Abstract
Vascular reactivity may affect the stiffness characteristics of the arterial wall. We investigated the association between forearm microcirculatory and conduit artery function and measures of arterial stiffness in 527 asymptomatic non-Hispanic white adults without known cardiovascular disease. High-resolution ultrasonography of the brachial artery (ba) was performed to assess forearm microcirculatory function (ba blood flow velocity, local shear stress, and forearm vascular resistance at rest and during reactive hyperemia) and conduit artery function (ba flow-mediated dilatation baFMD and ba nitroglycerin-mediated dilatation baNMD). Arterial stiffness was assessed by cuff-derived brachial pulse pressure and aortic pulse wave velocity (aPWV) measured by applanation tonometry. In regression analyses that adjusted for heart rate, mean arterial pressure, height, cardiovascular risk factors, and hypertension medication and statin use, higher baseline ba systolic velocity and systolic shear stress were associated with greater pulse pressure (P=0.0002 and P=0.006, respectively) and higher aPWV (each P<0.0001). During hyperemia, lower ba mean velocity and lower mean shear stress were associated with higher pulse pressure (P=0.045 and P=0.036, respectively) while both systolic and mean velocity (P<0.0001 and P=0.002, respectively) and systolic and mean shear stress (P<0.0001 and P=0.003, respectively) were inversely associated with aPWV. baFMD was not associated with pulse pressure but was inversely associated with aPWV (P=0.011). baNMD was inversely associated with pulse pressure (P=0.0002) and aPWV (P=0.008). Our findings demonstrate that impaired forearm microvascular function (in the form of elevated resting blood flow velocity and impaired flow reserve) and impaired brachial artery reactivity are associated with increased arterial stiffness.
Keywords: microvascular function, flow-mediated dilatation, nitroglycerin-mediated dilatation, arterial stiffness, pulse pressure, pulse wave velocity
Introduction
How arterial stiffness is related to vasoreactivity of conduit arteries and microcirculation is incompletely understood. Although arterial stiffening occurs mainly due to structural alterations in the scaffolding connective tissue proteins in the media1, endothelial and smooth muscle cells may influence the viscoelastic properties of the arterial wall and thereby affect arterial stiffness. Endothelium-derived factors including nitric oxide affect several biological processes in the arterial wall, including smooth muscle reactivity, and may have both short- and long-term effects on the degree of arterial stiffness.2,3 Smooth muscle cells in medium-sized and small arteries affect arterial stiffness through their regulatory influence on distending pressure as well as by affecting the physical properties of various arterial wall components.4,5 In turn, the hemodynamic abnormalities consequent to increased central arterial stiffness may have deleterious effects on the peripheral vasculature.6
An association between regional endothelial function and arterial stiffness in different vascular beds has been demonstrated using invasive studies in experimental animals7 as well as in human volunteers.8 However, the relationship between vasoreactivity of the microvasculature and peripheral conduit arteries and central arterial stiffness has not been adequately defined. In separate studies, microvascular function9 as well as conduit artery function10,11 have been associated with central arterial stiffness, but the evidence in this regard is controversial.12,13 The present study was undertaken to test the hypothesis that microvascular and conduit artery function is associated with central arterial stiffness. We investigated the cross-sectional association of forearm microvascular and brachial artery function assessed by brachial artery ultrasonography, with 2 measures of arterial stiffness – brachial pulse pressure and aortic pulse wave velocity (aPWV) – in a community-based sample of asymptomatic individuals.
Methods
Study Population
Participants belonged to a community-based study of subclinical coronary artery disease.14,15 From September 2002 to December 2005, 619 participants underwent arterial function testing including measurement of vascular reactivity in the forearm. Eleven participants with a history of stroke or myocardial infarction and 76 participants with incomplete data or outlier values were excluded. We also excluded 5 participants with technically suboptimal aPWV studies. The final study group consisted of 527 asymptomatic white individuals (254 men and 273 women). The study protocol was approved by the Mayo Clinic Institutional Review Board. Written informed consent was obtained from each subject.
Risk Factor Assessment
Information about the current use of medications, smoking, physician-diagnosed hypertension, myocardial infarction, stroke, or diabetes was obtained during the study interview. Resting blood pressure (BP) levels were measured as the average of second and third of 3 measurements taken at least 2 minutes apart in the right arm with a random-zero sphygmomanometer (Hawksley and Sons, London UK). Weight was measured by an electronic balance, height by a stadiometer, and body mass index (BMI) was calculated in units of kg/m2.
Blood samples were obtained after an overnight fast. Plasma glucose, total cholesterol, and high-density lipoprotein cholesterol were measured using standard enzymatic methods. Diabetes was considered present if a subject was being treated with insulin or oral agents or had a fasting glucose level >125 mg/dL. History of smoking was defined as having smoked >100 cigarettes in the past. The diagnosis of hypertension was determined based on BP levels measured at the study visit – systolic BP ≥140 mm Hg or diastolic BP ≥ 90 mm Hg – or report of a prior diagnosis of hypertension and current treatment with antihypertensive medications.
