Abstract
Recent studies suggest that weight suppression (WS), defined as the discrepancy between current and highest past weight, predicts short-term weight gain in bulimia nervosa (BN) during treatment. The current study was designed to build on this preliminary work by examining the relation between WS and long-term weight change in BN. Treatmentseeking women (N=97) with DSM-IV BN participated in a naturalistic longitudinal follow-up study of eating disorders. At intake, height and weight were measured and highest past weight was assessed. Self-reported weights were collected every 6 months for 5 years. Hierarchical Linear Modeling (HLM) estimated growth curves for weight change over time. Significant inter-person variability was detected for intercepts and slopes (p<0.001) so both were treated as random effects. Participants’ weights increased over the study course, moderated by baseline WS (p<0.001), such that higher WS predicted more rapid weight gain. Weight change was not associated with entry weight, height, or highest-ever weight, suggesting that WS per se predicted weight change. These findings complement previous short-term studies in BN by demonstrating that WS predicts weight gain over 5 years. Because weight gain could spur radical dieting that maintains BN, these results have important treatment implications.
Keywords: eating disorders, BMI, longitudinal
1. Introduction
Most individuals with bulimia nervosa (BN) are in the normal weight range, yet their apparently unremarkable body weight conceals a history of once weighing considerably more than they currently do. This discrepancy, which Lowe (1993) has labeled “weight suppression” (WS), was emphasized by Russell (1979) in his classic paper that first identified BN as an eating disorder, but has been little studied since. Weight suppression is defined as the difference between highest past weight (since reaching adult height) and current measured body weight. Individuals with BN are high in WS; Butryn et al. (2006) reported a mean WS of 9.6 kg in outpatients and Lowe et al. (2006) reported a mean WS of 12.0 kg in residential patients with BN. Because individuals in these studies had body mass indices (BMI; kg/m2) in the middle of the normal weight range, this means that many of those with BN were once overweight, an observation that coincides with the weight history findings from a community based study of BN (Fairburn et al., 1997).
Interestingly, though much has been written about how weight loss in obese individuals may set the stage for weight regain (Rosenbaum et al., 2008), few such concerns have been raised about the major weight losses most individuals with BN have undergone. The weight losses experienced by individuals with BN may be of even greater concern because, given that most women with BN have never been obese, a weight loss of a given size represents a larger percentage reduction in most individuals with BN than in obese individuals. Furthermore, if reducing weight well below its highest previous level makes weight gain more likely, such effects could exacerbate bulimic behavior because the dieting that may be required to avoid or reverse weight gain could drive the maintenance of binge eating and purging (Fairburn et al., 1993). Studies have in fact found that WS predicts weight gain in nonclinical women (Lowe et al., 2006), in outpatients with BN (Carter et al., 2008), and in residential patients with BN (Lowe et al., 2006). Lowe et al. (2007) found that WS was positively related to frequency of objective binge eating in individuals with BN and Butryn et al. (2006) found that greater WS predicted poorer outcome (study dropout and continuation of eating disorder symptoms at the end of treatment) in the cognitive-behavioral treatment of BN (though Carter et al. (2008) did not replicate this effect). These studies suggest that individuals with BN who are high in WS may be caught in a “biobehavioral bind”: Suppressing their weight may reduce their feelings of fatness and unattractiveness but may fuel binge eating, purging and eventual weight gain, leading to renewed intensive dieting and further binge eating and compensatory behavior.
Although WS has been consistently predictive of weight gain from periods ranging from several weeks to several months, no study has examined its longer-term relation to weight change. If WS as a predictor of weight gain is restricted to small amounts of weight over relatively brief periods of time, then its role in the perpetuation of BN may be limited. However, if WS predicts large weight gains over long periods of time, then it may play a more significant role in the maintenance of BN because such weight gain would presumably be anathema to these individuals, leading to intensified weight loss efforts that help perpetuate the disorder.
