Abstract
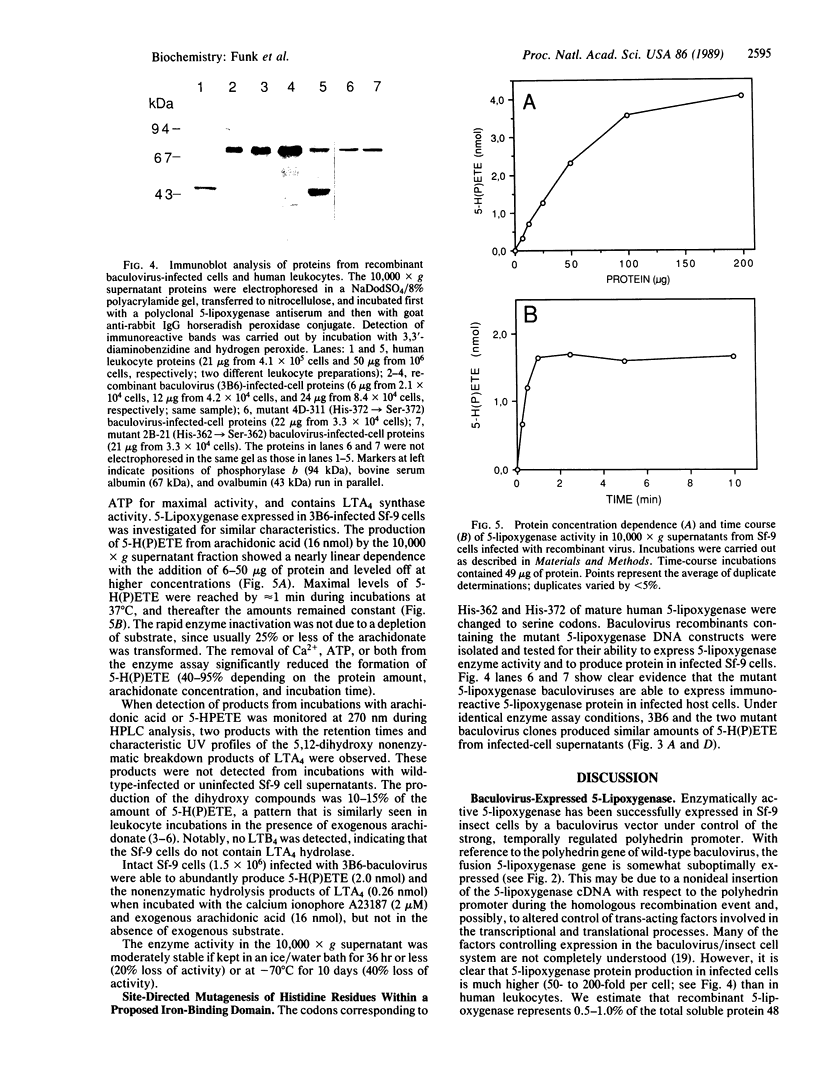
Human 5-lipoxygenase (EC 1.13.11.34), the key enzyme involved in the transformation of arachidonic acid to the potent biologically active leukotrienes, has been overexpressed in insect cells using a baculovirus expression system. A recombinant baculovirus (3B6) carrying the human 5-lipoxygenase coding sequence downstream of the strong polyhedrin protein promoter was isolated. Approximately 48 hr after infection of Spodoptera frugiperda cells with the recombinant baculovirus, maximal intracellular enzyme activity and protein levels were detected. The recombinant 5-lipoxygenase in 10,000 x g supernatant fractions was able to synthesize large amounts of 5-hydroperoxy-6,8,10,14-icosatetraenoic acid, together with smaller amounts of the nonenzymatic hydrolysis products of leukotriene A4, and exhibited a dependence on Ca2+ and ATP for maximal activity. Immunoblot analysis of supernatant proteins from human leukocytes and recombinant virus-infected cells indicated the presence of indistinguishable approximately 80-kDa bands. However, 5-lipoxygenase protein in recombinant-infected cells was found to be present in amounts 50-200 times that present in leukocytes on a per-cell basis. Histidine-362 and histidine-372, potential iron-atom ligands within a putative iron-binding domain, were changed to serine residues. Recombinant baculoviruses carrying the mutations were isolated and used to infect insect cells. Although infected cells were able to express mutant 5-lipoxygenase protein, enzyme activity was not substantially altered, suggesting the nonessential nature of these histidines in binding iron at the putative ferric catalytic site.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Jones R. E., Diehl R. E., Bennett C. D., Kargman S., Rouzer C. A. Cloning of the cDNA for human 5-lipoxygenase. Proc Natl Acad Sci U S A. 1988 Jan;85(2):416–420. doi: 10.1073/pnas.85.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ealing P. M., Casey R. The complete amino acid sequence of a pea (Pisum sativum) seed lipoxygenase predicted from a near full-length cDNA. Biochem J. 1988 Aug 1;253(3):915–918. doi: 10.1042/bj2530915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen P. D., Miller L. K. The regulation of baculovirus gene expression. Curr Top Microbiol Immunol. 1986;131:31–49. doi: 10.1007/978-3-642-71589-1_3. [DOI] [PubMed] [Google Scholar]