Aortic Pulse Wave Velocity (aPWV)
For arterial studies, each subject was examined in the morning after an overnight fast and at least 12 h off medications, smoking, or alcohol. aPWV was measured by applanation tonometry (Sphygmocor apparatus, AtCor Medical, Sydney Australia) as previously described.14 Briefly, ECG-gated carotid and femoral artery waveforms were sequentially recorded. Aortic length (D) was calculated as the difference in the distances (in meters) from the carotid sampling site to the manubrium sternum and from the manubrium sternum to the femoral artery, the time delay (t, seconds) between the onset of carotid and femoral waveforms (foot-to-foot) was determined from the average of 10 consecutive cardiac cycles, and aPWV was calculated as the ratio of distance to time: aPWV = D/t (m/s). In 10 volunteers, the within-subject standard deviation of aPWV measured on successive days using this method was 0.66 m/s, which was 7% of the mean aPWV in the present study.
Forearm Vascular Reactivity
Following the measurement of pulse pressure and aPWV, microvascular and conduit artery function in the forearm were assessed by non-invasive high-resolution ultrasound of the right brachial artery, using a hand-held ultrasound probe, as described previously.15 Microvascular function was assessed in terms of brachial artery blood flow velocity, local shear stress, and forearm vascular resistance before and after 5 min of ischemia induced by forearm arterial flow occlusion. Peak systolic flow velocity and flow velocity integrated over a cardiac cycle were measured at baseline and during the first 15 s after release of the BP cuff (taking the average of 3 consecutive velocity loops); velocity loops representing the maximum velocity during hyperemia, excluding the first one or two cycles after cuff release, were considered. Mean flow velocity at baseline and during reactive hyperemia was calculated from corresponding cardiac cycle lengths and time-velocity integrals. Local shear stress was calculated using the equation: shear stress = 8 × μ × V/D, where μ is the viscosity of blood (assumed to be 0.035 dynes.s/cm2), V is the velocity of blood (cm/s) and D is the brachial artery diameter (cm). Forearm vascular resistance was calculated as the ratio of mean arterial pressure (MAP, expressed in mm Hg, and assumed to remain constant throughout the test) to forearm blood flow (ml/min) and expressed as resistance units. Conduit artery function was assessed in terms of brachial artery flow-mediated dilatation (baFMD), an endothelium-dependent response, and brachial artery nitroglycerin-mediated dilatation (baNMD), an endothelium-independent response.
Statistical Methods
Descriptive statistics are given as mean ± standard deviation (as well as median and range) or as frequency (percent). The distributions of resting forearm vascular resistance, hyperemic forearm vascular resistance, baFMD, and aPWV were skewed and were log transformed. Because of sibships in the sample, generalized estimating equations (GEE) were used to account for the impact of familial correlations on the relationships between independent and dependent variables.16 We first assessed the association of heart rate, MAP, height, BMI, cardiovascular (CV) risk factors, and hypertension medication and statin use with pulse pressure and aPWV, in univariable GEE models. Next, independent predictors of pulse pressure and aPWV were identified using backward elimination. Finally, the association of vasoreactivity measures with measures of arterial stiffness was determined by including the former in the multivariable GEE models. For brachial flow velocity, shear stress, and forearm vascular resistance, both baseline and hyperemic values were included in the GEE models for pulse pressure and aPWV. The associations of baseline brachial artery diameter, baFMD, and baNMD with pulse pressure and aPWV were examined by including these variables one-at-a-time in the GEE models for pulse pressure and aPWV. The coefficient of determination (R2) was calculated using the likelihood ratio test statistic.17 We calculated R2 for the baseline model separately for pulse pressure and aPWV; the change in R2 value after adding the brachial vasoreactivity variables to the baseline models was then calculated. Statistical significance was determined at P <0.05.
Statistical analyses were performed with SAS v 9.1 (SAS Institute, Cary NC).
Results
Subject characteristics and the mean values of forearm vasoreactivity variables and arterial stiffness measures are shown in Tables 1 and 2, respectively. Mean baFMD and baNMD were 5.30% and 18.98%, respectively. Mean pulse pressure of the participants was 47 mm Hg and mean aPWV was 9.27 m/s.
TABLE 1.
Subject Characteristics (n = 527)
| Variable | Mean ± SD or no. (%) | Median (Min, Max) |
|---|---|---|
| Age, years | 59.8±10.1 | 60.0 (32.0, 86.0) |
| Men, no. (%) | 254 (48.2) | |
| BMI, kg/m2 | 28.0±4.8 | 27.4 (15.9, 49.4) |
| Height, cm | 169.4±9.5 | 169.0 (144.9, 198.1) |
| Total cholesterol, mg/dL | 198.9±33.3 | 197 (114, 301) |
| HDL cholesterol, mg/dL | 55.7±16.6 | 52.6 (21.2, 127.2) |
| Diabetes, no. (%) | 27 (5.1) | |
| History of smoking, no. (%) | 235 (44.6) | |
| Heart rate, bpm | 61.2±8.8 | 60 (40, 93) |
| Systolic BP, mm Hg | 121.7±16.7 | 120 (82, 176) |
| Diastolic BP, mm Hg | 74.6±9.1 | 74 (54, 108) |
| MAP, mm Hg | 90.3±10.4 | 90.0 (66.7, 122.0) |
| Statin use, no. (%) | 103 (19.5) | |
| Hypertension medication use, n (%) | 136 (25.81) |
BMI, body mass index; HDL, high-density lipoprotein; BP, blood pressure; MAP, mean arterial pressure. Continuous variables are presented as mean ± standard deviation and median (minimum (Min), maximum (Max)), whereas categorical variables are presented as counts and percentages.