The Massachusetts General Hospital (MGH) Longitudinal Study of Anorexia and Bulimia Nervosa is a prospective, naturalistic examination of 246 treatment-seeking women with anorexia nervosa (AN) or BN. The detailed longitudinal weekly data available from study participants offers a unique opportunity for investigating the relationship between WS among women with BN and prospective weight change. The availability of comprehensive assessment data at entry also allowed us to examine whether relevant covariates (i.e. age and entry BMI) might account for any prospective prediction of weight change by WS at entry. This study extends the design of previous studies by investigating weight change over a longer duration of follow-up and by assessing weight at frequent intervals. Based on the prediction of short-term weight gain in previous studies of WS, we hypothesized that weight suppression at study entry would predict greater weight gain over five years.
2. Method
2.1 Participants
Participants in the MGH Longitudinal Study of Anorexia and Bulimia Nervosa were recruited from MGH and other Boston-area treatment centers between October 1987 and June 1990. Five hundred and fifty-four women were screened for study inclusion. Of those, 229 women agreed to participate in the study after meeting criteria for AN or BN as detailed in the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (APA, 1987). Participants had to be English-speaking, live within 200 miles of the study site, be at least 12 years of age, and have no evidence of an organic brain syndrome or terminal illness. Prior to the first follow-up interview, four women discontinued participation, leaving a sample of 225 women. Twenty one additional participants with AN were recruited in 1991, increasing the sample to 246. When the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders was published (APA, 1994), participants’ eating disorder entry diagnoses were reclassified according to the new criteria; the sample comprised 51 women with AN restricting subtype, 85 women with AN binge/purge subtype, and 110 women with BN. Characteristics of the full sample at intake have been described elsewhere (Herzog et al., 1992, Herzog et al., 1999).
Participants for this report were drawn from the women with BN at study entry; thirteen participants were removed from the analyses because they became pregnant during the course of follow-up (i.e., between study entry and 5 years), leaving a final sample of 97. At entry, the mean duration of eating disorder illness was 6.2 years (SD = 6.6) and the mean age of the participants was 25.7 years (SD = 6.7). Women with BN were followed for a median of 9.5 years and 93.6% of these women were followed for 5 years (attrition rate = 7/110, 6.4%). On the basis of a Mann-Whitney U test, there were no significant differences between those who remained in the study and those who discontinued participation with regard to age, weight, or WS. A 5-year follow-up interval was used in the current report to maximize the available data and to avoid the widening confidence intervals that exist beyond 5 years due to attrition.
2.2 Procedure
Participants meeting inclusion criteria for the study were interviewed in person by a trained research assistant. Current and lifetime Axis I disorders were assessed, and data were gathered on current and past eating disorder symptomatology. Additionally, objective measurements of participants’ height and weight were taken during this interview. During follow-up, participants were interviewed every six months. Interviews were conducted in person whenever possible or by telephone. Approximately 90% of follow-up body weights were collected via self-report. However, because we found near-perfect agreement between self-reported and measured body weights at baseline (r = 0.96, p < 0.001), this procedure presumably introduced little bias. Furthermore, any bias would be consistent over time within participants. Compensation was provided for all interview assessments.
This study was approved by the MGH Institutional Review Board and written informed consent was obtained from all participants.
2.3 Measures
At entry, current and lifetime diagnoses were assessed with a modified version of the Schedule for Affective Disorders and Schizophrenia—Lifetime Version (Spitzer & Endicott, 1979), which included a section from the Diagnostic Interview Schedule (Robins et al., 1981) with criteria for AN and BN. Women completed the Eating Disorders Inventory questionnaire (EDI) (Garner et al., 1983), which assessed self-reported current weight, height, highest past weight (phrased to inform participants to exclude pregnancy), and lowest past weight. A calibrated stadiometer and balance beam scale were used to obtain current height and weight measurements. See Herzog et al. (1992) for a complete description of all measures completed during the entry interview.
Measurement of WS at study entry was calculated by subtracting participants’ current weight from their highest past weight (since reaching their adult or current height), as self-reported in the EDI. The validity of recalled past weights has been supported by a study that found a correlation of 0.85 between measured body weight at age 25 and recalled weights for age 25 that were collected an average of 20 years later (Tamakoshi et al., 2003); the mean error of recalled weights was just 1.28 kg, suggesting that the absolute size of the error in recalled weights was small. If on entry to the study a participant reported that she currently weighed her highest weight, WS equaled zero.
