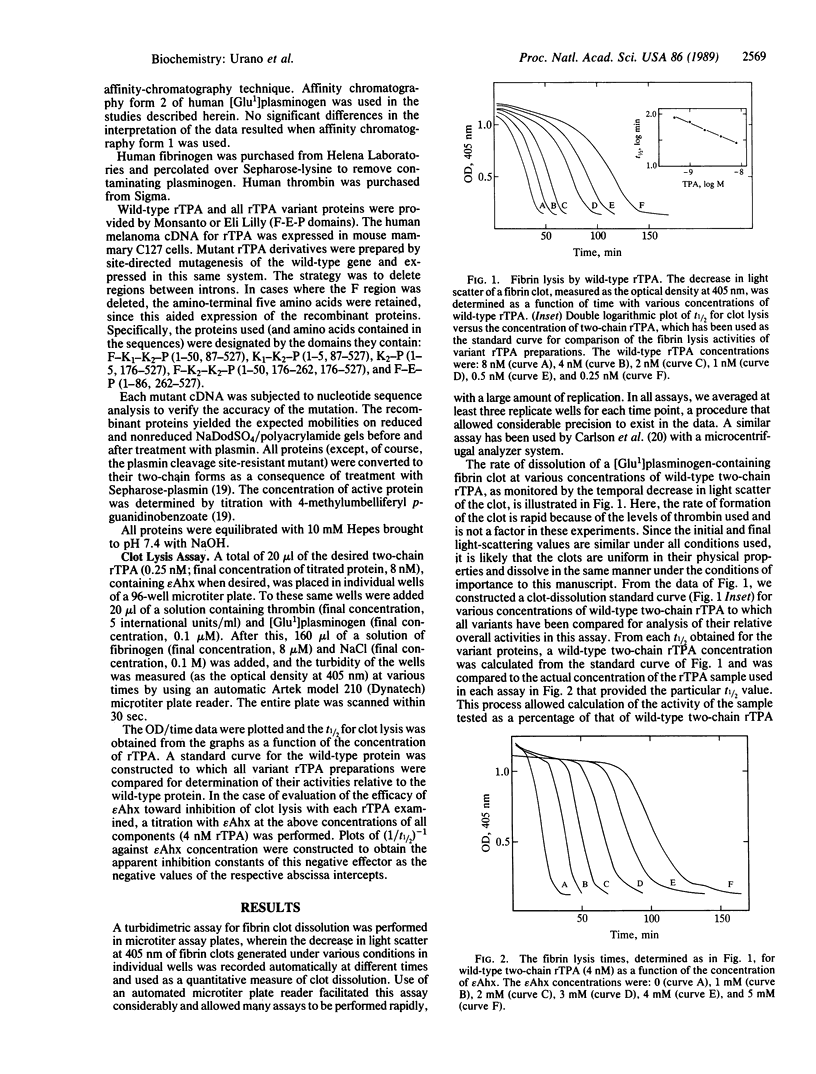
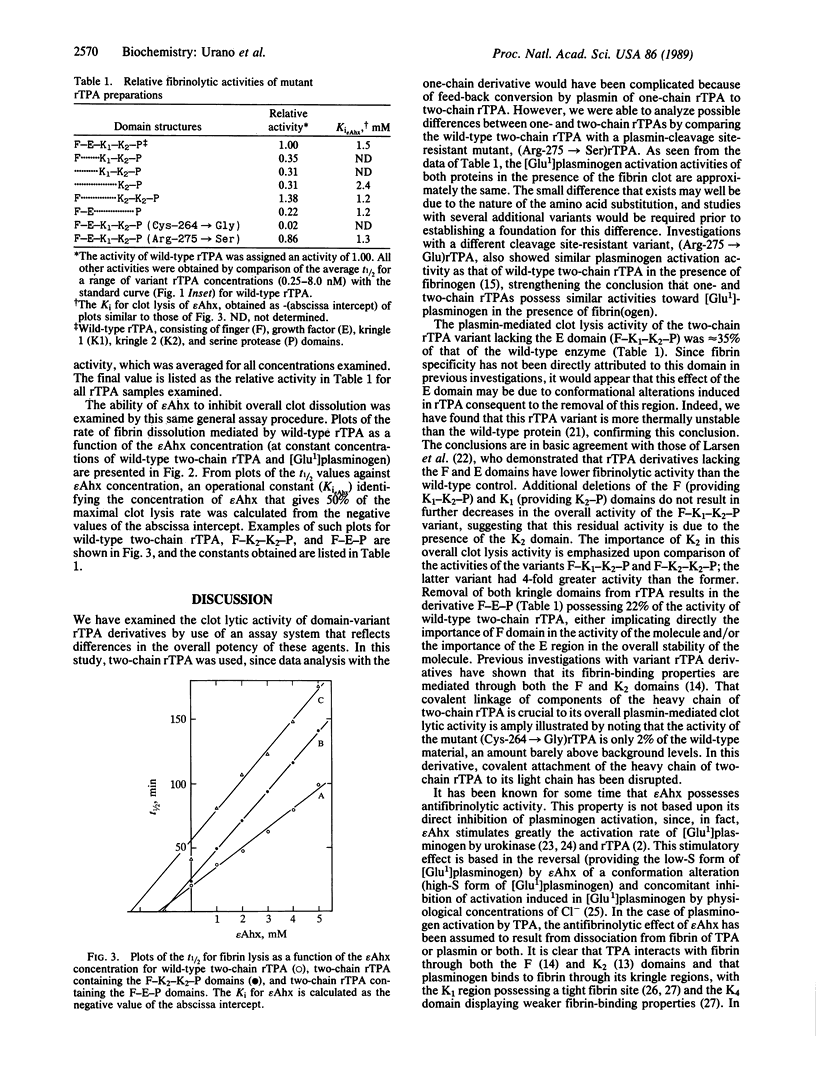
A rapid and quantitative fibrinolytic assay has been used to measure the overall activity of a recombinant tissue plasminogen activator (rTPA) preparation for dissolution of a fibrin clot by its ability to activate [Glu1]plasminogen (containing glutamic acid at position 1) to plasmin. A standard curve constructed for wild-type two-chain rTPA that contains, from the amino terminus, the finger (F)-growth factor (E)-kringle 1 (K1)-kringle 2 (K2)-serine protease (P) domains was used to assess the overall fibrin-dissolving abilities of variant recombinant molecules. Two-chain deletion mutants lacking the E domain, the F-E domains, the F-E-K1 domains, and the K1-K2 domains yielded activities ranging from 22% to 35% of the overall activity of wild-type two-chain rTPA, suggesting that both the K2 and F domains are individually responsible for a portion of the function of the molecule. Comparison of variant molecules containing F-K1-K2-P and F-K2-K2-P domains showed that the latter variant possessed a 4-fold higher activity (1.4-fold greater than that of wild-type two-chain rTPA), indicating that, for the activity measured, the presence of K2 leads to a greater effectiveness than that of K1. A plasmin cleavage-resistant mutant (Arg-275----Ser) has been used to assess possible differences in one- and two-chain rTPA in this overall activity, the former displaying 86% of the activity of the latter, suggesting that such differences are indeed small. Finally, the proper covalent attachment of the light and heavy chains of two-chain rTPA are very important to its overall fibrinolytic activity, since replacement of Cys-264 with glycine and concomitant disruption of one of the covalent attachment sites of the two chains provides a variant of rTPA with less than 2% of the activity of the wild-type two-chain molecule. The effector molecule, epsilon-amino hexanoic acid (epsilon Ahx; epsilon-aminocaproic acid), inhibits the overall fibrinolytic effect of rTPA in this system, with an effective Ki of approximately 1.5 mM. Its efficacy, as measured by the Ki, is independent of the presence of the epsilon Ahx binding regions of plasminogen and rTPA and is similar to the efficacy obtained when urokinase was the activator in place of wild-type two-chain rTPA or when activation of plasminogen was bypassed as a result