TABLE 2.
Measures of Forearm Vascular Reactivity and Arterial Stiffness.
| Variable | Mean ± SD or no. (%) | Median (Min, Max) |
|---|---|---|
| Pulse pressure, mm Hg | 47±13 | 46 (16, 100) |
| aPWV, m/s | 9.27±2.68 | 8.60 (5.20, 23.90) |
| Systolic blood flow velocity, m/s | ||
| Baseline | 0.64±0.16 | 0.62 (0.31, 1.25) |
| Hyperemic | 1.26±0.27 | 1.25 (0.58, 2.25) |
| Mean blood flow velocity, m/s | ||
| Baseline | 0.08±0.04 | 0.07 (0.01, 0.27) |
| Hyperemic | 0.66±0.18 | 0.65 (0.23, 1.75) |
| Systolic SS, dynes/cm2 | ||
| Baseline | 50.66±17.39 | 48.53 (18.07, 118.98) |
| Hyperemic | 99.17±31.65 | 96.99 (36.44, 226.25) |
| Mean SS, dynes/cm2 | ||
| Baseline | 6.24±3.76 | 5.43 (0.82, 28.61) |
| Hyperemic | 51.80±18.84 | 49.42 (16.41, 141.43) |
| FVR, resistance units | ||
| Baseline | 2.45±1.87 | 2.10 (0.42, 28.07) |
| Hyperemic | 0.25±0.11 | 0.23 (0.09, 0.82) |
| Baseline baD, mm | 3.71±0.69 | 3.62 (2.24, 5.69) |
| baFMD, % | 5.3±4.2 | 4.6 (−3.8, 27.5) |
| baNMD*, % | 19.0±7.4 | 18.7 (0.4, 42.3) |
aPWV, aortic pulse wave velocity; SS, shear stress; FVR, forearm vascular resistance; baD, brachial artery diameter; baFMD, brachial artery flow-mediated dilatation; baNMD, brachial artery nitroglycerin-mediated dilatation. Continuous variables are presented as means ± standard deviation and median (minimum (Min), maximum (Max)), whereas categorical variables are presented as counts and percentages.
For NMD, n=356.
Variables Associated with Pulse Pressure
In univariable analyses, variables associated with a higher pulse pressure included greater age, female sex, higher BMI, shorter height, diabetes, higher MAP, hypertension medication use, and statin use. In a multivariable analysis, greater age, shorter height, history of smoking, hypertension medication use, higher MAP, and lower heart rate were independently associated with higher pulse pressure (Table 3).
TABLE 3.
Variables Associated With Pulse Pressure and Aortic Pulse Wave Velocity (aPWV).
| Parameter | Pulse Pressure |
Log aPWV |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariable model |
Multivariable model* |
Univariable model |
Multivariable model† |
|||||
| β±SE | P | β±SE | P | β±SE | P | β±SE | P | |
| Age, years | 0.593±0.055 | <.0001 | 0.408±0.052 | <.0001 | 0.015±0.001 | <.0001 | 0.013±0.001 | <.0001 |
| Male sex | −2.387±1.132 | 0.035 | −0.03±0.026 | 0.26 | ||||
| BMI, kg/m2 | 0.414±0.113 | 0.0003 | 0.007±0.003 | 0.014 | ||||
| Height, cm | −0.273±0.06 | <.0001 | −0.161±0.049 | 0.0009 | −0.004±0.002 | 0.007 | ||
| Total cholesterol, mg/dL | 0.002±0.018 | 0.93 | 0.001±0.001 | 0.71 | ||||
| HDL cholesterol, mg/dL | 0.001±0.041 | 0.98 | −0.001±0.001 | 0.46 | ||||
| Hypertension medication use | 11.07±1.382 | <.0001 | 3.656±1.131 | 0.001 | 0.197±0.027 | <.0001 | 0.073±0.024 | 0.002 |
| Statin use | 3.898±1.388 | 0.005 | 0.085±0.027 | 0.002 | ||||
| Diabetes | 7.926±3.356 | 0.018 | 0.19±0.049 | <.0001 | 0.074±0.037 | 0.046 | ||
| History of smoking | 2.059±1.094 | 0.059 | 2.077±0.857 | 0.015 | −0.002±0.22 | 0.92 | ||
| MAP, mm Hg | 0.632±0.05 | <.0001 | 0.552±0.047 | <.0001 | 0.009±0.001 | <.0001 | 0.005±0.001 | <.0001 |
| Heart rate, per min | 0.018±0.066 | 0.79 | −0.159±0.05 | 0.002 | 0.006±0.002 | 0.0001 | 0.004±0.001 | <.0001 |
The multivariable regression models for both pulse pressure and log aPWV were built by including all the listed variables in the model and performing backward elimination; only variables significant at P <0.01 were included in the final model in each case. Abbreviations as in Tables 1 and 2.
Model R2 = 0.44;
Model R2 = 0.48
Variables Associated with aPWV
In univariable models, greater age, higher BMI, shorter height, diabetes, higher MAP, greater heart rate, hypertension medication use, and statin use were associated with higher aPWV. In a multivariable analysis, greater age, diabetes, higher MAP, higher heart rate, and hypertension medication use were independently associated with higher aPWV (Table 3).
