Abstract
While several clinical grade immunoassays exist for the specific measurement of hGH, or its isoforms in blood, there is an urgent need to apply these same reliable assays to the measurement of hGH in urine as a preferred non invasive biofluid. Unfortunately, conventional hGH immunoassays can not attain the required sensitivity to detect the low concentrations of hGH in urine. The lowest limit of sensitivity for existing hGH immunoassays is >50 pg/mL, while the estimated concentration of urinary hGH is about one pg/mL: fifty times lower than the sensitivity threshold. We have created novel N-isopropylacrylamide (NIPAm) based hydrogel nanoparticles functionalized with an affinity bait. When introduced into an analyte containing solution, the nanoparticles can perform, in one step, in solution, a) complete harvesting of all solution phase target analytes, b) fully protect the captured analyte from degradation and c) sequester the analyte and effectively increase the analyte concentration up to 100 fold. NIPAm nanoparticles functionalized with Cibacron Blue F3GA bait have been applied to raise the concentration of urinary hGH into the linear range of clinical grade immunoassays. This technology now provides an opportunity to evaluate the concentration of hGH in urine with high precision and accuracy.
Keywords: hGH, Immunoassay, Nanoparticles, Biomarker, Urine, Cibacron Blue F3GA
Introduction
Despite the ready availability of clinical grade human growth hormone (hGH) immunoassays,[1, 2] successful application of these platforms to the discovery of illegal doping cases has been rare. There are several reasons that hGH doping is difficult to detect. Firstly physiologic levels of hGH are normally low in blood, and can fluctuate widely or spike in concentration over the course of 24 hours.[3, 4] Secondly, normal physiologic levels of hGH levels can be influenced by exercise.[5, 6] Thirdly, the half-life of the persistence of elevated hGH in the blood, following the administration of a bolus of hGH is short and variable.[7] For these reasons it can be difficult to differentiate physiologic elevations of hGH from illegal hGH administration.
One way to overcome the deficiency in hGH anti-doping detection rates is to increase specificity for artificial versus native hGH. hGH is naturally present in the blood in different isoforms (22, 20, and 17 kDa), and may form dimers and multimers.[2, 8] Recombinant hGH (rhGH) consists of a unique isoform of 22 kDa and is monomeric only. The administration of the rhGH 22 kDa isoform and the reduction of endogenous hGH pituitary secretion due to negative feedback control increases the relative abundance of the 22 kDa in circulation.[9] Bidlingmaier and colleagues recently described a new high sensitivity chemiluminescence immunoassay exploiting antibodies directed against rhGH and pituitary derived hGH. The functional sensitivity of the assay was <50 ng/L (pg/mL).[10] After injection of rhGH, the ratio between recombinant and pituitary hGH remained significantly increased for 18–36 hours, depending on hGH dosage and sex of the patient.[10] The differential immunoassay test was applied at the 2004 Athens, 2006 Turin and 2008 Beijing Games but yielded no positives.[4] One possible reason for the lack of positive tests has been attributed to the short window of detection between the administration of rhGH and the ability to detect it.[11]
Measurement of hGH isoforms may improve the ability to distinguish normal from recombinant hGH in blood samples[9] but it will not solve the problem of the short half life of hGH elevation following an illegal administration. Consequently it has been proposed that urine testing could increase the timeframe in which hGH could potentially be detected. Unfortunately, currently, a precise and accurate clinical grade commercial urine hGH test does not exist. The first major reason is that the concentration of hGH in urine (approximately 1 pg/mL) is far below the detection limits of conventional clinical immunoassays. The principal metabolic clearance of hGH is glomerular filtration. hGH is efficiently re-absorbed and degraded in renal tubular cells, therefore only a small amount of hGH is present in urine (< 0.01 %).[4,11]
Beyond the extremely low concentration of hGH in the urine, other factors limit the ease of developing a standard assay for urine hGH. These factors include: a) A lack of information concerning the normal physiologic fluctuations of the levels of urine hGH isoforms of hGH,[12–15] the variable correlation of urine hGH with blood levels for samples collected at the same point in time, and the influence of exercise and kidney function on the urine hGH levels. b) Difficulties in preparing uniform standard preparations used to calibrate the assay, c) A majority of the hGH in the circulation forms complexes with high affinity circulating growth hormone binding proteins (GHBP).[16] The concentration of GHBP is highly variable and the complex formation can interfere with antibody recognition for the hGH epitope(s).[17]
In the past, a number of investigators have attempted to develop a highly sensitive immunoassay to detect hGH in human urine.[18] Saugy et al[11] employed two immunoassays, ELISA NordiTest (Novo Nordisk) and Delphia system (Pharmacia), with a dynamic range of 2–50 ng/L (pg/mL). Using this method, they measured the hGH concentration in urine donated by volunteer athletes before and after intense physical exercise (Table 1). The authors registered an increase of hGH levels in urine after exercise that did not depend on the specific gravity of urine or creatinine content. The authors observed that hGH release in urine depended on the type of athletic discipline practiced, and the levels increased in relatively short but very intense efforts.
Table 1.
