Abstract
Background
Postoperative pain remains a significant problem despite optimal treatment with current drugs. Nonsteroidal antiinflammatory drugs reduce inflammation and provide analgesia, but are associated with adverse side effects.
Methods
We tested low doses (0.5 – 5 mg/kg) of parenteral ketoprofen against pain related behaviors after plantar incision in rats. To further evaluate the potential sites of action of ketoprofen in our model, a novel, sustained-release microparticle formulation of ketoprofen was placed into the wound, and tested for its effects on pain behaviors. Intrathecal ketoprofen (150 mcg) was also studied. Plasma samples were assayed for drug concentrations.
Results
We found that low doses of parenterally administered ketoprofen produced a modality specific effect on pain behaviors; guarding after incision was decreased, whereas no inhibition of exaggerated responses to heat or mechanical stimuli was evident. Very low doses, 0.5 mg/kg, could produce inhibition of guarding. The locally applied sustained release ketoprofen-eluting microparticles and intrathecally administered ketoprofen also produced a modality-specific effect on pain behaviors after incision, inhibiting only guarding. Plasma levels of ketoprofen after parenteral or local administration were in the range of therapeutic blood levels in postoperative patients.
Conclusions
This study demonstrates that ketoprofen is an effective analgesic for nonevoked guarding in rats after plantar incision. There was no effect on mechanical or heat responses, which highlights the importance of multiple modality testing of pain behaviors for drug evaluation. We found efficacy at doses used clinically in postoperative patients.
Introduction
Therapeutic interventions aimed at reducing or eliminating postoperative pain are largely centered on opioids, nonsteroidal antiinflammatory drugs (NSAIDs) and nerve blockade with local anesthetics. Despite treatment with these drugs, postoperative pain remains a significant problem both immediately after surgery and, in some patients, it serves as the initiating event for chronic pain.1
New treatment strategies need to be developed which are effective with limited side effects. One avenue to accomplish this is to reduce pain by modifying nociceptor sensitization and perhaps reducing unwanted systemic or central nervous system side effects.
Ketoprofen has been used for the treatment of postoperative pain in patients.2 Ketoprofen, in doses of 1 to 2 mg/kg, consistently reduces pain and/or opioid requirements after many types of surgeries.2–7 In contrast, when ketoprofen is used in rodent postoperative studies, much larger doses (5–100 mg/kg) are administered.8–11 This suggests that there are shortcomings in translating from rodent postoperative pain models to clinical postoperative analgesia. Furthermore, such disparities in animal and human dosing may, in part, underlie some of the problems in analgesic development.
In the present study, we investigated the analgesic effect of ketoprofen on pain behaviors after plantar incision. We used 3 routes of administration; parenteral, intrathecal, and locally into the plantar incision to determine the site(s) of action. In our previous studies, we have found guarding pain behavior to be modifiable by clinically relevant doses of morphine.12,13 Because this particular guarding pain behavior and its response to low doses of other drugs may have strong clinical relevance, we further examined possible sites of action of the drug against these behaviors. First, to determine if a peripheral site of action is possible, a locally administered sustained release (SR) formula was placed inside the wound after the incision was made and pain behaviors were measured at varying timepoints. Second, to determine if a spinal site of action is plausible, the lowest dose of ketoprofen active parenterally was administered intrathecally; again pain behaviors were measured. Both were compared to the standard route of administration, parenteral. Plasma levels after local and parenteral administration were measured.
Methods
This study was approved by the Institutional Animal Care and Use Committee at the University of Iowa. Male Sprague-Dawley adult rats (Harlan, Indianapolis, IN) weighing 275 to 300 g were housed with a 12-hour light-dark cycle. Food and water were available ad libitum. All rats were euthanized at the end of the protocol. We used 73 rats; the intrathecal administration had 7 rats per group, the local and parenteral administration had 5 rats per group. We removed 4 rats from the intrathecal group due to epidural catheter placement (1 rat) and catheter dislodgement (3 rats). Data from all other rats were included in the analysis.
Plantar Incision
The rat hindpaw plantar incision model was performed as previously described.14 Briefly, rats were anesthetized with 2% halothane or 2 to 3% isoflurane delivered via nose cone. The plantar aspect of the right hindpaw was prepared in a sterile manner with a 10% povidone-iodine solution and draped. A 1 cm longitudinal incision was made with a number 11 blade through skin and fascia of the plantar aspect of the rat’s paw, starting 0.5 cm from the proximal edge of the heel and extending toward the toes. The flexor muscle was elevated and incised longitudinally and the muscle origin and insertion remained intact. After hemostasis with gentle pressure, the skin was apposed with 2 mattress sutures of 5-0 nylon on an FS-2 needle.
Pain Behaviors
Each rat underwent 3 pain behavior tests: guarding behavior, heat withdrawal latency, and mechanical sensitivity. Tests were always performed in this order each day by the same tester. In all experiments, persons who performed the tests were blinded to the dose of drug or vehicle administered.
Guarding Behavior
A cumulative pain score was used to assess non-evoked pain behaviors as described previously.14 Unrestrained rats were placed on an elevated plastic mesh floor (grid 8×8 mm) under a clear plastic cage (21 × 27 × 15 cm). The incised and nonincised paws were viewed. Both paws of each animal were closely observed during a 1 minute period repeated every 5 minutes for 1 hour. Depending on the position in which each paw was found during the majority of the 1-minute scoring period, a 0, 1, or 2 was given. Full weight-bearing of the paw (score =0) was present if the wound was blanched or distorted by the mesh. If the incision was completely off the mesh without any touch, a score of 2 was recorded. If the area of the wound touched the mesh gently without any blanching or distorting, a 1 was given. The sum of the 12 scores (0 – 24) obtained during the 1 hour session for each paw was obtained. The difference between the scores from the incised paw and nonincised paw was the cumulative pain score for that 1-hour period (ranges from −24 to +24).
Responses to Heat
Withdrawal latencies to heat stimuli were assessed by applying a focused radiant heat source to the unrestrained rat placed on a heat-tempered glass floor. The heat stimulus was a light from a 50 W projector lamp, with an aperture diameter of 6 mm, applied from underneath the glass floor (3 mm thick) on the middle of the incision. The stimulus intensity was adjusted so that sham rats withdrew to the heat stimulus after 10 to 12 seconds. The latency to evoke a withdrawal response was determined with a cut-off value of 20 seconds. Three trials 5–10 minutes apart were used to obtain average paw withdrawal latency.