Association between Measures of Forearm Vascular Reactivity and Arterial Stiffness
The associations between measures of forearm vascular reactivity and arterial stiffness are summarized in Table 4. Higher brachial artery systolic flow velocity at baseline and lower brachial artery mean flow velocity during reactive hyperemia were associated with higher pulse pressure and higher aPWV (Figure 1); these associations remained significant after adjustment for CV risk factors and hypertension medication and statin use (Table 4). Further, lower systolic flow velocity during hyperemia was also independently associated with higher aPWV. Higher systolic shear stress at baseline and lower mean shear stress during hyperemia were associated with higher pulse pressure and higher aPWV, independently of CV risk factors and hypertension medication and statin use; also lower systolic shear stress during hyperemia was independently associated with higher aPWV (Table 4).
TABLE 4.
Association Between Measures of Forearm Vasoreactivity and Arterial Stiffness.
| Model | Vasoreactivity Parameter | Pulse Pressure |
Log aPWV |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted* |
Unadjusted |
Adjusted* |
||||||||
| β±SE | P | β±SE | P | ΔR2 | β±SE | P | β±SE | P | ΔR2 | ||
| 1 | Systolic flow velocity, m/s | ||||||||||
| Baseline | 19.62±4.0 | <.0001 | 13.88±3.67 | 0.0002 | 0.04 | 0.31±0.08 | <.0001 | 0.28±0.06 | <.0001 | 0.04 | |
| Hyperemic | 6.94±2.17 | 0.001 | −0.82±2.01 | 0.68 | −0.03±0.04 | 0.46 | −0.15±0.04 | <.0001 | |||
| 2 | Mean flow velocity, m/s | ||||||||||
| Baseline | −0.01±0.14 | 0.96 | 0.13±0.12 | 0.27 | 0.01 | −0.002±0.003 | 0.42 | 0.002±0.002 | 0.49 | 0.02 | |
| Hyperemic | −0.06±0.03 | 0.069 | −0.06±0.03 | 0.045 | −0.002±0.001 | 0.0005 | −0.002±0.001 | 0.002 | |||
| 3 | Systolic shear stress, dyne/cm2 | ||||||||||
| Baseline | 0.10±0.03 | 0.002 | 0.12±0.04 | 0.006 | 0.02 | 0.002±0.001 | 0.023 | 0.003±0.001 | <.0001 | 0.04 | |
| Hyperemic | 0.03±0.02 | 0.07 | −0.03±0.02 | 0.17 | −0.001±0.001 | 0.29 | −0.002±0.000 | <.0001 | |||
| 4 | Mean shear stress, dyne/cm2 | ||||||||||
| Baseline | −0.06±0.17 | 0.74 | 0.17±0.15 | 0.25 | 0.01 | −0.003±0.003 | 0.26 | 0.002±0.003 | 0.54 | ||
| Hyperemic | −0.06±0.03 | 0.072 | −0.06±0.03 | 0.036 | −0.002±0.001 | 0.003 | −0.002±0.001 | 0.003 | 0.02 | ||
| 5 | Log FVR, resistance units | ||||||||||
| Baseline | 1.82±1.00 | 0.068 | −1.81±1.19 | 0.13 | 0.01 | 0.03±0.02 | 0.11 | −0.013±0.02 | 0.51 | 0.003 | |
| Hyperemic | 5.97±1.47 | <.0001 | 3.89±1.86 | 0.036 | 0.12±0.03 | <.0001 | 0.02±0.03 | 0.44 | |||
| 6 | Baseline baD, mm | 0.05±0.08 | 0.47 | 0.05±0.08 | 0.49 | 0.001 | 0.002±0.002 | 0.32 | 0.002±0.001 | 0.051 | 0.01 |
| 7 | Log (baFMD+10) | −2.39±2.57 | 0.35 | −1.76±1.87 | 0.35 | 0.002 | −0.10±0.05 | 0.039 | −0.09±0.04 | 0.011 | 0.01 |
| 8 | baNMD, % | −0.36±0.09 | 0.0001 | −0.25±0.07 | 0.0002 | 0.04 | −0.01±0.002 | 0.002 | −0.004±0.001 | 0.008 | 0.02 |
Models 1–8 are adjusted for other independent predictors of pulse pressure and aPWV (Table 3); Model 5 (for FVR) did not include mean arterial pressure as a covariate. Only summaries for brachial reactivity measures are shown; associations of other variables with pulse pressure and aPWV after adding vasoreactivity variables were not different from those in Table 3. For each model, ΔR2 represents change in R2 after adding the corresponding brachial vasoreactivity measure(s) to the respective multivariable model in Table 3. Abbreviations as in Table 2.
Figure 1.