Study on urinary hGH dependence on physical activity: Urinary hGH concentration+/−STD [pg/mL] / (number of athletes), adapted from Saugy and colleagues.[11]
| Type of athletic discipline | Before the competition [pg/mL] / (# athletes) |
After the competition [pg/mL] / (#athletes) |
|---|---|---|
| 17 km race | 4.0+/−0.2 / (130) | 62.8+/−7.9 / (130) |
| cycling (Tour the Swiss) | 71.6 / (60) | |
| Track and field (Switzerland) | 1513.9 / (60) | |
| Athletics* | 1514 / (50) | |
| Rowing* | 942 / (30) | |
| Weightlifting* | 6.2 / (30) | |
| Cycling* | 71.6 / (60) | |
| Volley team* | 4.4 / (179) | |
| Controls* | 3.7 / (114) | |
| Shot put, 1500 swimming, volleyball, basketball, 100 km cycling |
18+/−10 | |
| 800 m athletics, 1500 m athletics, 200 m swimming, 500 m canoe, 4000 m cycling |
1320+/−410 | |
| 400 m athletics, 400 m swimming, 1000 m canoe |
6437+/−2331 |
out of competition values for this set of measurement was 7.6 pg/mL on 50 athletes.
In another study,[19] the level of hGH in urine after administration of rhGH was evaluated (Table 2). The volunteers received subcutaneous or intramuscular hGH (to mimic both the therapeutic or the doping administration). In both treatments, the hGH concentration in urine increased 50–100 fold over the normal levels. In that study, urinary hGH peaks (300 ng/L (pg/mL)) appeared after 6–12 hours and disappear completely after 24 hours.[20]
Table 2.
Study on volunteers treated with rhGH subcutaneous injections: Urinary hGH concentration [pg/mL]+/−STD / (number of volunteers), adapted from Saugy and colleagues.[19]
| Before treatment [pg/mL] / (#athletes) |
Day 1 [pg/mL] / (#athletes) |
Day 2 [pg/mL] / (#athletes) |
Day 3 [pg/mL] / (#athletes) |
Day 4 [pg/mL] / (#athletes) |
Day 5 [pg/mL] / (#athletes) |
|
|---|---|---|---|---|---|---|
|
non treated non exercising volunteers |
4.2 / (196) | |||||
|
12 I.U. subcutaneous application |
4.3+/−4.1 / (9) |
136+/−84 / (9) |
185+/− (83) / (9) |
147+/−67 / (9) |
182+/−73 / (9) |
202+/−121 / (9) |
Past studies demonstrated that strong physical exercise could be accompanied by proteinuria and elevated levels of urinary hGH.[21] It is unclear if the increased levels of urinary hGH following exercise are a passive consequence of proteinuria, or reflect an increased rate of hGH production. Because of the influence of exercise on urine hGH, previous authors recommended that hGH should be measured only in the urine of athletes out of competition and that beta 2 microglobulin should be used as a control for normal kidney function. Previous authors recommended a cut off value of 100 pg/mL in the urine as a threshold for a positive screen.[11]
Former authors found positive correlation between serum and urinary hGH levels.[9, 22–29]
Elsewhere, the correlation between blood and urinary hGH levels was variable[30] and seemed to be impaired by factors such as sex (no correlation in males[31]), and disease state (no correlation in children with hGH deficiency,[32] individuals with thyroid disfunction,[33] renal insufficiency[16]).
Despite data gathered in the past employing research grade immunoassays,[34–39] no current clinically approved immunological test has the required sensitivity to reliably measure hGH in the urine. The estimated baseline concentration of urine hGH is approximately 1 pg/mL and falls fifty fold below the linear range and sensitivity threshold (50 pg/mL) of all commercially available clinical grade immunoassays for hGH, including isoform specific immunoassays.[10, 20, 40]
Hydrogel nanoparticles technology
We have applied nanoparticle technology (Figure 1) as a pre-processing step that can increase the sensitivity of any analyte immunoassay by 100 fold.[41–43] We originally proposed the novel core-shell affinity-bait nanotechnology as a means to overcome the three major hurdles to “disease-associated” biomarker discovery and measurement in blood and other body fluids. When placed in a biological fluid, the harvesting nanoparticles in one step rapidly conduct: 1) affinity capture, 2) concentration of target analytes, 3) complete exclusion of high molecular weight proteins such as immunoglobulins, albumin and GHBP, and 4) complete protection of captured analytes from degradation.[41–43] Hydrogel core-shell nanoparticles are constituted by poly(N-isopropylacrylamide) (NIPAm) and Methylenebisacrylamide (BIS) as a cross-linker.[43] No other technology exists that has a similar yield, concentration ability, and stabilization function. The nanoparticles incorporate a variety of very high affinity baits that target major categories of body fluid bioanalytes including: membrane proteins, nuclear proteins, cytoplasmic proteins and secreted soluble proteins; basic proteins and peptides, acidic proteins and peptides, and metabolites. The bait chemistries recognize the 3-D conformation of target proteins with very high affinity (greater than most antibodies) such that virtually the entire solution phase content of the target biomarker can be sequestered in five minutes.[41–43] The captured analytes can be readily eluted for analysis.[41–43] Core shell particles raised the concentration of extremely dilute undetectable serum platelet derived growth factor (PDGF-BB) signal pathway ligand into the detection range of a commercial clinical grade ELISA platform. Native PDGF-BB was captured and greatly concentrated by the particles.[42]
Figure 1.