Responses to Mechanical Stimuli
Rats were placed on an elevated plastic mesh floor covered with a clear plastic cage top. The animals were allowed to ambulate, explore, and eventually rest lying on the mesh. For testing pain behaviors, Von Frey filaments (thin plastic filaments with calibrated forces) were applied underneath the cage adjacent to the wound. Each filament was applied once starting with 10 mN and continuing until a withdrawal response occurred or 250 mN was reached. If a rat did not respond to the 250 mN filaments, 522 mN was recorded as the next filament. This was repeated 3 times with a 10 min test-free period between withdrawal responses. The lowest force from the 3 tests producing a response was considered the withdrawal threshold.
Drug Preparation
Parenteral administration of ketoprofen (100 mg/mL, Fort Dodge Animal Health, Fort Dodge, IA) was injected subcutaneously using a 25 gauge needle attached to a 1.0 ml syringe. Injections were made in the back of the neck in various doses (0.5, 1, 5, and 10 mg/kg) diluted in preservative-free sterile 0.9% saline to a final volume of 0.5–0.6 mL.
Three formulations of SR microparticles containing 3 different concentrations of ketoprofen in a matrix of bioresorbable tyrosine-derived polymer (Ketoprofen SR) were prepared by TyRx Pharma, Inc. The formulations were designed to release drug for at least 3 days and no more than 7 days. We placed the Ketoprofen SR or control polymer microparticles (no drug) into the wound at the time of surgery. Each SR release ketoprofen microparticle dose was 9 mg and contained either 5, 20, or 40% ketoprofen by weight. Bioresorbable microparticles containing no drug were also prepared and used as a control.
Ketoprofen for intrathecal administration (Sigma-Aldrich, Saint Louis, MO, USA) was reconstituted as 50 mg of ketoprofen, 3 ml of water, 0.2 ml of 1M NaOH until dissolved and the pH slowly adjusted to 7.4. The final volume was increased to 5 ml, resulting in a final concentration of 40 mM NaCl, and 10 mg/ml ketoprofen. The solution was filtered with a 0.2 micron syringe filter. The volume of drug injection was 15 microliters.
Intrathecal delivery
Intrathecal catheter placement was performed for subarachnoid ketoprofen administration as previously described.15 Under isoflurane anesthesia, the rat was placed in a kyphotic position, and a 3-cm longitudinal lumbar skin incision was made along the midline at the level of the iliac crests. The lumbar fifth and sixth intervertebral space was punctured with a 23-gauge hypodermic needle. Penetration into the subarachnoid space was indicated by a tail flick or hindpaw retraction. A 10-cm-long 32-gauge polyurethane catheter (Micor, Allison Park, PA) was threaded through the needle cranially for approximately 3 cm. The 32-gauge catheter was fixed to the fascia and connected to a PE-10 catheter (Becton Dickinson, Sparks, MD) and tunneled under the skin to the nape of the neck. The catheter was flushed with sterile saline and closed by heating the plastic. The dead-space of the catheter was approximately 5 μL. Before each injection, the heat-sealed catheter tip was cut off and injections were made with a sterile needle and syringe. To confirm the catheter location, a lidocaine test (2% lidocaine, 20 μL) was performed after the animal emerged from anesthesia. All drugs were flushed with 10 μL of sterile, preservative-free normal saline. Experimental protocols began on day 3 after intrathecal catheterization. At the end of the experiment, 30 μL methylene blue dye was injected intrathecally followed by spinal dissection to confirm the location of the intrathecal catheter. If the lumbar spinal cord did not stain with the dye, the animal was excluded from the study.
Experimental protocols
In one group of rats, ketoprofen was administered parenterally. Saline or various concentrations of ketoprofen were injected subcutaneously and rats were allowed to rest in their cages for 30 minutes after injection before undergoing plantar incision. Based on previous pharmacokinetic studies in humans and rats, 30 minutes would allow for some effect of ketoprofen at the time of incision, and likely a peak effect at the time of pain behavior testing16–18 On the operative day, rats were tested twice, at 2h and 4h after incision. Subsequent behavioral testing was performed 2h after repeat injection of ketoprofen or saline on postoperative days (POD) 1–4. Rats were tested for 5 days and then euthanized.
In a second group of rats, Ketoprofen SR or the no-drug control microparticles were applied into the wound before closure. The onset time of this experimental drug is not known, however based on previous studies in humans and rats, ketoprofen’s effects would likely be present with application into the wound and reach peak effects at 2 hours when behaviors were studied.16–18 There were 6 groups studied. Three groups received 9 mg of Ketoprofen SR (5%, 20%, or 40%) into the wound and 3 control groups which consisted of: nothing in the wound, 9 mg of control microparticles alone placed into the wound, and 9 mg of 40% Ketoprofen SR (40% Ketoprofen SR) placed subcutaneously under a small incision at the neck. They were tested 2 and 4 hours after incision, twice on POD 1 and once on POD 2, 3, 4, 6 and 8.
In a third group of rats, intrathecal ketoprofen was injected. There were 2 groups studied, the intrathecal ketoprofen and vehicle control. Thirty minutes before surgery, animals received either 15 μl ketoprofen or vehicle intrathecally. This allowed for some effect of the ketoprofen at the time of pain behavior testing.16–18 We administered ketoprofen at a dose of 150 μg/15 μl. This dose (150 μg ≈ 0.40 – 0.45 mg/kg) was slightly less than the lowest effective dose administered parenterally (0.5 mg/kg). Animals were tested for pain behaviors 2 hours after surgery. Subsequent behavioral testing was performed 30 minutes after repeat intrathecal injection of ketoprofen or vehicle on POD 1 and 2.