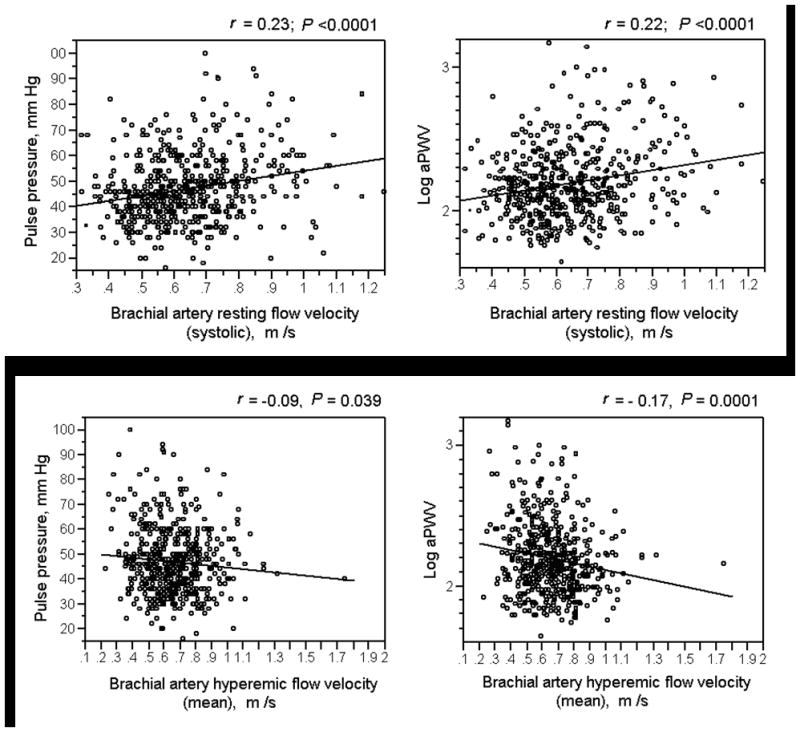
Correlation between forearm blood flow and arterial stiffness. Higher resting brachial artery blood flow velocity during systole (upper panel) and lower mean hyperemic brachial artery flow velocity (lower panel) were associated with greater pulse pressure and aortic pulse wave velocity (aPWV).
Higher forearm vascular resistance (both resting and hyperemic) was associated with higher pulse pressure and higher aPWV in univariable models. After adjustment for other covariates, a greater hyperemic forearm vascular resistance was associated with higher pulse pressure (P = 0.036); however, the association of forearm vascular resistance (both resting and hyperemic) with aPWV was not statistically significant in the adjusted model.
baFMD was not associated with pulse pressure but was inversely associated with aPWV in the unadjusted model (P = 0.039) and after adjustment for age, sex, height, heart rate, MAP, CV risk factors, and hypertension medication and statin use (P = 0.011). Lower baNMD was associated with higher pulse pressure before (P < 0.0001, Figure 2) and after adjustment for other known determinants of pulse pressure and CV risk factors (P = 0.0002). Lower baNMD was associated with higher aPWV in univariable regression analysis (P = 0.006, Figure 2); the association remained significant after adjustment for other determinants of aPWV (P = 0.008) (Table 4).
Figure 2.
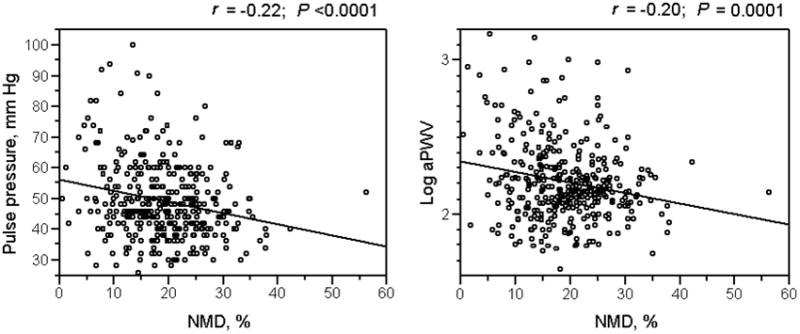
Correlation between brachial artery nitroglycerin-mediated dilatation (baNMD) and arterial stiffness. A higher baNMD was associated with lower pulse pressure and aortic pulse wave velocity (aPWV).
Discussion
Impaired vascular reactivity and arterial stiffening are important manifestations of vascular dysfunction in the presence of CV risk factors or established CV disease. While stiffening predominantly affects the central elastic arteries, impaired reactivity to various stimuli can be demonstrated in the microvasculature and conduit arteries of several arterial beds. The results of the present study demonstrate that impaired forearm microvascular function and reduced brachial artery reactivity are associated with increased arterial stiffness.
Normal resting flow and hyperemic response reflect the integrity of vascular function in the microcirculation. Elevated resting forearm blood flow may represent a state of ‘hyperperfusion’, analogous to the increased renal blood flow and glomerular hyperfiltration noted in early stages of diabetes,18 essential hypertension,19 and obesity.20 Reactive hyperemia is due mainly to ischemia-induced local release of vasodilator substances, including nitric oxide.21,22 We23 and others24 have previously shown that CV risk factors are associated with higher resting forearm blood flow and an impaired flow reserve. In the present study, a higher resting brachial artery blood flow velocity was associated with higher brachial pulse pressure and higher aPWV while a lower hyperemic flow velocity was associated with higher aPWV, even after adjustment for CV risk factors. Peak systolic flow velocity at rest and during hyperemia and mean flow velocity during hyperemia were associated with measures of arterial stiffness. Such a pattern of association between brachial artery flow velocity and arterial stiffness may be due to forward blood flow in the brachial artery being mainly systolic at rest but throughout the cardiac cycle immediately after ischemia.