During follow-up, participants were interviewed with the Longitudinal Interval Follow-up Evaluation—Eating Disorders Version (LIFE-EAT II), a modified version of the LIFE II interview (Keller et al., 1987). The LIFE-EAT II was used to assess symptoms of eating disorders, comorbid diagnoses, pharmacological and psychological treatment participation, and psychosocial functioning. Self-reported weights were recorded at each follow-up interview and were analyzed in this report at 6-month intervals.
Entry weight and follow-up weights were calculated into BMI measurements (kilograms divided by (meters)2).
2.4 Data Analytic Procedure
The broad analytic plan used followed Hedeker and Gibbons (2006) and Peugh and Enders (2005). SPSS version 14 was used to complete all analyses. As a first step, descriptive statistics were generated to identify the distribution of scores and the need for transformation. Unless noted otherwise, the distribution of all variables was sufficiently normal.
Hierarchical linear modeling (HLM) was used to investigate changes in BMI over time. HLM is a form of multi-level modeling that expands upon traditional regression techniques by (a) more accurately modeling changes in individuals, (b) treating time as continuous rather than a fixed set of points, (c) allowing inclusion of individuals with missing data, and (d) allowing greater flexibility in the specification of covariance structures among repeated measures.
Our HLM analyses included two levels. Changes in the outcome over time (weight) were modeled at level-1 for each individual. Specifically, participants’ BMI was measured every six months for 60 months. BMI at intake was treated as time 0, thereby setting baseline BMI as the model intercept. Participant characteristics (i.e., WS and age) were modeled at level-2. HLM assumes that, for each individual, the dependent variable (i.e., BMI) is a function of intraindividual variables (i.e., time), person-level variables (i.e., WS), and error.
Our HLM analysis included two major steps. In the first step, unconditional models were specified to determine whether patterns could be identified in the mean change in BMI over time (fixed effects), and whether these patterns varied by individual (random effects). Covariates representing linear and non-linear (quadratic, cubic, and quartic) patterns of change were included in this step. This was done to determine whether significant interindividual variability exists in BMI slopes (i.e., change in BMI over time), which may be accounted for by level-2 covariates. Models with good fit are generally identified by parsimony, the presence of significant variability in slopes, and reliable coefficients (i.e., reliability > 0.1).
In the second step, level-2 covariates (WS and age) were added to the model to predict variability in BMI slopes. Level-2 predictors were mean centered before entry into the analysis. These models tested the hypotheses that baseline WS and age predict the direction and rate of change in BMI after a diagnosis of BN. Full maximum likelihood estimation was used in all HLM models to facilitate model comparison.
3. Results
3.1 Descriptives
Ninety-seven participants with a mean age of 25.47 years (SD = 6.66) were assessed at baseline. Mean BMI and the number of participants who contributed data at each assessment, are reported in Table 1. Mean baseline weight was 62.78 kg (SD = 9.63). Mean baseline WS was 3.68 (SD = 4.33) BMI units, or 10.11 kg (SD = 12.12). Baseline WS and BMI were uncorrelated (r = −0.01, p > 0.1, n = 97). Baseline WS was positively skewed, so all HLM analyses incorporating WS as a covariate were repeated using a root transformation of the WS variable. The pattern of results was completely consistent whether using the untransformed or transformed WS variable, so only the analyses using the untransformed WS variable are reported.
Table 1.
BMI at Each Assessment
| Assessment | n | Mean (SD) | Range |
|---|---|---|---|
| Entry | 97 | 23.25 (3.38) | 18.02 – 33.89 |
| 6 Months | 97 | 23.07 (3.03) | 16.83 – 33.30 |
| 12 Months | 96 | 23.45 (3.24) | 18.56 – 35.42 |
| 18 Months | 96 | 23.30 (3.38) | 18.29 – 37.12 |
| 24 Months | 96 | 23.36 (3.46) | 18.84 – 35.33 |
| 30 Months | 94 | 23.31 (3.60) | 16.37 – 35.33 |
| 36 Months | 94 | 23.53 (3.84) | 15.90 – 35.33 |
| 42 Months | 94 | 23.57 (4.26) | 15.90 – 43.07 |
| 48 Months | 93 | 23.81 (4.62) | 16.21 – 46.98 |
| 54 Months | 93 | 23.86 (4.75) | 16.21 – 46.98 |
Note: BMI = Body Mass Index (kg/m2)
At entry, the majority of participants (76.3%) had a BMI in the normal weight range (i.e. 18–24.99 kg/m2), 19.6% had a BMI in the overweight range (25–29.99 kg/m2), and 4.1% had a BMI in the obese range (greater than 30 kg/m2) (National Institute of Health, 1998).