Drug Assays
In rats receiving Ketoprofen SR, blood samples (0.5 ml) were obtained from the tail vein after behavioral testing on days 2 and 8. Samples were centrifuged at 12,000 g for 5 minutes and 250 μl of serum was frozen at −70°C. Ketoprofen blood levels were assayed by Covance, Inc. (Princeton, NJ) using a high performance liquid chromatography methodology. We measured ketoprofen levels in rats 2 hours after parenteral administration using a Waters Q-Tof Premier mass spectrometer which is a hybrid mass spectrometer with a quadrupole mass analyzer in-line with a time-of-flight mass analyzer. The Q-Tof Premier was interfaced with a Waters Acquity UPLC (ultra-high pressure liquid chromatograph) equipped with an autosampler. A Waters Acquity UPLC BEH C18 (1.7μm, 2.1 × 100mm) column was used for analyses. All comparisons were made to ketoprofen and fenoprofen standard curves.
Statistics
All data were analyzed by Prism 5.0 software (GraphPad Software, Inc., San Diego, CA). Withdrawal threshold to mechanical stimuli are non-continuous and therefore were analyzed with nonparametric tests. The data were expressed as median and interquartile range. Differences were determined by Kruskal-Wallis test followed by Dunn’s post hoc test for comparing paw withdrawal threshold after drug to vehicle. For guarding pain scores and withdrawal latencies to heat, data were expressed as mean ± SEM. One-way ANOVA followed by Dunnett’s post hoc test was used for comparisons versus vehicle. Un-paired t test was performed for two groups’ comparison. P<0.05 was considered statistically significant.
Results
Effect of ketoprofen on non-evoked guarding behavior caused by plantar incision
In the group of rats treated with saline, the mean cumulative pain score increased from 0.0 ± 1.0 to 17.9 ± 3.2 2 hours after incision (Fig. 1A). These scores in the control group were similar to scores in untreated rats from previous studies.19,20 There was a significant dose-dependent decrease in cumulative pain score for parenteral ketoprofen at 2h up to 5 mg/kg. Ketoprofen at 5 mg/kg decreased the pain score to 4.8 ± 6.2 and 10 mg/kg decreased the pain score to 4.2 ± 1.9. The effect of parenterally administered ketoprofen on guarding was evident the first POD for all doses studied, and for the first 2 PODs for the 5 mg/kg and 10 mg/kg groups. Guarding had resolved by days 3 and 4 (in parenteral group the baseline score was: 0 ± 0.38, day 3 score: 4.7 ± 0.75, day 4 score: 3.0 ± 1.4, p>0.05, not significant from baseline).
Figure 1.
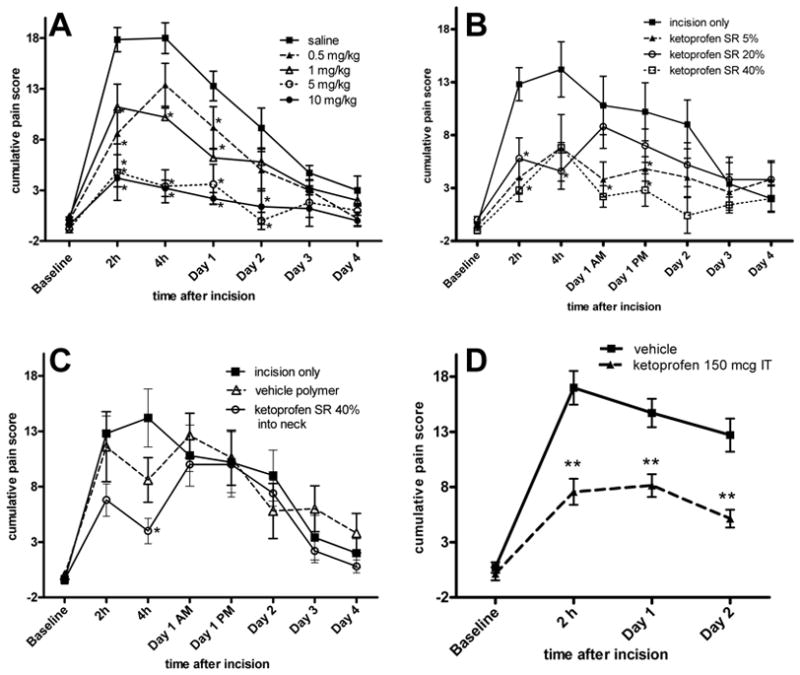
Effect of ketoprofen on non-evoked, guarding pain behavior caused by plantar incision. A: Parenteral administration of ketoprofen produced a dose-dependent decrease in guarding pain behavior. B: Effect of locally administered Ketoprofen sustained-release (SR) polymer on non-evoked, guarding pain behavior caused by plantar incision. Drug was effective in the early postoperative period. C: Control groups for locally administered Ketoprofen SR polymer on non-evoked guarding. Administration of the highest dose of Ketoprofen SR polymer parenterally (subcutaneously into the neck) attenuated guarding pain behavior at the 4h time point only. The incision only group is the same as shown in part B. D: Effect of intrathecal (IT) ketoprofen on on-evoked guarding caused by plantar incision. IT administration of ketoprofen produced a highly significant decrease in pain behavior for the first 2 postoperative days. Data are expressed as mean ± SEM. ANOVA followed by the Dunnett test was used for comparisons versus controls (incision only, saline, or vehicle.) *p<0.05 versus controls, **p<0.001 versus controls, n=5 per group (A, B, C) or n=7 per group (D).
For the locally treated groups, the mean cumulative pain score for the control (incision only) increased from −0.4 ± 0.6 to 12.8 ± 3.5 2 hours after incision (Fig. 1B) in the incision only group. Pain scores for the incision only and the empty (control) microparticles only group were not statistically different. The lowest dose Ketoprofen SR (5%) significantly decreased guarding pain scores at 2h to 4.0 ± 3.54. There was no dose-dependent effect; further reductions in pain scores with higher doses of Ketoprofen SR (5.8 ± 4.3 for 20% compound and 2.8 ± 2.4 for 40% compound) were not evident. By the first POD, the highest dose of Ketoprofen SR attenuated guarding pain behavior caused by plantar incision. Later timepoints (days 2–4) did not reveal a statistically significant difference between any dose of sustained-release ketoprofen and the control group; however, all scores trended to be lower than the controls. Pain scores in the control groups had decreased by almost 50% on day 2.
There was a significant reduction in non-evoked guarding pain behavior 4 hours after incision in the group of rats treated with Ketoprofen SR polymer at the highest dose (40%) injected into the subcutaneous tissue at the neck (Fig. 1C). There was no other effect at other timepoints on guarding with this dose compared to the vehicle polymer placed in the incision.