The association of brachial artery flow velocity with arterial stiffness was similar to the association of local shear stress with arterial stiffness – higher shear stress (particularly systolic shear stress) at rest and lower shear stress during hyperemia were independently associated with higher pulse pressure and higher aPWV. Local shear stress reflects the flow characteristics better than blood flow velocity, incorporating vessel diameter and blood viscosity which are important determinants of flow velocity in themselves. The Framingham Heart Study investigators24 have shown that during brachial vasoreactivity testing, changes in local shear stress may be a better measure of microvascular function compared to changes in mean volume flow. Local shear stress has been shown to promote endothelial dysfunction.25 However, the implications of local brachial artery shear stress for systemic arterial physiology are not known. Our finding of an association of higher resting shear stress with greater pulse pressure and aPWV suggest that elevated shear stress in the brachial artery may be a marker of greater arterial stiffness. Our results also suggest that impaired forearm vasodilator function, as reflected by reduced shear stress response to ischemia, is associated with increased arterial stiffness.
A prior study of 2045 asymptomatic adults by Mitchell et al9 found no association between resting blood flow velocity and pulse pressure, a weak association between resting blood flow velocity and aPWV, and a significant inverse association between hyperemic flow velocity and both measures of arterial stiffness; a stronger association was noted between resting and hyperemic forearm vascular resistance and measures of aortic stiffness. In contrast, in our study, the association of forearm vascular resistance with pulse pressure and aPWV was modest. Both forearm vascular resistance and aPWV (but not flow velocity) are directly related to MAP, setting the stage for possible confounding. Furthermore, unlike vascular resistance, flow velocity is not significantly influenced by tissue volume, making it a better surrogate of microvascular function.
Taken together, these findings suggest that forearm blood flow and flow resistance at rest and during reactive hyperemia are associated with arterial stiffness. A potential mechanism underlying the association may involve the effect of the risk factors shared between these arterial phenotypes. Risk factors associated with arterial stiffness26,27 are also associated with structural microvascular abnormalities in the form of increased media-lumen ratio and rarefaction of the microvascular and resistance arteries.28,29 The presence of such structural abnormalities could blunt the hyperemic response through a deficient recruitment of microvascular tributaries during ischemia-reperfusion or physical limitation of the vasorelaxant reserve of the microvasculature.30 However, the association of brachial artery flow velocity with measures of arterial stiffness persisted even after adjustment for CV risk factors, suggesting an independent relationship between these arterial phenotypes.
An alternative basis for the association between forearm microcirculatory dysfunction and arterial stiffness may be the direct transmission of pressure pulsatility into the microvasculature. Stiffening of the central arteries decreases the gradient of stiffness that normally exists between the central and peripheral arteries. With loss of the centrifugal gradient of arterial stiffness, reflection of the arterial pressure wave may be reduced, leading to increased transmission of pressure pulse energy into distal microvasculature with possible deleterious consequences for microvascular structure and function.6
Both constitutive8,31 and stimulated7,32 production of nitric oxide from the endothelium may be important for the functional regulation of arterial stiffness. Further, nitric oxide may modulate the biomechanical properties of arterial wall components, resulting in long-term alterations in the degree of arterial stiffness.33 baFMD is mediated predominantly through endothelial release of nitric oxide34 and is considered a measure of systemic endothelial function. We found baFMD to be inversely associated with aPWV but not with pulse pressure. Some earlier studies have also reported an inverse association between baFMD and measures of large artery stiffness.10,11,35 However, Liang et al12 did not find a correlation between baFMD and aPWV in healthy subjects. Wiesman et al13 also found no significant correlation between baFMD and aortic distensibility in healthy young volunteers. The reason for the varying observations is not clear and could be partly due to differences in sample characteristics.
A novel finding of the present study is the inverse association of baNMD with pulse pressure and aPWV. The association of baNMD with both measures of arterial stiffness was independent of other factors known to influence arterial stiffness. Arterial smooth muscle reactivity may directly affect arterial stiffness, for example by altering the strain distribution among different arterial wall components.36 Vasodilatation to nitroglycerin is an ‘endothelium-independent’ response that depends upon the reactivity of arterial smooth muscle to nitric oxide released from the intracellular conversion of nitroglycerin37 and is known to be impaired in presence of CV risk factors.38 In previous studies, we have shown impaired baNMD to be associated with urinary albumin excretion39 (a measure of subclinical renal disease) and coronary artery calcium15 (a measure of subclinical coronary plaque burden), both of which are associated with increased arterial stiffness. Our finding in the present study that arterial smooth muscle dysfunction may be a marker of increased arterial stiffness suggests one possible explanation for the association of this phenotype with CV disease.
Strengths and Limitations
Due to the cross-sectional design of the present study, we cannot confirm the direction of association between microvascular and conduit artery vasoreactivity and arterial stiffness. The present study included non-Hispanic white subjects and the results may not be applicable to other racialor ethnic subgroups. Nearly a quarter of the study participants were on BP-lowering medications which could have affected arterial reactivity or measures of arterial stiffness. To minimize the impact of any acute vascular effect of these medications on measures of arterial function, brachial artery ultrasound and measurement of pulse pressure and aPWV were performed at least 12 h off of any medications. A strength of the present study is its relatively large sample size and inclusion of asymptomatic individuals from the community.