Self-reported weights were compared to objectively measured weights at study entry in 82 (85% of 97) women who agreed to provide self-reported weight in addition to being weighed by the assessor. The mean discrepancy between self-reported and measured weights was 0.02 kg (SD = 2.68).
3.2 Unconditional Growth Model
In the first step of our HLM analysis, we evaluated linear, quadratic, cubic, and quartic models of the change in BMI over time. The mean change in BMI units was 0.66 (SD = 3.78), or a gain of 1.84 kg (SD = 10.45). Only the linear model showed significant variability in both intercepts (variance component = 8.392, SD = 2.897, χ2 = 1540.72, df = 96, p < 0.001) and slopes (variance component = 0.003, SD = 0.059, χ2 = 837.19, df = 96, p < 0.001), with reliable estimates of coefficients (intercept = 0.936, slope = 0.868). The coefficient for the slope of change in BMI over time was of borderline significance (coefficient = 0.0132, SE = 0.006, df = 96, t-ratio = 1.951, p = 0.054), with a trend towards increasing BMI over time. Intercepts (i.e., baseline BMI) and slopes (i.e., change in BMI over time) were not associated (coefficient = 0.009, SE = 0.019, χ2 = 90.04, df = 96, p = 0.652).
3.3 Conditional Growth Models
In the second step of our HLM analysis, level-2 covariates were added to the model in an attempt to account for the variability in slopes. The addition of age at baseline significantly improved model fit (χ2 = 12.22, df = 2, p = 0.002), and the addition of baseline WS (measured in BMI units) further improved model fit (χ2 = 44.58, df = 2, p < 0.001). For the model that included both baseline WS and age, age (coefficient = 0.169, SE = 0.046, df = 94, t-ratio = 3.703, p = 0.001), but not WS (coefficient = −0.112, SE = 0.070, df = 94, t-ratio = 1.601, p = 0.112) had a significant effect on the level-1 intercept (coefficient = 23.033, SE = 0.290, df = 94, t-ratio = 79.444, p < 0.001). In contrast, WS (coefficient = 0.009, SE = 0.001, df = 94, t-ratio = 6.854, p < 0.001), but not age (coefficient = 0.0003, SE = 0.001, df = 94, t-ratio = 0.388, p = 0.698) had an effect on the slope representing change in BMI over time (coefficient = 0.013, SE = 0.005, df = 94, t-ratio = 2.420, p = 0.018). Even after baseline WS and age were added to the unconditional model, there remained significant variability in intercepts (variance component = 7.593, SD = 2.756, χ2 = 1371.40, df = 94, p < 0.001) and slopes (variance component = 0.002, SD = 0.046, χ2 = 527.47, df = 94, p < 0.001). Intercepts (i.e., baseline BMI) and slopes (i.e., change in BMI over time) were not associated (coefficient = 0.014, SE = 0.014, χ2 = 100.02, df = 94, p = 0.316).
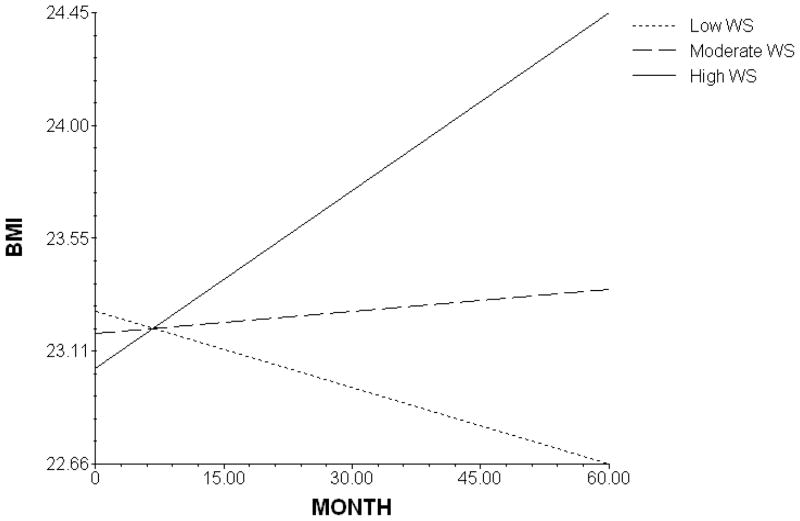
The above findings suggest that, at baseline, women who were older also had higher BMIs. Furthermore, baseline WS was a significant predictor of the change in BMI in the 5 years following a diagnosis of BN. However, age and WS did not completely account for the individual differences in BMI trajectories. Also, BMI at baseline did not predict BMI change over the 5-year period. As depicted in Figure 1, weight change over 5 years was differentially related to baseline WS, such that the direction and extent of weight change depended on WS level at intake into the study.