In the group of rats treated with intrathecal ketoprofen, the mean cumulative pain score for the control animals increased from 0.7 ± 0.4 to 17.0 ± 1.5 2 hours after incision (Fig. 1D). Treatment with intrathecal ketoprofen resulted in a significant decrease in guarding pain score at 2h (7.6 ± 1.2). The effect of ketoprofen remained significantly different from vehicle control for the first 2 PODs; 8.1 ± 1.3 versus 14.7 ± 1.3 on POD 1 and 5.2 ± 0.8 versus 12.7 ± 1.5 on POD 2 (Fig. 1D).
Effect of ketoprofen on withdrawal latency to radiant heat caused by plantar incision
In the saline-treated group, the mean withdrawal latency to radiant heat decreased from 12.9 ± 0.7 sec to 3.3 ± 0.8 sec 2 hours after incision (Fig. 2A). Generally, all doses of parenteral ketoprofen (0.5, 1, 5 and 10 mg/kg) did not affect heat withdrawal latency. Only on POD 3, did the 5 mg/kg parenteral dose increase withdrawal latency compared to saline control (p < 0.01).
Figure 2.
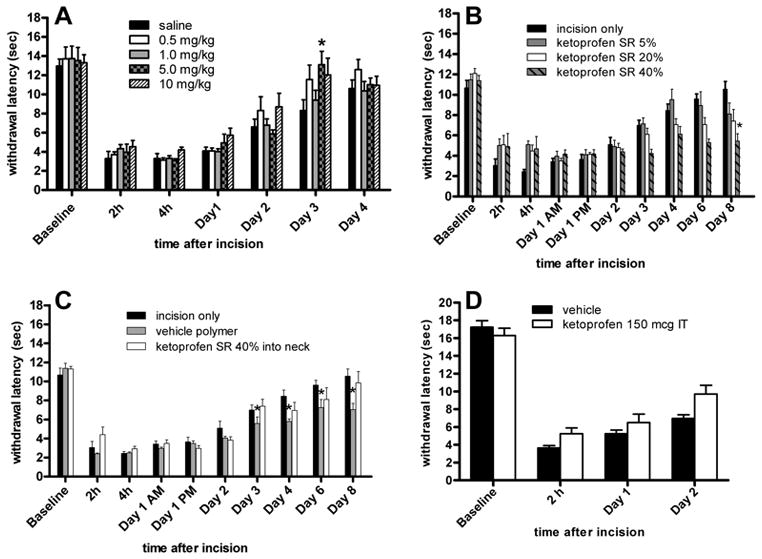
Effect of ketoprofen on withdrawal latency to heat after plantar incision. A: Parenteral ketoprofen did not increase the withdrawal latency. B: Effect of locally administered Ketoprofen sustained-release (SR) polymer on withdrawal latency to heat after plantar incision. Locally administered Ketoprofen SR polymer did not increase the withdrawal latency after incision. C: Control groups for locally administered Ketoprofen SR polymer on withdrawal latency to heat. There was no increase in the withdrawal latency. The incision only group is the same as shown in part B. D: Effect of intrathecal (IT) ketoprofen on withdrawal latency to heat after plantar incision. Ketoprofen did not increase the withdrawal latency. Data are expressed as mean ± SEM. ANOVA followed by the Dunnett test was used for comparisons versus controls (incision only, saline or vehicle). *p<0.05 versus control, n=5 per group (A, B, C) or n=7 per group (D).
None of the doses of locally administered, Ketoprofen SR (40%, 20% and 5%) increased withdrawal latency at any timepoint studied. At some times, the highest dose of Ketoprofen SR decreased the withdrawal latency on PODs 3, 6 and 8. Locally administered vehicle polymer also decreased the withdrawal latency at intermittent times (Fig. 2B), which may have been due to local irritation by the polymer. Ketoprofen SR polymer injected at the neck did not affect heat withdrawal latency (Fig. 2C). Intrathecal ketoprofen did not increase withdrawal latency at the timepoints studied (Fig. 2D).
Effect of ketoprofen on mechanical hypersensitivity caused by plantar incision
In the saline treated incised group, the median withdrawal threshold to von Frey filament decreased from 522 mN to 13 mN 2 hours after incision (Fig. 3A). All doses of parenteral ketoprofen (0.5, 1, 5 and 10 mg/kg) did not increase the withdrawal threshold at any timepoints studied. There was only a trend toward increasing withdrawal threshold in the highest doses of parenteral ketoprofen (5 mg/kg and 10 mg/kg) at all timepoints studied (Fig. 3A). We may have been able to detect a difference if more animals were studied.
Figure 3.
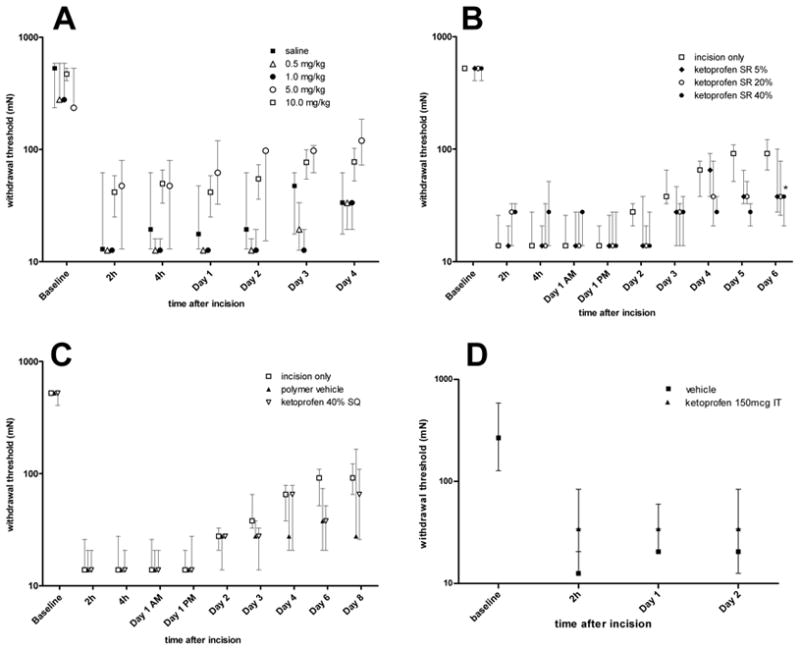
Effect of ketoprofen on mechanical withdrawal threshold after plantar incision. A: Parenteral ketoprofen did not increase the withdrawal threshold. B: Effect of locally administered sustained-release ketoprofen polymer on withdrawal threshold after plantar incision. Locally administered sustained-release ketoprofen polymer did not increase the withdrawal threshold after incision. C: Control groups for locally administered Ketoprofen sustained-release polymer on withdrawal latency to heat. There was no increase in the withdrawal threshold. The incision only group is the same as shown in part B. D: Effect of intrathecal (IT) ketoprofen on withdrawal threshold after plantar incision. Ketoprofen did not increase the withdrawal threshold. Data are expressed as median and interquartile range (25th –75th percentile), n=5 per group (A, B, C) or n=7 per group (D).