Schematic representation of hydrogel nanoparticle functioning. Adapted from Longo and colleagues.[42]
Concentration and preservation of urinary human growth hormone by Cibacron Blue F3G-A loaded hydrogel particles
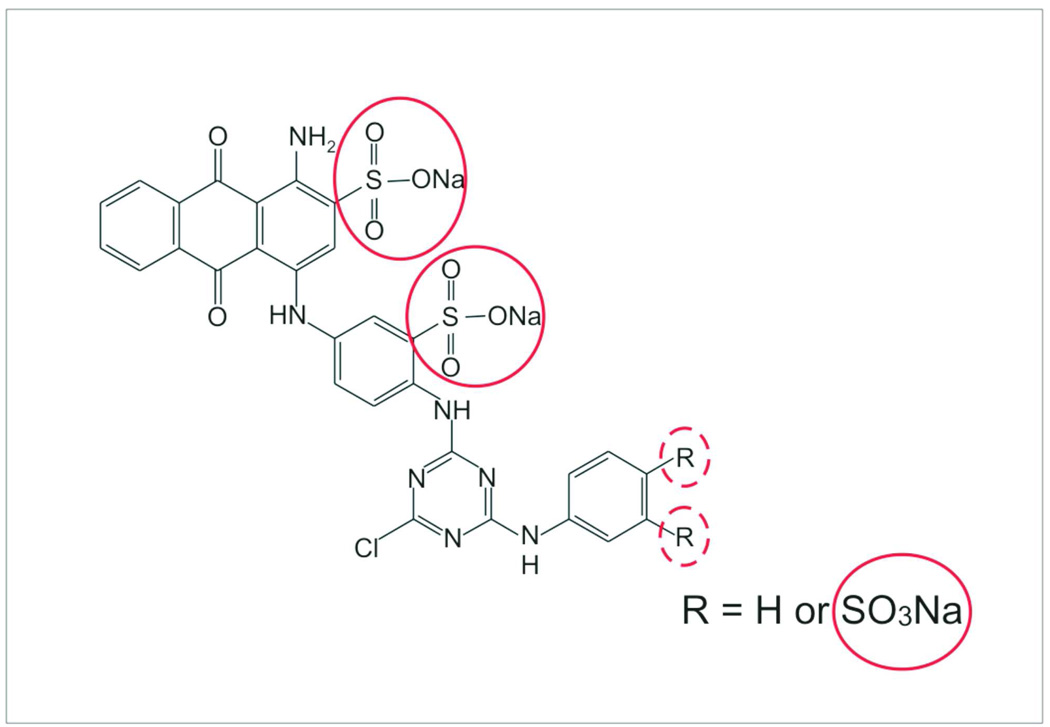
Among the variety of chemical baits that have been covalently bound to the core shell nanoparticles.[44] Cibacron Blue F3GA (CB) was successfully employed in order to capture, preserve and concentrate hGH in urine.[41] CB is a monochlorotriazinyl textile dye with well-known ability to interact with protein,[45, 46] already used in solid phase affinity chromatography, and with proven affinity for hGH.[47] Previous studies have demonstrated that the interaction between deprotonated SO3 groups of CB and protonated sites of the protein have an important role in the protein-dye complex formation.[48] Figure 2 shows the location of the putative binding site on the CB molecule. In addition to CB, we have identified several different novel bait chemistries that will bind hGH with high affinity, including all the isoforms.
Figure 2.
Chemical formula of Cibacron Blue F3GA (CB). Sulfonate groups, which are thought to play a fundamental role in CB-hGH complex formation, are highlighted with a red circle.
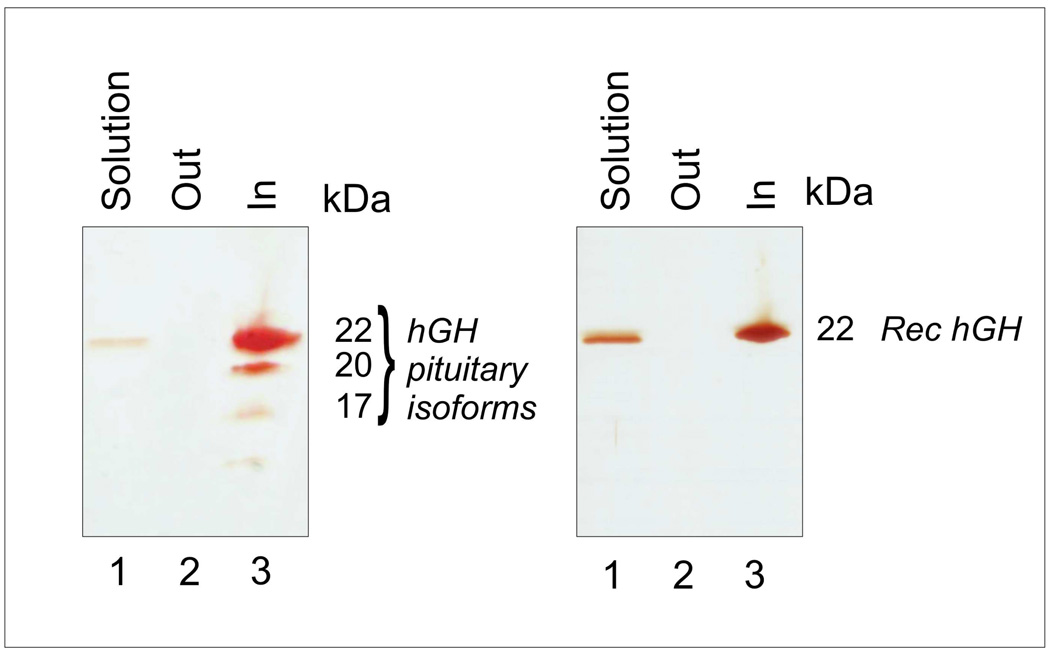
NIPAm/CB nanoparticles have submicron dimensions (diameter 600 nm), are characterized by high homogeneity in size distribution and form a stable suspension in aqueous solution. These characteristics expedite the kinetics of capturing process. In Figure 3, atomic force microscope pictures of NIPAm/CB particles are shown. NIPAm/CB nanoparticles perform, in solution, molecular size sieving with a molecular cut off of 45 kDa.[41] NIPAm/CB nanoparticles captured and concentrated rhGH (22 kDa) and all the three major isoforms (22, 20, 17 kDa) of pituitary hGH (Figure 4). NIPAm/CB nanoparticles are specific for hGH but have the affinity for a broad class of proteins. NIPAm/CB nanoparticles completely sequestered IGF-1 and other proteins from solution.[41] This property extends NIPAm/CB nanoparticles applicability to broader testing. The uptake of hGH by particles is a very fast process. Time course studies suggested that after only one minute of incubation most of the hormone is trapped into the particles.[41]
Figure 3.