of provision of preformed plasmin to the assay. The results suggest that in the overall clot lysis system, an important epsilon Ahx binding site may exist on fibrin that inhibits its dissolution by plasmin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brockway W. J., Castellino F. J. Measurement of the binding of antifibrinolytic amino acids to various plasminogens. Arch Biochem Biophys. 1972 Jul;151(1):194–199. doi: 10.1016/0003-9861(72)90488-2. [DOI] [PubMed] [Google Scholar]
- Carlson R. H., Garnick R. L., Jones A. J., Meunier A. M. The determination of recombinant human tissue-type plasminogen activator activity by turbidimetry using a microcentrifugal analyzer. Anal Biochem. 1988 Feb 1;168(2):428–435. doi: 10.1016/0003-2697(88)90340-5. [DOI] [PubMed] [Google Scholar]
- Chibber B. A., Morris J. P., Castellino F. J. Effects of human fibrinogen and its cleavage products on activation of human plasminogen by streptokinase. Biochemistry. 1985 Jul 2;24(14):3429–3434. doi: 10.1021/bi00335a006. [DOI] [PubMed] [Google Scholar]
- Claeys H., Vermylen J. Physico-chemical and proenzyme properties of NH2-terminal glutamic acid and NH2-terminal lysine human plasminogen. Influence of 6-aminohexanoic acid. Biochim Biophys Acta. 1974 Apr 11;342(2):351–359. doi: 10.1016/0005-2795(74)90090-7. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Fu K. P., Lee S., Hum W. T., Kalyan N., Rappaport R., Hetzel N., Hung P. P. Disposition of a novel recombinant tissue plasminogen activator, delta 2-89 TPA, in mice. Thromb Res. 1988 Apr 1;50(1):33–41. doi: 10.1016/0049-3848(88)90172-7. [DOI] [PubMed] [Google Scholar]
- Gaffney P. J., Urano T., de Serrano V. S., Mahmoud-Alexandroni M., Metzger A. R., Castellino F. J. Roles for chloride ion and fibrinogen in the activation of [Glu1]plasminogen in human plasma. Proc Natl Acad Sci U S A. 1988 May;85(10):3595–3598. doi: 10.1073/pnas.85.10.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoylaerts M., Rijken D. C., Lijnen H. R., Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982 Mar 25;257(6):2912–2919. [PubMed] [Google Scholar]
- Ichinose A., Takio K., Fujikawa K. Localization of the binding site of tissue-type plasminogen activator to fibrin. J Clin Invest. 1986 Jul;78(1):163–169. doi: 10.1172/JCI112546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen G. R., Henson K., Blue Y. Variants of human tissue-type plasminogen activator. Fibrin binding, fibrinolytic, and fibrinogenolytic characterization of genetic variants lacking the fibronectin finger-like and/or the epidermal growth factor domains. J Biol Chem. 1988 Jan 15;263(2):1023–1029. [PubMed] [Google Scholar]