All doses of Ketoprofen SR (40%, 20% and 5%) did not increase the withdrawal threshold at any time tested (Fig. 3B). The highest dose of Ketoprofen SR resulted in a significant reduction in the withdrawal threshold on POD 6. Neither Ketoprofen SR injected at the neck nor empty vehicle polymer applied into the wound changed the withdrawal threshold compared with control incision only (Fig. 3C).
In the intrathecal group, the median withdrawal threshold to von Frey filaments decreased from 267 mN to 13 mN 2 hours after incision and ketoprofen did not increase the mechanical withdrawal threshold at any timepoint studied (Fig. 3D).
Ketoprofen plasma levels
Two hours after subcutaneous ketoprofen administration, plasma levels were 0.73 ± 0.19, 1.79 ± 0.58 and 8.43 ± 0.68 mcg/ml after 0.5, 1.0 and 5.0 mg/kg (Fig 4A). Plasma concentrations of Ketoprofen SR placed in the plantar incision varied with dose. The plasma concentration was 0.24 ± 0.16 mcg/ml after the largest dose of polymer was placed into the plantar incision. The smaller doses of Ketoprofen SR resulted in proportional decreases in plasma levels of the drug (0.10 ± 0. 04 mcg/ml and 0.02 ± 0.01 mcg/ml) for the 20% and 5% polymers, respectively). By day 8, the plasma levels of ketoprofen were negligible for all groups, which suggests that Ketoprofen SR worked as designed and reinforced the concept of a local site of action for ketoprofen. As shown in figure 4C, the highest plasma concentration of ketoprofen was evident after the largest dose of Ketoprofen SR polymer (40%) was placed into the subcutaneous tissue of the neck (0.63 ± 0.22 mcg/ml).
Figure 4.
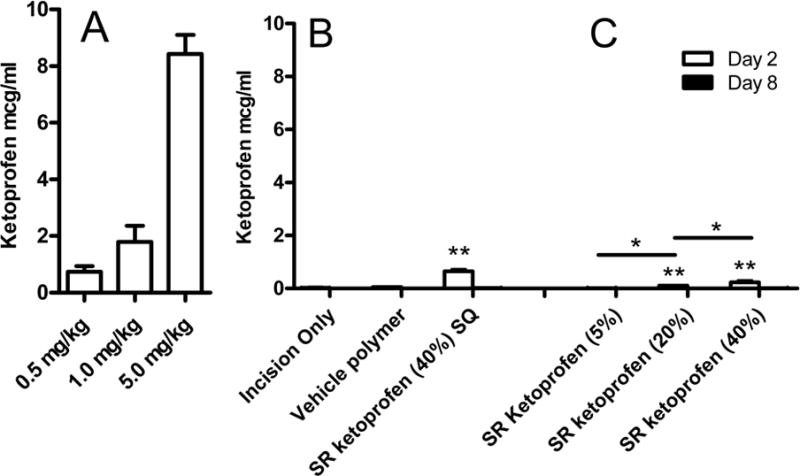
Plasma levels (mean ± SD) of ketoprofen. A: Dose-dependent increase in plasma concentration of ketoprofen measured 2 hours after subcutaneous injection. B: On day 2 and day 8 after local administration of Ketoprofen SR, the highest blood concentrations were seen in animals that received Ketoprofen SR polymer parenterally as a subcutaneous (SQ) placement into the neck (SR ketoprofen (40%) SQ). C: All concentrations of Ketoprofen SR placed locally into the wound had lower blood values than parenteral administration. Plasma values after local administration were dose-dependent. Ketoprofen levels were nearly undetectable in all groups by day 8. *p<0.05 between groups receiving ketoprofen in the wound, **p<0.05 versus incision only or vehicle polymer, n=5 per group (A, B, C) or n=7 per group (D).
Discussion
This study demonstrates that parenteral ketoprofen, in clinically relevant doses (0.5 to 1.0 mg/kg), produces a modality-specific effect on pain behaviors using 3 different routes of administration. Guarding after incision was decreased in all 3 groups. Parenteral administration produced a dose-dependent effect, whereas locally administered ketoprofen had the same modality-specific effect on pain behaviors after incision, without consistent dose-dependent effects. We found ketoprofen to be effective against guarding using doses that produce analgesia and similar plasma levels to those measured in patients. Intrathecal dosing decreased guarding pain behavior as well. Dosing regimens were designed to allow for peak effects during pain behavior testing. All routes of ketoprofen administration had minimal effects on heat hyperalgesia. Mechanical hyperalgesia was influenced by ketoprofen administration, but not to a significant extent compared to the control groups. The current study may have been under-powered to detect a significant difference in mechanical withdrawal threshold due to the variance inherent in von Frey testing.