Atomic force microscopy (AFM) images of NIPAm/CB nanoparticles show that the size distribution of NIPAm/CB nanoparticles is very uniform. Aqueous nanoparticle suspension was deposited under humid atmosphere at room temperature on freshly cleaved mica. After 15 minute incubation, water was removed under nitrogen flow. AFM images were acquired on particles using an NSCRIPTOR™ DPN® System (NanoInk). Images were acquired under AC mode using a silicon tip with a typical resonance frequency of 300 kHz and a radius smaller than 10 nm.
Figure 4.
SDS PAGE analysis shows that NIPAm/CB nanoparticles completely sequestered and concentrated pituitary hGH (the three major isoforms 22, 20, and 17 kDa are clearly visible by silver staining) and recombinant hGH from synthetic urine solution. Adapted from Fredolini and colleagues.[41]
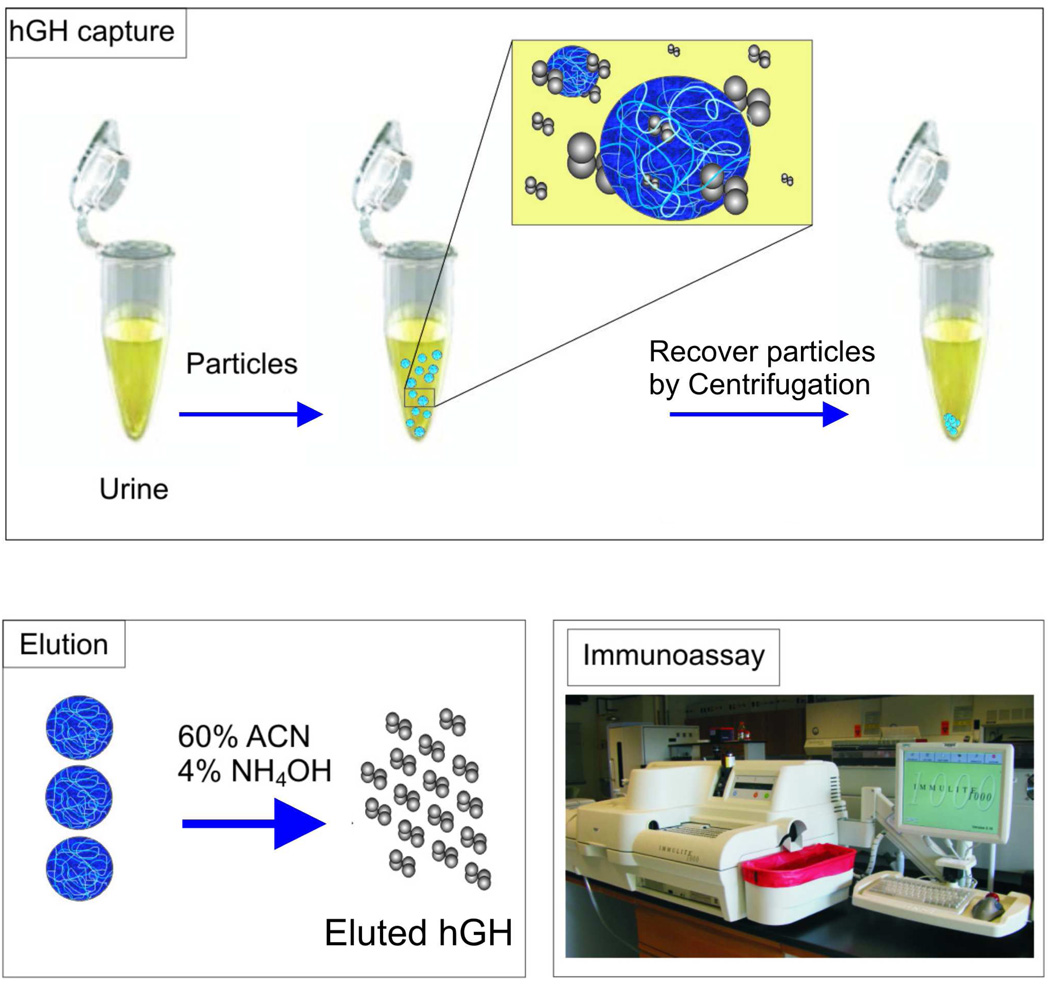
The protocol that was used by Fredolini et al.[41] consists of three steps (Figure 5): 1) Urine was incubated with NIPAm/CB nanoparticles and the nanoparticles were recovered by centrifugation. 2) Proteins were eluted from the nanoparticles by means of a chemical solvent. 3) The nanoparticle eluate was loaded into a clinical immunometric assay (Immulite 1000, Siemens Medical Solution Diagnostic), which is approved for the clinical measurement of hGH in serum. The Immulite 1000 test for hGH is linear from 0.05 to 40 ng/mL. Only 570 µg of particles were necessary to completely sequester hGH from 1 mL of synthetic or biologic urine in which hGH was spiked in at a concentration of 80 pg/mL. Cibacron Blue F3GA has affinity for a wide group of proteins and is not specific for hGH. It was important to understand if the binding capacity of NIPAm/CB nanoparticles was sufficient to capture all the hGH present in solution even in presence of vast excess of competing protein analytes. Synthetic urine containing a mixture of low molecular weight protein (total concentration 1 µg/mL) and hGH in a ratio of 10,000:1 hGH:total protein was incubated with the particles. The presence of competitors did not affect the efficiency of the particles in capturing hGH in solution (Figure 6, Left panel) or the concentration ability.