- Lerch P. G., Rickli E. E., Lergier W., Gillessen D. Localization of individual lysine-binding regions in human plasminogen and investigations on their complex-forming properties. Eur J Biochem. 1980;107(1):7–13. doi: 10.1111/j.1432-1033.1980.tb04617.x. [DOI] [PubMed] [Google Scholar]
- Lucas M. A., Fretto L. J., McKee P. A. The binding of human plasminogen to fibrin and fibrinogen. J Biol Chem. 1983 Apr 10;258(7):4249–4256. [PubMed] [Google Scholar]
- Lucas M. A., Straight D. L., Fretto L. J., McKee P. A. The effects of fibrinogen and its cleavage products on the kinetics of plasminogen activation by urokinase and subsequent plasmin activity. J Biol Chem. 1983 Oct 25;258(20):12171–12177. [PubMed] [Google Scholar]
- Maxwell R. E., Allen D. Interactions of epsilon-aminocaproic acid with the thrombin clotting and fibrinolytic systems. Nature. 1966 Jan 8;209(5019):211–213. doi: 10.1038/209211a0. [DOI] [PubMed] [Google Scholar]
- Ny T., Elgh F., Lund B. The structure of the human tissue-type plasminogen activator gene: correlation of intron and exon structures to functional and structural domains. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5355–5359. doi: 10.1073/pnas.81.17.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patthy L. Evolution of the proteases of blood coagulation and fibrinolysis by assembly from modules. Cell. 1985 Jul;41(3):657–663. doi: 10.1016/s0092-8674(85)80046-5. [DOI] [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., Bennett W. F., Yelverton E., Seeburg P. H., Heyneker H. L. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983 Jan 20;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- Radek J. T., Castellino F. J. A differential scanning calorimetric investigation of the domains of recombinant tissue plasminogen activator. Arch Biochem Biophys. 1988 Dec;267(2):776–786. doi: 10.1016/0003-9861(88)90087-2. [DOI] [PubMed] [Google Scholar]
- Rijken D. C., Hoylaerts M., Collen D. Fibrinolytic properties of one-chain and two-chain human extrinsic (tissue-type) plasminogen activator. J Biol Chem. 1982 Mar 25;257(6):2920–2925. [PubMed] [Google Scholar]