Perspective
The results of the present study indicate that elevated resting forearm blood flow velocity and impaired flow reserve (markers of microvascular function) and lower brachial artery reactivity (a marker of conduit artery function) are associated with higher brachial pulse pressure and aPWV in asymptomatic adult subjects from the community. These findings highlight the association between microvascular and conduit artery function and central arterial stiffness, suggesting that vascular abnormalities in the presence of CV risk factors may be widespread within the arterial system, involving the central large arteries, the medium-sized conduit arteries, and the microvasculature. Abnormalities in peripheral arterial reactivity may affect the stiffness characteristics of the central arterial system, which in turn, may lead to abnormalities in microvascular structure and function, leading to continuing cycle of increasing arterial stiffness and vascular dysfunction.
Acknowledgments
This work was supported by a K-23 Award (RR17720) from the National Center for Research Resources, National Institutes of Health.
Sources of Funding
This work was supported by a Mentored Patient-Oriented Research Career Development Award (K-23, RR17720) to I.J.K. from the National Center for Research Resources, National Institutes of Health.
Footnotes
Conflicts of Interest/Disclosures
None
References
- 1.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 2.Wilkinson IB, Franklin SS, Cockcroft JR. Nitric oxide and the regulation of large artery stiffness: from physiology to pharmacology. Hypertension. 2004;44:112–116. doi: 10.1161/01.HYP.0000138068.03893.40. [DOI] [PubMed] [Google Scholar]
- 3.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 4.Bank AJ, Wang H, Holte JE, Mullen K, Shammas R, Kubo SH. Contribution of collagen, elastin, and smooth muscle to in vivo human brachial artery wall stress and elastic modulus. Circulation. 1996;94:3263–3270. doi: 10.1161/01.cir.94.12.3263. [DOI] [PubMed] [Google Scholar]
- 5.Bank AJ, Kaiser DR, Rajala S, Cheng A. In vivo human brachial artery elastic mechanics: effects of smooth muscle relaxation. Circulation. 1999;100:41–47. doi: 10.1161/01.cir.100.1.41. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR. Nitric oxide regulates local arterial distensibility in vivo. Circulation. 2002;105:213–217. doi: 10.1161/hc0202.101970. [DOI] [PubMed] [Google Scholar]
- 8.Kinlay S, Creager MA, Fukumoto M, Hikita H, Fang JC, Selwyn AP, Ganz P. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension. 2001;38:1049–1053. doi: 10.1161/hy1101.095329. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, Vasan RS, Levy D, Benjamin EJ. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation. 2005;112:3722–3728. doi: 10.1161/CIRCULATIONAHA.105.551168. [DOI] [PubMed] [Google Scholar]
- 10.Kidawa M, Krzeminska-Pakula M, Peruga JZ, Kasprzak JD. Arterial dysfunction in syndrome X: results of arterial reactivity and pulse wave propagation tests. Heart. 2003;89:422–426. doi: 10.1136/heart.89.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nigam A, Mitchell GF, Lambert J, Tardif JC. Relation between conduit vessel stiffness (assessed by tonometry) and endothelial function (assessed by flow-mediated dilatation) in patients with and without coronary heart disease. American Journal of Cardiology. 2003;92:395–399. doi: 10.1016/s0002-9149(03)00656-8. [DOI] [PubMed] [Google Scholar]
- 12.Liang YL, Teede H, Kotsopoulos D, Shiel L, Cameron JD, Dart AM, McGrath BP. Non-invasive measurements of arterial structure and function: repeatability, interrelationships and trial sample size. Clinical Science. 1998;95:669–679. doi: 10.1042/cs0950669. [DOI] [PubMed] [Google Scholar]
- 13.Wiesmann F, Petersen SE, Leeson PM, Francis JM, Robson MD, Wang Q, Choudhury R, Channon KM, Neubauer S. Global impairment of brachial, carotid, and aortic vascular function in young smokers: direct quantification by high-resolution magnetic resonance imaging. J Am Coll Cardiol. 2004;44:2056–2064. doi: 10.1016/j.jacc.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 14.Kullo IJ, Bielak LF, Turner ST, Sheedy PF, II, Peyser PA. Aortic pulse wave velocity is associated with the presence and quantity of coronary artery calcium: A Community-Based Study. Hypertension. 2006;47:174–179. doi: 10.1161/01.HYP.0000199605.35173.14. [DOI] [PubMed] [Google Scholar]
- 15.Kullo IJ, Malik AR, Bielak LF, Sheedy PF, Turner ST, Peyser PA. Brachial artery diameter and vasodilator response to nitroglycerin but not flow-mediated dilation are associated with presence and quantity of coronary artery calcium in asymptomatic adults. Clin Sci (Lond) 2007;112:175–182. doi: 10.1042/CS20060131. [DOI] [PubMed] [Google Scholar]
- 16.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 17.Magee L. R2 measures based on Wald and likelihood ratio joint significance tests. Am Stat. 1990;44:250–253. [Google Scholar]