Figure 5.
Our new strategy for immunological detection of human growth hormone (hGH) in urine involves three steps 1) NIPAm/CB nanoparticles are briefly incubated with urine and separated via high speed centrifugation. 2) hGH that was captured by NIPAm/CB nanoparticles is eluted from the nanoparticles with an acetonitrile (ACN)-NH4OH buffer. 3) The elution process does not compromise the immunogenicity of hGH, which is measured by means of a clinical grade immunoassay (Immulite 1000, Siemens). Adapted from Fredolini and colleagues.[41]
Figure 6.
Left panel: Interfering proteins do not diminish the concentration capabilities of NIPAm/CB nanoparticles. Solutions of synthetic urine (Surine) containing hGH alone and hGH mixed with interfering proteins were incubated with NIPAm/CB nanoparticles. The nanoparticles captured and concentrated hGH 30 fold with no interference by competing proteins, showing a very high binding capacity.
Right panel: The sensitivity of hGH immunoassay is amplified. hGH is spiked in 10 mL of synthetic urine and mixed with NIPAm/CB nanoparticles. All solution phase hGH is rapidly sequestred within the nanoparticles. hGH concentration in the particle eluate is linearly dependent on hGH concentration in the original urine solution. UD means undetectable (below the detection limits of the Immulite, 50 pg/mL) Adapted from Fredolini and colleagues.[41]
The capacity of NIPAm/CB nanoparticles to increase the concentration of the target analyte is a function of the starting volume of urine and the final volume in which the proteins that are captured by the nanoparticles are collected. The concentration mechanism is based on this aforementioned volumetric ratio. A mathematical equation can be used to describe the increased detection limit of immunoassay when the sample is pretreated with the nanoparticles: Cmin = T/ (V/v) = T/c where Cmin is the lowest concentration detectable, T the limit of sensitivity of the immunoassay, V the starting volume of urine and v the volume of eluate.
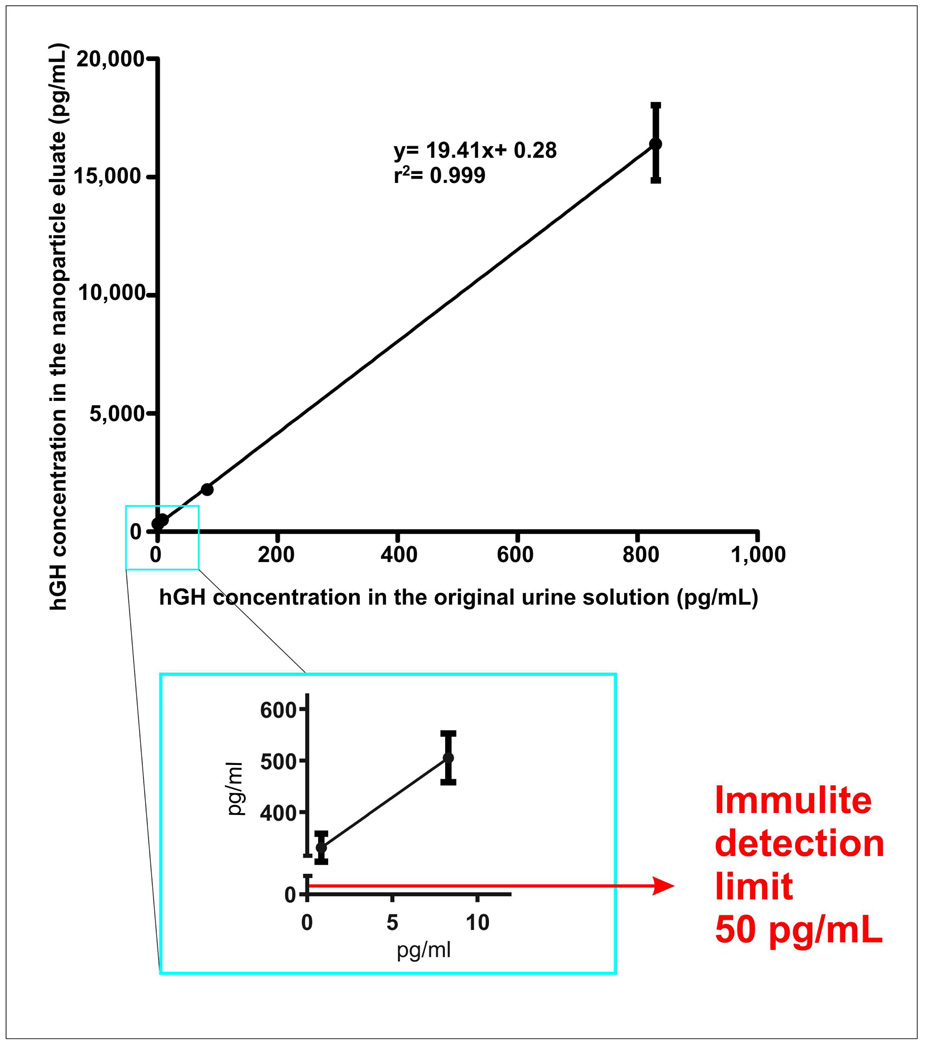
NIPAm/CB nanoparticles showed concentration capability from ten to one hundred fold depending on the starting urine volume. When the starting concentration of hGH was in the linear range of the immunoassay (170 pg/mL and 800 pg/mL), NIPAm/CB nanoparticles increased the hGH concentration over forty fold, well above the upper detection limit of the Immulite assay (Figure 6, Right panel). When the hGH concentration was 0.8 pg/mL (obtained by 4 sequential 1:10 dilutions of an original solution measured by Immulite at 8.3 ng/mL), NIPAm/CB nanoparticles increased the hGH concentration up to 300 pg/mL, a value that is well within the linear range of Immulite, with highly acceptable precision (Figure 7, insert). Very importantly, the relationship between the hGH concentration in the NIPAm/CB nanoparticle eluate and the concentration of hGH in the starting solution was linear (r2= 0.999, Figure 7). The linearity of the NIPAm/CB nanoparticles preprocessing step coupled to the linearity and precision and accuracy of an accepted approved clinical immunoassay is a prerequisite to develop a clinical urine hGH test. The concentration of hGH in the starting urine solution can calculated from the value of hGH concentration in the NIPAm/CB nanoparticle eluate by means of the mathematical equation that describes the standard curve in Figure 7. We are currently applying the nanoparticle pre-processing step to achieve a routine urine test for hGH that can be applied to random urine collection volumes of less than 30 mL. As part of an ongoing IRB approved clinical research study we are evaluating the correlation of hGH blood levels with urine levels collected at the same time. Example data from normal volunteers in the 18 to 30 year old age group who are not currently exercising is shown in Table 3. The data indicates that there is at least a one thousand fold difference in the concentration of physiologic hGH in the urine compared to the blood, when measured at the same point in time. The nanoparticle based urine assay for hGH can now serve as a means to answer a series of important previously unknown questions concerning the normal fluctuations of hGH in the urine, correlation with blood levels, the proportion of hGH isoforms in the urine, and the interfering factors that influence the level of hGH in the urine.