- Rånby M., Bergsdorf N., Nilsson T. Enzymatic properties of the one- and two-chain form of tissue plasminogen activator. Thromb Res. 1982 Jul 15;27(2):175–183. doi: 10.1016/0049-3848(82)90197-9. [DOI] [PubMed] [Google Scholar]
- Rånby M. Studies on the kinetics of plasminogen activation by tissue plasminogen activator. Biochim Biophys Acta. 1982 Jun 24;704(3):461–469. doi: 10.1016/0167-4838(82)90068-1. [DOI] [PubMed] [Google Scholar]
- Tate K. M., Higgins D. L., Holmes W. E., Winkler M. E., Heyneker H. L., Vehar G. A. Functional role of proteolytic cleavage at arginine-275 of human tissue plasminogen activator as assessed by site-directed mutagenesis. Biochemistry. 1987 Jan 27;26(2):338–343. doi: 10.1021/bi00376a002. [DOI] [PubMed] [Google Scholar]
- Urano T., Chibber B. A., Castellino F. J. The reciprocal effects of epsilon-aminohexanoic acid and chloride ion on the activation of human [Glu1]plasminogen by human urokinase. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4031–4034. doi: 10.1073/pnas.84.12.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano T., Sator de Serrano V., Chibber B. A., Castellino F. J. The control of the urokinase-catalyzed activation of human glutamic acid 1-plasminogen by positive and negative effectors. J Biol Chem. 1987 Nov 25;262(33):15959–15964. [PubMed] [Google Scholar]
- Urano T., Sator de Serrano V., Gaffney P. J., Castellino F. J. Effectors of the activation of human [Glu1]plasminogen by human tissue plasminogen activator. Biochemistry. 1988 Aug 23;27(17):6522–6528. doi: 10.1021/bi00417a049. [DOI] [PubMed] [Google Scholar]
- Wallén P., Bergsdorf N., Rånby M. Purification and identification of two structural variants of porcine tissue plasminogen activator by affinity adsorption on fibrin. Biochim Biophys Acta. 1982 Nov 24;719(2):318–328. doi: 10.1016/0304-4165(82)90105-2. [DOI] [PubMed] [Google Scholar]
- van Zonneveld A. J., Veerman H., Pannekoek H. Autonomous functions of structural domains on human tissue-type plasminogen activator. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4670–4674. doi: 10.1073/pnas.83.13.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zonneveld A. J., Veerman H., Pannekoek H. On the interaction of the finger and the kringle-2 domain of tissue-type plasminogen activator with fibrin. Inhibition of kringle-2 binding to fibrin by epsilon-amino caproic acid. J Biol Chem. 1986 Oct 25;261(30):14214–14218. [PubMed] [Google Scholar]