We have suggested that guarding pain behavior is continuous, unprovoked pain in our postoperative model. It is likely transmitted by continuing neural activity, which is evident in nociceptors21 and dorsal horn neurons22 after incision and may be a correlate to pain at rest in patients after surgery. Guarding pain behavior is inhibited by very low doses of morphine, 0.03 to 0.1 mg/kg12, much lower doses than those used in most other analgesia studies using preclinical pain models.23–25 These lower doses of morphine are within a clinical dosing range for postoperative patients (0.05 to 0.2 mg/kg).26 Guarding is also inhibited by local anesthetic administration, nerve growth factor sequestration, and spinal morphine administration.12,20,22,27
NSAIDs like ketoprofen are often tested in preclinical pain models as a positive control and validation step in analgesic target development. Typically, very large doses of NSAIDs are used to inhibit pain-related behaviors in a variety of models of persistent pain.28 In postoperative patients, much smaller doses of NSAIDS are used.2,29–32
Ketoprofen has been extensively studied in rat models of persistent pain, with much higher doses needed to reduce pain behaviors (30–100 mg/kg).8 In the hindpaw incision model, parenteral ketoprofen did not affect heat hyperalgesia for the first 3 hours postoperatively, despite much higher doses than those used in our study (30 mg/kg and 100 mg/kg).8 Prado and Pontes reported that doses of 10 mg/kg and 20 mg/kg ketoprofen were effective at blunting the mechanical allodynia after hindpaw incision.9 This tendency was only evident in our highest dose.
Veterinarians customarily recommend doses of ketoprofen in the range of 5 mg/kg after surgery in rats.10,11 We found that this dose provided a maximal reduction in guarding pain behavior and that further treatment with larger doses (10 mg/kg) did not reduce the pain score further. The 5 mg/kg dose produced no significant effect on heat or mechanical hyperalgesia. In human trials for postoperative pain, ketoprofen has been used to reduce pain scores and for its opioid-sparing properties. Dosages in humans range from 0.5 to 2 mg/kg3–6 although other studies have shown the median effective dose in postoperative patients to be 30 mg, approximately 0.3–0.5 mg/kg.33 It is significant that we used dosages of 0.5 and 5.0 mg/kg, which are in the range of those used in postoperative patients. These dosages reduced pain behaviors and produced plasma levels of 0.25 to 8 mcg/ml, which is in the range of analgesic human plasma levels of 2 to 10 mcg/ml.16,17,34,35
We demonstrate analgesic effects against guarding only. These data add further evidence that guarding, reduced by clinically relevant doses of morphine, may be an important preclinical pain behavior in our model that translates to pain at rest in patients after surgery.
A recent clinical study supports our findings that guarding in rats may have significant translational relevance. Lavand’homme et al 36 evaluated the postoperative analgesic effect of a continuous infusion of diclofenac into the wound for 48 hours after elective cesarean delivery. The infusion significantly reduced postoperative morphine consumption by 18 mg. Thus, in patients, a locally administered NSAID, diclofenac, produced clinically significant analgesia and opioid-sparing effects.
The effect of ketoprofen on guarding pain behavior may not be entirely through inhibition of prostaglandin synthesis. Ketoprofen is a NSAID that inhibits the synthesis of arachidonic acid metabolites, including prostaglandins and leukotrienes. Leukotrienes are necessary for the maintenance of inflammatory and cellular proliferation pathways.37 Leukotrienes have been implicated in the pain caused by nerve growth factor38 and platelet activating factor.39 Therefore, the analgesic effect from ketoprofen may be due to an effect on leukotriene synthesis in addition to the reduction in prostaglandins. Other mechanisms, like N-methy-D-aspartate receptor blockade, are also possible.40
Spinal cyclooxygenase inhibitors and prostanoid pharmacology have recently been reviewed by Svensson and Yaksh.41 Cyclooxgenase isozymes are constitutively expressed in the dorsal root ganglion and spinal cord dorsal horn and can be increased in a variety of pain models. Collectively, intrathecal NSAIDs have been shown to have little or no effect on pain behaviors in models without a component of hyperalgesia and sensitization. However, for models such as the acetic acid writhing test, phase II of the formalin test, and thermal hyperalgesia by inflammation, intrathecal NSAIDs are very effective.41 Our postoperative pain model also displayed central sensitization22 and this may explain why intrathecal ketoprofen was effective at reducing guarding pain behavior in our model. Our study is also in agreement with others that the antinociceptive effects of intrathecal NSAIDs occur at doses that are largely ineffective systemically.42
The present study demonstrates that ketoprofen is an effective analgesic for non-evoked guarding in rats after plantar incision. There was no analgesic effect on mechanical or heat responses, which highlights the importance of multiple modality testing of pain behaviors. We found efficacy at doses that produce plasma levels that are similar to the levels produced by dosages that are used for pain relief in patients.
Acknowledgments
This work is supported by Foundation for Anesthesia Education and Research (FAER) Mentored Research Training Grant (CMS), NIH T32 NS045549 (CMS), NIH RO1 GM55831 (TJB) and TyRx Pharma, Inc. (TJB). The authors acknowledge the support of the University of Iowa High Resolution Mass Spectrometry Facility via an NCRR shared instrument grant S10 RR02338-01.
The authors are grateful to Lynn Teesch and the High Resolution Mass Spectroscopy Core Facility at the University of Iowa for technical assistance with ketoprofen measurements.
Footnotes
Conflict of Interest/Disclosures: Dr. Brennan has served as a consultant to TyRx Pharma, Inc. and Drs. Buevich and Moses are staff scientists at TyRx Pharma, Inc.
Author Roles: Dr. Spofford did the parenteral ketoprofen experiments and wrote the manuscript. Dr. Ashmawi did the intrathecal ketoprofen experiments. Mr. Subieta did the local ketoprofen experiments. Drs. Buevich and Moses constructed the ketoprofen sustained release preparation and consulted on experimental design. Dr. Baker measured the ketoprofen levels after parenteral administration. Dr. Brennan developed the model, constructed the experimental design, supervised all aspects of data collection and analysis and edited the manuscript.
Contributor Information
Christina M. Spofford, Associate, Department of Anesthesia, University of Iowa, Iowa City, IA, 52242.
Hazem Ashmawi, Visiting Professor, Department of Anesthesia, University of Iowa, Iowa City, IA, 52242.
Alberto Subieta, Research Assistant Department of Anesthesia, University of Iowa, Iowa City, IA, 52242.
Fatima Buevich, Senior Scientist TyRx Pharma, Inc, Monmouth Junction, NJ.
Arikha Moses, Chief Scientific Officer TyRx Pharma, Inc, Monmouth Junction, NJ.
Max Baker, Associate Professor, Department of Anesthesia, University of Iowa, Iowa City, IA, 52242.