Figure 1.
The modeled effect of weight suppression (WS) on BMI slope over 5 years.
4. Discussion
The current results extend past findings by showing that WS among individuals with BN predicts weight change not only over periods of a few months (Lowe et al., 2006; Carter et al., 2008) but over a period of 5 years. While the average weight change among individuals with BN in our sample was minimal, the trajectory of 5-year weight change differed dramatically, depending on initial WS level (Figure 1). Those high in WS gained a substantial amount of weight, those with moderate WS gained a little weight, and those with the lowest WS lost a modest amount of weight. These effects were still evident when age and initial BMI were controlled.
Our findings are consistent with the literature, which concludes that on average individuals with BN experience relatively little change in BMI over time. For example, Fairburn et al. (1993) reported that patients with BN lost a small (0.6 BMI units or about 1.5 kg) but significant amount of weight from treatment start to a one-year follow-up. In a much longer follow-up also following outpatient treatment done by Fairburn et al. (1995), there was a modest (and significant) increase in weight (1.6 kg over a 6-year follow-up).
Two aspects of these findings suggest that the influence of WS on weight change is powerful. One, the fact that high levels of WS predicted weight gain over such a lengthy period of time suggests that the biological and behavioral consequences of WS are persistent and that whatever tactics high-WS BN individuals use to avoid weight gain are not very effective. Two, because individuals with BN evaluate themselves largely in terms of weight and shape and abhor weight gain (Fairburn, 2008), the weight gain observed in high-WS participants presumably occurred despite an intense fear of weight gain and fatness. Two factors that may have contributed to this weight gain are binge eating and metabolic efficiency. Past research has found a positive relationship between WS and frequency of objective binge eating in those with BN (Lowe et al., 2007), and significant weight loss is known to produce an exaggerated reduction in metabolic rate (i.e., beyond that expected based on loss of lean tissue (Rosenbaum et al., 2008)), which means that those higher in weight suppression would be more prone to store (as body fat) rather than oxidize energy consumed beyond energy needs.
The weight loss observed in those low in WS, unlike the weight gain of those high in WS, was presumably intentional in nature. Being at a weight that is at or near one’s highest weight ever would presumably be distressing to those with BN, which might result in weight loss efforts involving caloric restriction, purging, physical activity or some combination of these. Although volitional weight loss would generally be viewed as counter-therapeutic in the treatment of BN (Fairburn, 2008), future research should determine how weight loss relates to treatment outcome among BN individuals low in WS.
It is possible that our findings do not reflect participants’ current level of WS so much as their inability to keep their weight at a stable level. The fact that those lowest in WS lost weight over time is consistent with this interpretation. However, WS has also predicted weight gain in normal weight college students without disordered eating (Lowe & Kral, 2006) suggesting that weight suppression itself may be problematic.
It is notable that BMI did not predict weight change over 5 years and that BMI and WS at entrance into the study were uncorrelated. Thus absolute BMI was not informative about the extent of future weight change, but current BMI relative to highest ever BMI was predictive. The prediction of future weight change by WS indicates, in line with Russell’s (1979) original theorizing, that therapists should assess the weight history of their bulimic patients because such information may reflect the direction and extent of future weight change, changes that could undermine therapeutic progress. For instance, despite other positive changes that may be occurring, a patient who is gaining weight may attribute it to the changes they are making, become alarmed, and drop out of treatment.