Figure 7.
The concentration of hGH in the urine can be calculated from the concentration of hGH in NIPAm/CB nanoparticle eluate by means of a calibration curve. Solutions obtained by dissolving rhGH in synthetic urine at decreasing concentration from 830 to 0.83 pg/mL were incubated with NIPAm/CB nanoparticles. Standard deviation of the concentration of hGH in NIPAm/CB nanoparticle eluate is always below 10% of the average value (3 replicates). Linearity between the concentrations in urine and particle eluate is evident, and the relationship can be mathematically described by y= 19.41x+ 0.28 (r= 0.999). In the blue square a magnification of the curve for the low concentration range (83 pg/mL - 0.8 pg/mL). For hGH concentration in the original urine solution of 0.8 pg/mL a concentration of 0.3 ng/mL was measured by Immulite in the NIPAm/CB nanoparticle eluate.
Table 3.
Human Growth Hormone was measured in blood and urine at the same time from healthy donors.
| Healthy Donor hGH + |
Blood (ng/ml) |
Direct Measurement in Urine by the Immunoassay¶ (ng/ml) |
Particle Eluate (ng/ml) |
Calculated actual urine concentration of hGH following nanoparticles harvesting (Particle Eluate / Concentration Factor) (pg/ml) |
|---|---|---|---|---|
| 1 | 2.830 | <0.050 | 0.107 | 0.973 |
| 2 | 0.095 | <0.050 | 0.056 | 0.509 |
| 3 | 10.136 | <0.050 | 0.078 | 0.100 |
| 4 | 1.880 | <0.050 | 0.058 | 0.527 |
| 4 repeat | 2.04 | <0.050 | 0.063 | 0.572 |
Measurements were done using the Immulite hGH serum Immunoassay (Siemens) with a lower detection limit of 50 pg/ml and a linear range of 50 pg-40 ng per ml.
Prior to nanoparticle sequestration of hGH from the urine, the concentration of hGH in all urine samples was below the detection limit (<0.050 ng/mL) of the immulite assay.
The actual concentration in urine was calculated as (particle eluate concentration) / CF, where CF is the Concentration Factor = (Starting volume of urine) / (Volume of eluate) = 33ml/0.3 ml = 110.
Conclusions
Testing for hGH in urine presents two main disadvantages: hGH is present in urine at very low concentration (1000 times less than in blood) and previous studies demonstrated that the level of urinary hGH is not clearly correlated to the level of hGH in blood.[19] We propose a new strategy applicable to the measurement of hGH in urine employing NIPAm/CB hydrogel nanoparticles technology. Hydrogel nanoparticles capture, stabilize and concentrate (as much as 100 fold depending on the starting urine volume) hGH from very diluted urine solutions, so that it is possible to measure urinary hGH with a standardized clinical grade immunoassay. Studies are ongoing in order to understand the baseline value of hGH in the urine among individuals that are not undergoing administration of rhGH, to characterize the physiological variability and the correlation with hGH levels in blood, and to evaluate the normal physiologic proportion of hGH isoforms in urine. Our nanotechnology, when introduced into urine, can harvest and concentrate all the major isoforms of hGH in addition to other hGH related protein analytes such as IGF-1. Nanoparticle harvesting technology can vastly improve the sensitivity of any immunoassay with the existing linear range of the assay. Thus increased sensitivity can be achieved with no increase in background signal.
Our results strongly suggest that nanoparticle technology could be applied routinely as a preprocessing step for the routine measurement of urinary hGH.