Timothy J. Brennan, Samir Gergis Professor of Anesthesia, Department of Anesthesia, University of Iowa, Iowa City, IA, 52242.
References
- 1.Perkins F, Kehlet H. Chronic Pain as an Outcome of Surgery: A Review of Predictive Factors. Anesthesiology. 2000;93:1123–33. doi: 10.1097/00000542-200010000-00038. [DOI] [PubMed] [Google Scholar]
- 2.Sunshine A, Olson NZ. Analgesic efficacy of ketoprofen in postpartum, general surgery, and chronic cancer pain. J Clin Pharmacol. 1988;28:S47–54. doi: 10.1002/j.1552-4604.1988.tb05977.x. [DOI] [PubMed] [Google Scholar]
- 3.Wnek W, Zajaczkowska R, Wordlicezek J, Dobrogowski J, Korbut R. Influence of Pre-Operative Ketoprofen Administration (Preemptive Analgesia) on Analgesic Requirement and the Level of Prostaglandins in the Early Postoperative Period. Polish Journal of Pharmacology. 2004;56:547–552. [PubMed] [Google Scholar]
- 4.Boccara G, Chaumeron A, Pouzeratte Y, Mann C. The preoperative administration of ketoprofen improves analgesia after laparoscopic cholecystectomy in comparison with propacetamol or postoperative ketoprofen. Br J Anaesth. 2005;94:347–351. doi: 10.1093/bja/aei056. [DOI] [PubMed] [Google Scholar]
- 5.Basto ER, Waintrop C, Mourey FD, Landru JP, Eurin B, Jacob LP. Intravenous Ketoprofen in Thyroid and Parathyroid Surgery. Anesth Analg. 2001;92:1052–1057. doi: 10.1097/00000539-200104000-00047. [DOI] [PubMed] [Google Scholar]
- 6.Hiller A, Meretoja OA, Korpela R, Piiparinen S, Taivainen T. The Analgesic Efficacy of Acetaminophen, Ketoprofen, or Their Combination for Pediatric Surgical Patients Having Soft Tissue or Orthopedic Procedures. Anesth Analg. 2006;102:1365–1371. doi: 10.1213/01.ane.0000204278.71548.bf. [DOI] [PubMed] [Google Scholar]
- 7.Aubrun F, Langeron O, Heitz D, Coriat P, Riou B. Randomised, placebo-controlled study of the postoperative analgesic effects of ketoprofen after spinal fusion surgery. Acta Anaesthesiologica Scandinavica. 2000;44:934–939. doi: 10.1034/j.1399-6576.2000.440807.x. [DOI] [PubMed] [Google Scholar]
- 8.Girard P, Verniers D, Coppé M-C, Pansart Y, Gillardin J-M. Nefopam and ketoprofen synergy in rodent models of antinociception. European Journal of Pharmacology. 2008;584:263–271. doi: 10.1016/j.ejphar.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Prado W, Pontes R. Presurgical ketoprofen, but not morphine, dipyrone, diclofenac or tenoxicam, preempts post-incisional mechanical allodynia in rats. Brazilian Journal of Medical and Biological Research. 2002;35:111–119. doi: 10.1590/s0100-879x2002000100016. [DOI] [PubMed] [Google Scholar]
- 10.Roughan JV, Flecknell PA. Behavioural effects of laparotomy and analgesic effects of ketoprofen and carprofen in rats. Pain. 2001;90:65–74. doi: 10.1016/s0304-3959(00)00387-0. [DOI] [PubMed] [Google Scholar]
- 11.Roughan JV, Flecknell PA. Effects of surgery and analgesic administration on spontaneous behaviour in singly housed rats. Research in Veterinary Science. 2000;69:283–288. doi: 10.1053/rvsc.2000.0430. [DOI] [PubMed] [Google Scholar]
- 12.Wu C, Gavva NR, Brennan TJ. Effect of AMG0347, a Transient Receptor Potential Type V1 Receptor Antagonist, and Morphine on Pain Behavior after Plantar Incision. Anesthesiology. 2008;108:1100–1108. doi: 10.1097/ALN.0b013e31817302b3. [DOI] [PubMed] [Google Scholar]
- 13.Zahn PK, Gysbers D, Brennan TJ. Effect of systemic and intrathecal morphine in a rat model of postoperative pain. Anesthesiology. 1997;86:1066–77. doi: 10.1097/00000542-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 15.Pogatzki E, Zahn P, Brennan T. Lumbar catheterization of the subarachnoid space with a 32-gauge polyurethane catheter in the rat. Eur J Pain. 2000;4:111–113. doi: 10.1053/eujp.1999.0157. [DOI] [PubMed] [Google Scholar]
- 16.Roda A, Sabatini L, Mirasoli M, Baraldini M, Roda E. Bioavailability of a new ketoprofen formulation for once-daily oral administration. International Journal of Pharmaceutics. 2002;241:165–172. doi: 10.1016/s0378-5173(02)00230-2. [DOI] [PubMed] [Google Scholar]
- 17.Kokki H, Karvinen M, Suhonen P. Pharmacokinetics of intravenous and rectal ketoprofen in young children. Clinical Pharmacokinetics. 2003;42:373–379. doi: 10.2165/00003088-200342040-00005. [DOI] [PubMed] [Google Scholar]
- 18.Mannila A, Kokki H, Heikkinen M, Laisalmi M, Lehtonen M, Louhisto HL, Jarvinen T, Savolainen J. Cerebrospinal fluid distribution of ketoprofen after intravenous administration in young children. Clinical Pharmacokinetics. 2006;45:737–743. doi: 10.2165/00003088-200645070-00008. [DOI] [PubMed] [Google Scholar]
- 19.Wu C, Boustany L, Liang H, Brennan TJ. Nerve growth factor expression after plantar incision in the rat. Anesthesiology. 2007;107:128–35. doi: 10.1097/01.anes.0000267512.08619.bd. [DOI] [PubMed] [Google Scholar]
- 20.Banik RK, Subieta AR, Wu C, Brennan TJ. Increased nerve growth factor after rat plantar incision contributes to guarding behavior and heat hyperalgesia. Pain. 2005;117:68–76. doi: 10.1016/j.pain.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Pogatzki EM, Gebhart GF, Brennan TJ. Characterization of Adelta - and C-Fibers Innervating the Plantar Rat Hindpaw One Day After an Incision. J Neurophysiol. 2002;87:721–731. doi: 10.1152/jn.00208.2001. [DOI] [PubMed] [Google Scholar]