The cognitive-behavioral model of BN views over-concern with weight and shape as the “core psychopathology” of BN (Fairburn, 2008). However, a patient’s level of weight suppression is not taken into account in the assessment or treatment of the disorder. One implication of the present results is that concern with weight should be evaluated in relation to a patient’s current body mass and especially to the difference between their current and highest-ever body weight. The greater a patient’s level of weight suppression, the more likely it is that she may be caught in a “biobehavioral bind” (Butryn et al., 2006) from which she cannot extricate herself. That is, a (realistic) fear of weight gain may lead to vigorous dieting, which could exacerbate binge eating and purging. The way out of this dilemma is not clear, but it could involve preparing the patient to accept the possibility that some weight gain may occur during treatment and in fact may be helpful in reducing the pattern of restricting, binge eating and purging that comprise BN. Although CBT for BN does inform patients that their weight may change during treatment (Fairburn, 2008), the likelihood and direction of future weight change based on a patient’s weight suppression level is not considered.
Two qualifications need to be taken into account when interpreting the present results. First, the construct of current dieting is different from the construct of WS (Lowe, 1993). In fact, current dieting among those with bulimia nervosa has been found to be associated with reduced levels of binge eating (Lowe et al., 1998; Lowe et al., 2007) whereas WS was found to be positively related to binge eating frequency (Lowe et al., 2007). Second, the consequences of WS in bulimia nervosa appear to be different than in obesity. Studies from the National Weight Control Registry indicate that previously obese individuals who lose substantial weight and keep it off do not appear to be more susceptible to disinhibited or binge eating (Wing & Hill, 2001).
Strengths of this study include the use of structured diagnostic interviews, frequent assessments of body weight, and the long-term follow-up. A possible limitation of the study is that follow-up weights were collected via self-report, though this concern is mitigated somewhat because there was almost no discrepancy between the average of measured and self-reported weights at baseline. The results are based on women with BN who were willing to join the study and report repeatedly on their clinical status over many years. The generalizability of the results to other populations with BN cannot be assumed.
In conclusion, the present study adds to several others indicating that bulimic patients on average maintain body weights well below their highest historical weights and that such weight suppression may contribute to weight gain and help perpetuate their eating disorder. Future research should explore the therapeutic implications of treating BN patients with elevated levels of weight suppression.
Acknowledgments
This work was supported by the National Institute of Mental Health grant 5R01 MH 38333 05 (DBH). These data were presented in part at the Eating Disorder Research Society Annual Meeting in Montreal, Quebec (September 2008). The authors would like to thank Stephanie Ross for her assistance in editing and preparing the final version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. American Psychiatric Association; Washington, DC: 1987. Revised. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Butryn ML, Lowe MR, Safer DL, Agras WS. Weight suppression is a robust predictor of outcome in the cognitive-behavioral treatment of bulimia nervosa. Journal of Abnormal Psychology. 2006;115:62–67. doi: 10.1037/0021-843X.115.1.62. [DOI] [PubMed] [Google Scholar]
- Carter FA, McIntosh VV, Joyce PR, Bulik CM. Weight suppression predicts weight gain over treatment but not treatment completion or outcome in bulimia nervosa. Journal of Abnormal Psychology. 2008;117:936–940. doi: 10.1037/a0013942. [DOI] [PubMed] [Google Scholar]
- Fairburn CG. Cognitive Behavior Therapy and Eating Disorders. Guilford Press; New York: 2008. [Google Scholar]
- Fairburn CG, Jones R, Peveler RC, Hope RA, O’Connor M. Psychotherapy and bulimia nervosa. Longer-term effects of interpersonal psychotherapy, behavior therapy, and cognitive behavior therapy. Archives of General Psychiatry. 1993;50:419–428. doi: 10.1001/archpsyc.1993.01820180009001. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Marcus MD, Wilson GT. Cognitive–behavioral therapy for binge eating and bulimia nervosa: A comprehensive treatment manual. In: Fairburn CG, Wilson GT, editors. Binge Eating: Nature, Assessment, and Treatment. Guilford Press; New York: 1993. pp. 361–404. [Google Scholar]