Large scale manufacture of the nanoparticles is relatively low cost, and rapid, with very good batch to batch reproducibility. The monomeric chemistries are largely available. The storage of nanoparticles in lyophilized form preserves the nanoparticles for over 1 year. A commercial kit would incorporate the nanoparticles directly in the urine collection vessel; the nanoparticles would immediately capture and stabilize hGH and other proteins so that the sample would be shipped and analyzed in the laboratory without any significant analyte degradation. Rigorous quality assurance and quality control methods have been applied as we scale up of the nanoparticle production, and utilize the qualified nanoparticles for ongoing IRB approved clinical research trials investigating the baseline physiologic levels of urinary hGH isoform concentration.
Acknowledgements
Supported partially by 1) George Mason University, 2) the Italian Istituto Superiore di Sanita’ in the framework of the Italy/USA cooperation agreement between the U.S. Department of Health and Human Services, George Mason University, and the Italian Ministry of Public Health, 2). US Anti-Doping Agency, 3) U.S. Department of Energy DE-FC52-04NA2545, 4) NIH, NCI grant 1R21CA137706-01. The funding organizations did not have any role in data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- CB
Cibacron Blue F3GA
- HGH
human growth hormone
- NIPAm
N-isopropylacrylamide
- rhGH
recombinant human growth hormone
- BIS
N,N’-methylenebisacrylamide
Footnotes
Competing Interests: The technology described herein is licensed (patent pending) to the author’s institutions (George Mason University, USA and Istituto Superiore di Sanita’, Italy) under faculty guidelines.
References
- 1.Bidlingmaier M, Strasburger CJ. Pituitary. 2007;10:115. doi: 10.1007/s11102-007-0030-1. [DOI] [PubMed] [Google Scholar]
- 2.Popii V, Baumann G. Clin Chim Acta. 2004;350:1. doi: 10.1016/j.cccn.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Giustina A, Veldhuis JD. Endocr Rev. 1998;19:717. doi: 10.1210/edrv.19.6.0353. [DOI] [PubMed] [Google Scholar]
- 4.Saugy M, Robinson N, Saudan C, Baume N, Avois L, Mangin P. Br J Sports Med. 2006;40 Suppl 1:i35. doi: 10.1136/bjsm.2006.027573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felsing NE, Brasel JA, Cooper DM. J Clin Endocrinol Metab. 1992;75:157. doi: 10.1210/jcem.75.1.1619005. [DOI] [PubMed] [Google Scholar]
- 6.Lassarre C, Girard F, Durand J, Raynaud J. J Appl Physiol. 1974;37:826. doi: 10.1152/jappl.1974.37.6.826. [DOI] [PubMed] [Google Scholar]
- 7.Parker ML, Utiger RD, Daughaday WH. J Clin Invest. 1962;41:262. doi: 10.1172/JCI104479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Palo EF, Gatti R, Antonelli G, Spinella P. Clin Chim Acta. 2006;364:77. doi: 10.1016/j.cca.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Momomura S, Hashimoto Y, Shimazaki Y, Irie M. Endocr J. 2000;47:97. doi: 10.1507/endocrj.47.97. [DOI] [PubMed] [Google Scholar]
- 10.Bidlingmaier M, Suhr J, Ernst A, Wu Z, Keller A, Strasburger CJ, Bergmann A. Clin Chem. 2009;55:445. doi: 10.1373/clinchem.2008.112458. [DOI] [PubMed] [Google Scholar]
- 11.Saugy M, Cardis C, Rivier L, Brisson G, Ayotte C, Hemmersbach Haug PE, Segura J. Recent Advances in Doping analysis. 1995;2:223. [Google Scholar]
- 12.Baumann G, Abramson EC. J Clin Endocrinol Metab. 1983;56:305. doi: 10.1210/jcem-56-2-305. [DOI] [PubMed] [Google Scholar]
- 13.Skinner AM, Clayton PE, Price DA, Addison GM, Mui CY. J Endocrinol. 1993;138:337. doi: 10.1677/joe.0.1380337. [DOI] [PubMed] [Google Scholar]
- 14.Turner G, Brown RC, Weeks I, Butler GE, Creagh FN, Woodhead JS. Ann Clin Biochem. 1993;30(Pt 2):180. doi: 10.1177/000456329303000212. [DOI] [PubMed] [Google Scholar]
- 15.Main K, Philips M, Jorgensen M, Skakkebaek NE. Horm Res. 1991;36:174. doi: 10.1159/000182156. [DOI] [PubMed] [Google Scholar]
- 16.Hattori N, Shimatsu A, Kato Y, Imura H. Kidney Int. 1990;37:951–954. doi: 10.1038/ki.1990.70. [DOI] [PubMed] [Google Scholar]
- 17.Bidlingmaier M. Eur J Endocrinol. 2008;159 Suppl 1:S41. doi: 10.1530/EJE-08-0284. [DOI] [PubMed] [Google Scholar]
- 18.Hourd P, Edwards R. J Endocrinol. 1989;121:167. doi: 10.1677/joe.0.1210167. [DOI] [PubMed] [Google Scholar]
- 19.Saugy M, Cardis C, Schweizer C, Veuthey JL, Rivier L. J Chromatogr B Biomed Appl. 1996;687:201. doi: 10.1016/s0378-4347(96)00331-3. [DOI] [PubMed] [Google Scholar]