- Funk C. D., Hoshiko S., Matsumoto T., Rdmark O., Samuelsson B. Characterization of the human 5-lipoxygenase gene. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2587–2591. doi: 10.1073/pnas.86.8.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk C. D., Rådmark O., Fu J. Y., Matsumoto T., Jörnvall H., Shimizu T., Samuelsson B. Molecular cloning and amino acid sequence of leukotriene A4 hydrolase. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6677–6681. doi: 10.1073/pnas.84.19.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M. Metabolism of prostaglandins in rat liver mitochondria. Eur J Biochem. 1968 Oct 17;6(1):135–146. doi: 10.1111/j.1432-1033.1968.tb00430.x. [DOI] [PubMed] [Google Scholar]
- Hogaboom G. K., Cook M., Newton J. F., Varrichio A., Shorr R. G., Sarau H. M., Crooke S. T. Purification, characterization, and structural properties of a single protein from rat basophilic leukemia (RBL-1) cells possessing 5-lipoxygenase and leukotriene A4 synthetase activities. Mol Pharmacol. 1986 Dec;30(6):510–519. [PubMed] [Google Scholar]
- Luckow V. A., Summers M. D. High level expression of nonfused foreign genes with Autographa californica nuclear polyhedrosis virus expression vectors. Virology. 1989 May;170(1):31–39. doi: 10.1016/0042-6822(89)90348-6. [DOI] [PubMed] [Google Scholar]
- Maclouf J., de Laclos B. F., Borgeat P. Stimulation of leukotriene biosynthesis in human blood leukocytes by platelet-derived 12-hydroperoxy-icosatetraenoic acid. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6042–6046. doi: 10.1073/pnas.79.19.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T., Funk C. D., Rådmark O., Hög J. O., Jörnvall H., Samuelsson B. Molecular cloning and amino acid sequence of human 5-lipoxygenase. Proc Natl Acad Sci U S A. 1988 Jan;85(1):26–30. doi: 10.1073/pnas.85.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaratnam S., Feiters M. C., Al-Hakim M., Allen J. C., Veldink G. A., Vliegenthart J. F. Iron environment in soybean lipoxygenase-1. Biochim Biophys Acta. 1988 Aug 31;956(1):70–76. doi: 10.1016/0167-4838(88)90299-3. [DOI] [PubMed] [Google Scholar]
- Persson H., Gray H. E., Godeau F. Growth-dependent synthesis of c-myc-encoded proteins: early stimulation by serum factors in synchronized mouse 3T3 cells. Mol Cell Biol. 1985 Nov;5(11):2903–2912. doi: 10.1128/mcb.5.11.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer C. A., Matsumoto T., Samuelsson B. Single protein from human leukocytes possesses 5-lipoxygenase and leukotriene A4 synthase activities. Proc Natl Acad Sci U S A. 1986 Feb;83(4):857–861. doi: 10.1073/pnas.83.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer C. A., Rands E., Kargman S., Jones R. E., Register R. B., Dixon R. A. Characterization of cloned human leukocyte 5-lipoxygenase expressed in mammalian cells. J Biol Chem. 1988 Jul 25;263(21):10135–10140. [PubMed] [Google Scholar]