- Fairburn CG, Norman PA, Welch SL, O’Connor ME, Doll HA, Peveler RC. A prospective study of outcome in bulimia nervosa and the long-term effects of three psychological treatments. Archives of General Psychiatry. 1995;52:304–312. doi: 10.1001/archpsyc.1995.03950160054010. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Welch SL, Doll HA, Davis BA, O’Connor ME. Risk factors for bulimia nervosa: A community-based case-control study. Archives of General Psychiatry. 1997;54:509–517. doi: 10.1001/archpsyc.1997.01830180015003. [DOI] [PubMed] [Google Scholar]
- Garner DM, Olmstead M, Polivy J. The eating disorders inventory: a measure of cognitive-behavioral dimensions of anorexia nervosa and bulimia. In: Darby PL, editor. Anorexia Nervosa: Recent Developments in Research. Alan R. Liss; New York: 1983. pp. 173–184. [Google Scholar]
- Hedeker D, Gibbons RD. Longitudinal Data Analysis. John Wiley & Sons, Inc; Hoboken, NJ: 2006. [Google Scholar]
- Herzog DB, Dorer DJ, Keel PK, Selwyn SE, Ekeblad ER, Flores AT, Greenwood DN, Burwell RA, Keller MB. Recovery and relapse in anorexia and bulimia nervosa: a 7.5-year follow-up study. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:829–837. doi: 10.1097/00004583-199907000-00012. [DOI] [PubMed] [Google Scholar]
- Herzog DB, Keller MB, Sacks NR, Yeh CJ, Lavori PW. Psychiatric comorbidity in treatment-seeking anorexics and bulimics. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:810–818. doi: 10.1097/00004583-199209000-00006. [DOI] [PubMed] [Google Scholar]
- Keel PK, Dorer DJ, Franko DL, Jackson SC, Herzog DB. Postremission predictors of relapse in women with eating disorders. American Journal of Psychiatry. 2005;162:2263–2268. doi: 10.1176/appi.ajp.162.12.2263. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreason NC. The longitudinal interview follow-up evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Archives of General Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Lowe MR. The effects of dieting on eating behavior: a three-factor model. Psychological Bulletin. 1993;114:100–121. doi: 10.1037/0033-2909.114.1.100. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Davis W, Lucks D, Annunziato R, Butryn ML. Weight suppression predicts weight gain during inpatient treatment of bulimia nervosa. Physiology & Behavior. 2006;87:487–492. doi: 10.1016/j.physbeh.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Gleaves DH, Murphy-Eberenz KP. On the relation of dieting and bingeing in bulimia nervosa. Journal of Abnormal Psychology. 1998;107:263–271. doi: 10.1037//0021-843x.107.2.263. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Kral TV. Stress-induced eating in restrained eaters may not be caused by stress or restraint. Appetite. 2006;46:16–21. doi: 10.1016/j.appet.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Thomas JG, Safer DL, Butryn ML. The relationship of weight suppression and dietary restraint to binge eating in bulimia nervosa. International Journal of Eating Disorders. 2007;40:640–644. doi: 10.1002/eat.20405. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report [published correction appears in Obesity Research. 1998;6:464] Obesity Research. 1998;6(suppl 2):51S–209S. [PubMed] [Google Scholar]
- Peugh JL, Enders CK. Using the SPSS Mixed procedure to fit cross-sectional and longitudinal multilevel models. Educational and Psychological Measurement. 2005;65:717–741. [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Radcliff KS. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Archives of General Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. American Journal of Clinical Nutrition. 2008;88:906–12. doi: 10.1093/ajcn/88.4.906. [DOI] [PubMed] [Google Scholar]
- Russell G. Bulimia nervosa: an ominous variant of anorexia nervosa. Psychological Medicine. 1979;9:429–448. doi: 10.1017/s0033291700031974. [DOI] [PubMed] [Google Scholar]
- Spitzer R, Endicott J. Schedule for Affective Disorders and Schizophrenia. New York State Psychiatric Institute; New York: 1979. [Google Scholar]
- Tamakoshi K, Yatsuya H, Kondo T, Hirano T, Hori Y, Yoshida T, Toyoshima H. The accuracy of long-term recall of past body weight in Japanese adult men. International Journal of Obesity and Related Metabolic Disorders. 2003;27:247–252. doi: 10.1038/sj.ijo.802195. [DOI] [PubMed] [Google Scholar]
- Wing RR, Hill JO. Successful weight loss maintenance. Annual Review of Nutrition. 2001;21:323–341. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]