- 20.Wu Z, Bidlingmaier M, Dall R, Strasburger CJ. Lancet. 1999;353:895. doi: 10.1016/S0140-6736(99)00775-8. [DOI] [PubMed] [Google Scholar]
- 21.Flanagan DE, Taylor MC, Parfitt V, Mardell R, Wood PJ, Leatherdale BA. Clin Endocrinol (Oxf) 1997;46:425. doi: 10.1046/j.1365-2265.1997.1410966.x. [DOI] [PubMed] [Google Scholar]
- 22.Bates AS, Evans AJ, Jones P, Clayton RN. Clin Endocrinol (Oxf) 1995;42:425. doi: 10.1111/j.1365-2265.1995.tb02652.x. [DOI] [PubMed] [Google Scholar]
- 23.Bates AS, Evans AJ, Jones P, Clayton RN. Clin Endocrinol (Oxf) 1995;42:417. doi: 10.1111/j.1365-2265.1995.tb02651.x. [DOI] [PubMed] [Google Scholar]
- 24.Girard J. Nucl Med Biol. 1994;21:381. doi: 10.1016/0969-8051(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 25.Girard J, Fischer-Wasels T. Horm Res. 1990;33 Suppl 4:12. doi: 10.1159/000181578. [DOI] [PubMed] [Google Scholar]
- 26.Main KM, Lindholm J, Vandeweghe M, Skakkebaek NE. Acta Endocrinol (Copenh) 1993;129:409. doi: 10.1530/acta.0.1290409. [DOI] [PubMed] [Google Scholar]
- 27.Pirazzoli P, Mandini M, Zucchini S, Gualandi S, Vignutelli L, Capelli M, Cacciari E. Arch Dis Child. 1996;75:228. doi: 10.1136/adc.75.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porquet D, Rigal O, Brion DE, Valade F, Leger J, Czernichow P. Clin Chem. 1992;38:1717. [PubMed] [Google Scholar]
- 29.Sartorio A, Palmieri E, Vangeli V, Conte G, Narici M, Faglia G. J Endocrinol Invest. 2001;24:515. doi: 10.1007/BF03343885. [DOI] [PubMed] [Google Scholar]
- 30.Winer LM, Shaw MA, Baumann G. J Endocrinol Invest. 1989;12:461. doi: 10.1007/BF03350732. [DOI] [PubMed] [Google Scholar]
- 31.Main KM, Jansson C, Skakkebak N, Albertsson-Wikland K. Horm Res. 1997;48:16. doi: 10.1159/000185604. [DOI] [PubMed] [Google Scholar]
- 32.Leger J, Reverchon C, Porquet D, Noel M, Czernichow P. Horm Res. 1995;44:57. doi: 10.1159/000184593. [DOI] [PubMed] [Google Scholar]
- 33.Murao K, Takahara J, Sato M, Tamaki M, Niimi M, Ishida T. Endocr J. 1994;41:517. doi: 10.1507/endocrj.41.517. [DOI] [PubMed] [Google Scholar]
- 34.Aman J, Jones I. Scand J Clin Lab Invest. 1994;54:227. doi: 10.3109/00365519409088429. [DOI] [PubMed] [Google Scholar]
- 35.Braschi S, Faivre A, Charbonnel B. Pathol Biol (Paris) 1994;42:621. [PubMed] [Google Scholar]
- 36.Geller J, Loh A. J Clin Endocrinol Metab. 1963;23:1107. doi: 10.1210/jcem-23-11-1107. [DOI] [PubMed] [Google Scholar]
- 37.Hourd P, Edwards R. Clin Endocrinol (Oxf) 1994;40:155. doi: 10.1111/j.1365-2265.1994.tb02462.x. [DOI] [PubMed] [Google Scholar]
- 38.Lin MH, Lin DY. J Formos Med Assoc. 1993;92:807. [PubMed] [Google Scholar]
- 39.McConway MG, Smith KA, Beastall GH. Ann Clin Biochem. 1999;36(Pt 5):649. doi: 10.1177/000456329903600514. [DOI] [PubMed] [Google Scholar]
- 40.Nelson AE, Ho KK. Asian J Androl. 2008;10:416. doi: 10.1111/j.1745-7262.2008.00395.x. [DOI] [PubMed] [Google Scholar]
- 41.Fredolini C, Meani F, Reeder KA, Rucker S, Patanarut A, Botterell PJ, Bishop B, Longo C, Espina V, Petricoin EFI, Liotta LA, Luchini A. Nano Research. 2008;1:502. doi: 10.1007/s12274-008-8054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Longo C, Patanarut A, George T, Bishop B, Zhou W, Ross MM, Espina V, Pellacani G, Petricoin EFI, Liotta LA, Luchini A. PLoS one. 2009;4:e4763. doi: 10.1371/journal.pone.0004763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luchini A, Geho DH, Bishop B, Tran D, Xia C, Dufour RL, Jones CD, Espina V, Patanarut A, Zhou W, Ross MM, Tessitore A, Petricoin EF, 3rd, Liotta LA. Nano Lett. 2008;8:350. doi: 10.1021/nl072174l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luchini A, Longo C, Espina V, Petricoin EI, Liotta L. Journal of Materials Chemistry. 2009 doi: 10.1039/b822264a. DOI: 10.1039/b822264a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denizli A, Piskin E. J Biochem Biophys Methods. 2001;49:391–416. doi: 10.1016/s0165-022x(01)00209-3. [DOI] [PubMed] [Google Scholar]
- 46.Lowe CR, Pearson JC. Methods Enzymol. 1984;104:97. doi: 10.1016/s0076-6879(84)04085-4. [DOI] [PubMed] [Google Scholar]
- 47.Sereikaite J, Bumelis VA. Biomed Chromatogr. 2006;20:195. doi: 10.1002/bmc.552. [DOI] [PubMed] [Google Scholar]
- 48.Salih B, Zenobi R. Anal Chem. 1998;70:1536. doi: 10.1021/ac9708506. [DOI] [PubMed] [Google Scholar]