- Rouzer C. A., Samuelsson B. On the nature of the 5-lipoxygenase reaction in human leukocytes: enzyme purification and requirement for multiple stimulatory factors. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6040–6044. doi: 10.1073/pnas.82.18.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
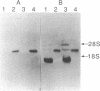
- Rouzer C. A., Shimizu T., Samuelsson B. On the nature of the 5-lipoxygenase reaction in human leukocytes: characterization of a membrane-associated stimulatory factor. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7505–7509. doi: 10.1073/pnas.82.22.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B., Dahlén S. E., Lindgren J. A., Rouzer C. A., Serhan C. N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987 Sep 4;237(4819):1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983 May 6;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata D., Steczko J., Dixon J. E., Andrews P. C., Hermodson M., Axelrod B. Primary structure of soybean lipoxygenase L-2. J Biol Chem. 1988 May 15;263(14):6816–6821. [PubMed] [Google Scholar]
- Shibata D., Steczko J., Dixon J. E., Hermodson M., Yazdanparast R., Axelrod B. Primary structure of soybean lipoxygenase-1. J Biol Chem. 1987 Jul 25;262(21):10080–10085. [PubMed] [Google Scholar]
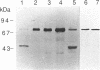
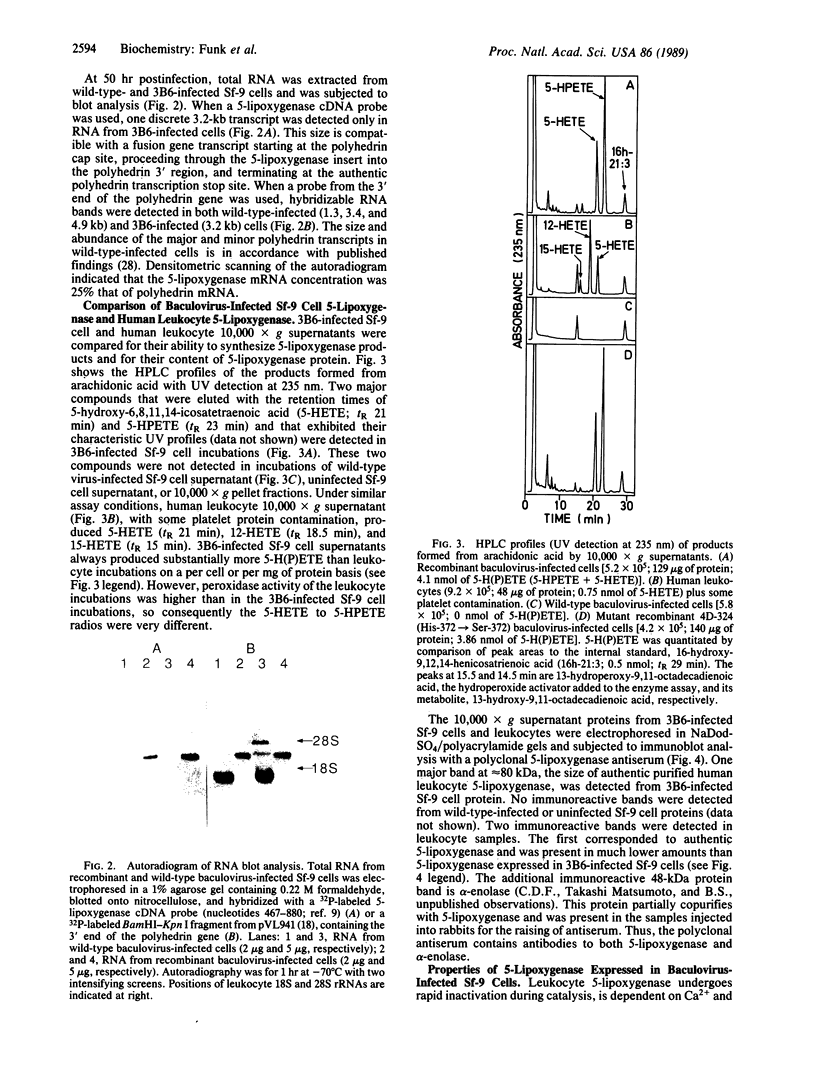
- Shimizu T., Izumi T., Seyama Y., Tadokoro K., Rådmark O., Samuelsson B. Characterization of leukotriene A4 synthase from murine mast cells: evidence for its identity to arachidonate 5-lipoxygenase. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4175–4179. doi: 10.1073/pnas.83.12.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D., Fraser M. J. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol Cell Biol. 1983 Dec;3(12):2156–2165. doi: 10.1128/mcb.3.12.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H., Pohl G., Gunne H., Hellers M., Elhammer A., Hansson L. Human tissue-type plasminogen activator synthesized by using a baculovirus vector in insect cells compared with human plasminogen activator produced in mouse cells. Gene. 1988 Dec 20;73(2):449–457. doi: 10.1016/0378-1119(88)90509-4. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda N., Kaneko S., Yoshimoto T., Yamamoto S. Purification of arachidonate 5-lipoxygenase from porcine leukocytes and its reactivity with hydroperoxyeicosatetraenoic acids. J Biol Chem. 1986 Jun 15;261(17):7982–7988. [PubMed] [Google Scholar]