- 18.Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983;32 (Suppl 2):64–78. doi: 10.2337/diab.32.2.s64. [DOI] [PubMed] [Google Scholar]
- 19.Schmieder RE, Veelken R, Gatzka CD, Ruddel H, Schachinger H. Predictors for hypertensive nephropathy: results of a 6-year follow-up study in essential hypertension. J Hypertens. 1995;13:357–365. [PubMed] [Google Scholar]
- 20.Ribstein J, du Cailar G, Mimran A. Combined renal effects of overweight and hypertension. Hypertension. 1995;26:610–615. doi: 10.1161/01.hyp.26.4.610. [DOI] [PubMed] [Google Scholar]
- 21.Monsuez JJ. Mediators of reactive hyperemia. Arch Mal Coeur Vaiss. 2001;94:591–599. [PubMed] [Google Scholar]
- 22.Meredith IT, Currie KE, Anderson TJ, Roddy MA, Ganz P, Creager MA. Postischemic vasodilation in human forearm is dependent on endothelium-derived nitric oxide. Am J Physiol. 1996;270:H1435–1440. doi: 10.1152/ajpheart.1996.270.4.H1435. [DOI] [PubMed] [Google Scholar]
- 23.Kullo IJ, Malik AR, Santos S, Ehrsam JE, Turner ST. Association of cardiovascular risk factors with microvascular and conduit artery function in hypertensive subjects. Am J Hypertens. 2007;20:735–742. doi: 10.1016/j.amjhyper.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 25.Gimbrone MA, Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci. 2000;902:230–239. doi: 10.1111/j.1749-6632.2000.tb06318.x. discussion 239–240. [DOI] [PubMed] [Google Scholar]
- 26.Cohn JN. Arteries, myocardium, blood pressure and cardiovascular risk: towards a revised definition of hypertension. J Hypertens. 1998;16:2117–2124. [PubMed] [Google Scholar]
- 27.Glasser SP, Arnett DK, McVeigh GE, Finkelstein SM, Bank AJ, Morgan DJ, Cohn JN. Vascular compliance and cardiovascular disease: A risk factor or a marker? Am J Hypertens. 1997;10:1175–1189. doi: 10.1016/s0895-7061(97)00311-7. [DOI] [PubMed] [Google Scholar]
- 28.Mulvany MJ, Aalkjaer C. Structure and function of small arteries. Physiol Rev. 1990;70:921–961. doi: 10.1152/physrev.1990.70.4.921. [DOI] [PubMed] [Google Scholar]
- 29.Serne EH, Stehouwer CDA, ter Maaten JC, ter Wee PM, Rauwerda JA, Donker AJM, Gans ROB. Microvascular Function Relates to Insulin Sensitivity and Blood Pressure in Normal Subjects. Circulation. 1999;99:896–902. doi: 10.1161/01.cir.99.7.896. [DOI] [PubMed] [Google Scholar]
- 30.Leeson P, Thorne S, Donald A, Mullen M, Clarkson P, Deanfield J. Non-invasive measurement of endothelial function: effect on brachial artery dilatation of graded endothelial dependent and independent stimuli. Heart. 1997;78:22–27. doi: 10.1136/hrt.78.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmitt M, Avolio A, Qasem A, McEniery CM, Butlin M, Wilkinson IB, Cockcroft JR. Basal NO locally modulates human iliac artery function in vivo. Hypertension. 2005;46:227–231. doi: 10.1161/01.HYP.0000164581.39811.bd. [DOI] [PubMed] [Google Scholar]
- 32.Joannides R, Richard V, Haefeli WE, Benoist A, Linder L, Luscher TF, Thuillez C. Role of nitric oxide in the regulation of the mechanical properties of peripheral conduit arteries in humans. Hypertension. 1997;30:1465–1470. doi: 10.1161/01.hyp.30.6.1465. [DOI] [PubMed] [Google Scholar]
- 33.Boutouyrie P, Bezie Y, Lacolley P, Challande P, Chamiot-Clerc P, Benetos A, de la Faverie JF, Safar M, Laurent S. In vivo/in vitro comparison of rat abdominal aorta wall viscosity. Influence of endothelial function. Arterioscler Thromb Vasc Biol. 1997;17:1346–1355. doi: 10.1161/01.atv.17.7.1346. [DOI] [PubMed] [Google Scholar]
- 34.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 35.McEniery CM, Wallace S, Mackenzie IS, McDonnell B, Yasmin, Newby DE, Cockcroft JR, Wilkinson IB. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension. 2006;48:602–608. doi: 10.1161/01.HYP.0000239206.64270.5f. [DOI] [PubMed] [Google Scholar]
- 36.Bank AJ, Kaiser DR. Smooth muscle relaxation: effects on arterial compliance, distensibility, elastic modulus, and pulse wave velocity. Hypertension. 1998;32:356–359. doi: 10.1161/01.hyp.32.2.356. [DOI] [PubMed] [Google Scholar]
- 37.Mehta JL. Endothelium, coronary vasodilation, and organic nitrates. Am Heart J. 1995;129:382–391. doi: 10.1016/0002-8703(95)90021-7. [DOI] [PubMed] [Google Scholar]
- 38.Adams MR, Robinson J, McCredie R, Seale JP, Sorensen KE, Deanfield JE, Celermajer DS. Smooth muscle dysfunction occurs independently of impaired endothelium-dependent dilation in adults at risk of atherosclerosis. J Am Coll Cardiol. 1998;32:123–127. doi: 10.1016/s0735-1097(98)00206-x. [DOI] [PubMed] [Google Scholar]
- 39.Malik AR, Sultan S, Turner ST, Kullo IJ. Urinary albumin excretion is associated with impaired flow- and nitroglycerin-mediated brachial artery dilatation in hypertensive adults. J Hum Hypertens. 2007;21:231–238. doi: 10.1038/sj.jhh.1002143. [DOI] [PubMed] [Google Scholar]