- 22.Pogatzki EM, Vandermeulen EP, Brennan TJ. Effect of plantar local anesthetic injection on dorsal horn neuron activity and pain behaviors caused by incision. Pain. 2002;97:151–161. doi: 10.1016/s0304-3959(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 23.Hama A, Sagen J. Behavioral characterization and effect of clinical drugs in a rat model of pain following spinal cord compression. Brain Research. 2007;1185:117–128. doi: 10.1016/j.brainres.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 24.van der Kam EL, Vry JD, Schiene K, Tzschentke TM. Differential effects of morphine on the affective and the sensory component of carrageenan-induced nociception in the rat. Pain. 2008;136:373–379. doi: 10.1016/j.pain.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 25.Lemberg K, Kontinen VK, Viljakka K, Kylanlahti I, Yli-Kauhaluoma J, Kalso E. Morphine, Oxycodone, Methadone and Its Enantiomers in Different Models of Nociception in the Rat. Anesth Analg. 2006;102:1768–1774. doi: 10.1213/01.ane.0000205751.88422.41. [DOI] [PubMed] [Google Scholar]
- 26.Aubrun F, Salvi N, Coriat P, BR Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology. 2005;103:156–60. doi: 10.1097/00000542-200507000-00023. [DOI] [PubMed] [Google Scholar]
- 27.Zahn PK, Subieta A, Park SS, Brennan TJ. Effect of blockade of nerve growth factor and tumor necrosis factor on pain behaviors after plantar incision. The Journal of Pain. 2004;5:157–163. doi: 10.1016/j.jpain.2004.02.538. [DOI] [PubMed] [Google Scholar]
- 28.Whiteside G, Harrison J, Boulet J, Mark L, Pearson M, Gottshall S, Walker K. Pharmacological consideration of a rat model of incisional pain. British Journal of Pharmacology. 2004;141:85–91. doi: 10.1038/sj.bjp.0705568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derry S, Barden J, McQuay H, Moore R. Single dose oral celecoxib for acute postoperative pain in adults. Cochrane Database Syst Rev. 2008;4:CD004233. doi: 10.1002/14651858.CD004233.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Mason L, Edwards J, Moore R, McQuay H. Single dose oral naproxen and naproxen sodium for acute postoperative pain. Cochrane Database Syst Rev. 2004;4:CD004234. doi: 10.1002/14651858.CD004234.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barden J, Edwards J, McQuay H, Moore R. Single dose oral celecoxib for postoperative pain. Cochrane Database Syst Rev. 2003;2:CD004233. doi: 10.1002/14651858.CD004233. [DOI] [PubMed] [Google Scholar]
- 32.Mason L, Edwards J, Moore R, McQuay H. Single dose oral indometacin for the treatment of acute postoperative pain. Cochrane Database Syst Rev. 2004 Oct;18(4):CD004308. doi: 10.1002/14651858.CD004308.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delage N, Maaliki H, Beloeil H, Benhamou D, Mazoit J-X. Median Effective Dose (ED50) of Nefopam and Ketoprofen in Postoperative Patients: A Study of Interaction Using Sequential Analysis and Isobolographic Analysis. Anesthesiology. 2005;102:1211–1216. doi: 10.1097/00000542-200506000-00022. [DOI] [PubMed] [Google Scholar]
- 34.Kokki H, Karvinen M, Suhonen P. Pharmacokinetics of intravenous and rectal ketoprofen in young children. Clin Pharmacokinet. 2003;42:373–9. doi: 10.2165/00003088-200342040-00005. [DOI] [PubMed] [Google Scholar]
- 35.Roda A, Sabatini L, Mirasoli M, Baraldini M, Roda E. Bioavailability of a new ketoprofen formulation for once-daily oral administration. Int J Pharm. 2002;241:165–72. doi: 10.1016/s0378-5173(02)00230-2. [DOI] [PubMed] [Google Scholar]
- 36.Lavand’homme P, Roelants F, Waterloos H, De Kock MF. Postoperative Analgesic Effects of Continuous Wound Infiltration with Diclofenac after Elective Cesarean Delivery. [Miscellaneous Article] Anesthesiology. 2007;106:1220–5. doi: 10.1097/01.anes.0000267606.17387.1d. [DOI] [PubMed] [Google Scholar]
- 37.Massoumi R, Sjölander A. The Role of Leukotriene Receptor Signaling in Inflammation and Cancer. ScientificWorldJournal. 2007;7:1413–21. doi: 10.1100/tsw.2007.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennett G, al-Rashed S, Hoult JRS, Brain SD. Nerve growth factor induced hyperalgesia in the rat hind paw is dependent on circulating neutrophils. Pain. 1998;77:315–322. doi: 10.1016/S0304-3959(98)00114-6. [DOI] [PubMed] [Google Scholar]
- 39.Dallob A, Guindon Y, Goldenberg MM. Pharmacological evidence for a role of lipoxygenase products in platelet-activating factor (PAF)-induced hyperalgesia. Biochemical Pharmacology. 1987;36:3201–3204. doi: 10.1016/0006-2952(87)90633-2. [DOI] [PubMed] [Google Scholar]
- 40.Lizarraga I, Chambers JP, Johnson CB. Prevention of N-Methyl-d-Aspartate-Induced Mechanical Nociception by Intrathecal Administration of Ketoprofen and Ketamine in Sheep. Anesth Analg. 2008;107:2061–2067. doi: 10.1213/ane.0b013e318187ac06. [DOI] [PubMed] [Google Scholar]
- 41.Svensson CI, Yaksh TL. The Spinal Phospholipase Cyclooxygenase Prostanoid Cascade in Nociceptive Processing. Annual Review of Pharmacology and Toxicology. 2002;42:553–583. doi: 10.1146/annurev.pharmtox.42.092401.143905. [DOI] [PubMed] [Google Scholar]
- 42.Malmberg AB, Yaksh TL. Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exp Ther. 1992;263:136–146. [PubMed] [Google Scholar]


