Abstract
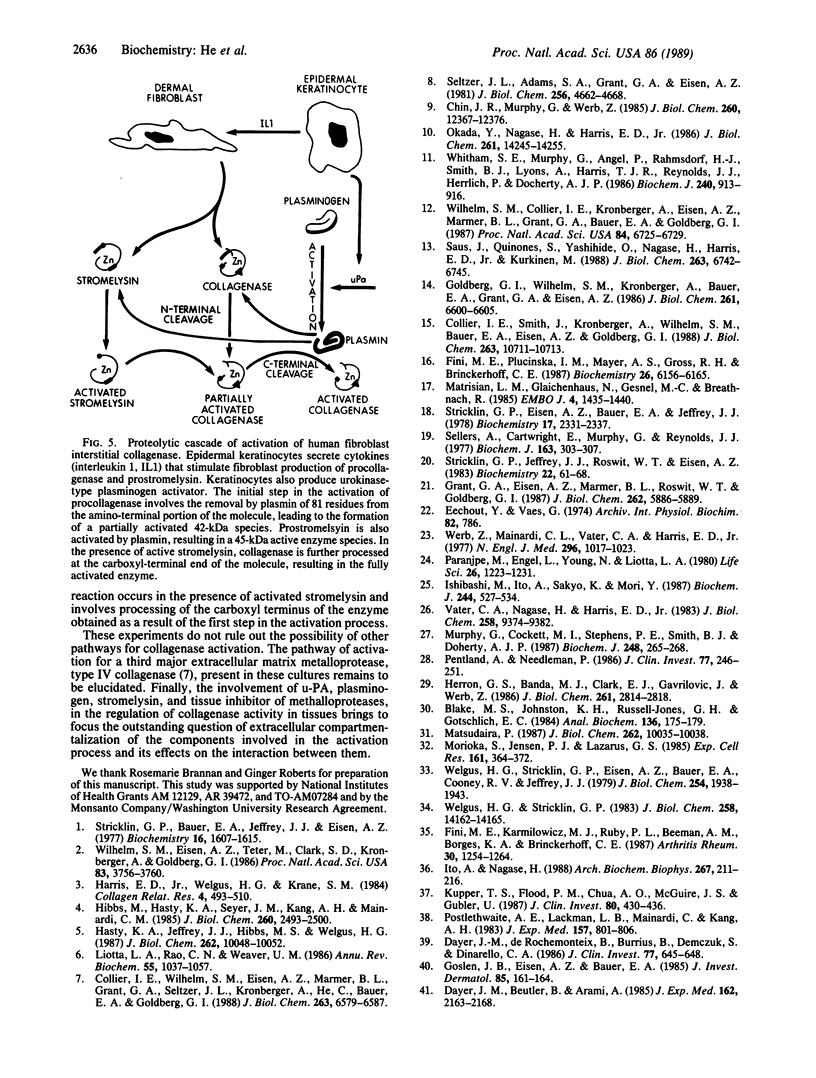
We present a cascade of proteolytic events catalyzed by the proteases secreted by cultured keratinocytes and fibroblasts that results in the activation of interstitial procollagenase. Cultured human skin fibroblasts constitutively secrete interstitial collagenase and stromelysin as proenzymes. In contrast, interstitial collagenase found in serum-free skin organ culture conditioned medium is activated. Cocultivation of the major cellular components of skin organ culture, dermal fibroblasts and epidermal keratinocytes, induces activation of interstitial procollagenase and prostromelysin in the presence of plasminogen. This activation occurs through a urokinase-dependent pathway where added keratinocytes secrete the plasminogen activator urokinase, which converts plasminogen into plasmin. Plasmin is capable of activating purified procollagenase and prostromelysin. Plasmin-dependent activation of procollagenase generates an enzyme species, by amino-terminal processing, identical to those generated by limited proteolysis with trypsin or treatment with organomercurial compounds. Catalytic amounts of activated stromelysin can in turn convert plasmin- or trypsin-activated collagenase into a fully active enzyme by removal of approximately 15 amino acid residues from the carboxyl end of the enzyme. This results in a 5- to 8-fold increase in collagenase specific activity that is due to its proteolytic cleavage and not to the presence of the activator stromelysin. Stromelysin alone in both pro- and activated forms is not capable of efficient activation of human fibroblast interstitial procollagenase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Chin J. R., Murphy G., Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. Biosynthesis, isolation, characterization, and substrates. J Biol Chem. 1985 Oct 5;260(22):12367–12376. [PubMed] [Google Scholar]
- Collier I. E., Smith J., Kronberger A., Bauer E. A., Wilhelm S. M., Eisen A. Z., Goldberg G. I. The structure of the human skin fibroblast collagenase gene. J Biol Chem. 1988 Aug 5;263(22):10711–10713. [PubMed] [Google Scholar]
- Collier I. E., Wilhelm S. M., Eisen A. Z., Marmer B. L., Grant G. A., Seltzer J. L., Kronberger A., He C. S., Bauer E. A., Goldberg G. I. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988 May 15;263(14):6579–6587. [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., de Rochemonteix B., Burrus B., Demczuk S., Dinarello C. A. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986 Feb;77(2):645–648. doi: 10.1172/JCI112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhout Y., Vaes G. Proceedings: Activation of the inactive precursor of collagenase by kallikrein and plasmin. Arch Int Physiol Biochim. 1974 Oct;82(4):786–786. [PubMed] [Google Scholar]
- Fini M. E., Karmilowicz M. J., Ruby P. L., Beeman A. M., Borges K. A., Brinckerhoff C. E. Cloning of a complementary DNA for rabbit proactivator. A metalloproteinase that activates synovial cell collagenase, shares homology with stromelysin and transin, and is coordinately regulated with collagenase. Arthritis Rheum. 1987 Nov;30(11):1254–1264. doi: 10.1002/art.1780301108. [DOI] [PubMed] [Google Scholar]
- Fini M. E., Plucinska I. M., Mayer A. S., Gross R. H., Brinckerhoff C. E. A gene for rabbit synovial cell collagenase: member of a family of metalloproteinases that degrade the connective tissue matrix. Biochemistry. 1987 Sep 22;26(19):6156–6165. doi: 10.1021/bi00393a032. [DOI] [PubMed] [Google Scholar]
- Goldberg G. I., Wilhelm S. M., Kronberger A., Bauer E. A., Grant G. A., Eisen A. Z. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem. 1986 May 15;261(14):6600–6605. [PubMed] [Google Scholar]
- Goslen J. B., Eisen A. Z., Bauer E. A. Stimulation of skin fibroblast collagenase production by a cytokine derived from basal cell carcinomas. J Invest Dermatol. 1985 Aug;85(2):161–164. doi: 10.1111/1523-1747.ep12276589. [DOI] [PubMed] [Google Scholar]
- Grant G. A., Eisen A. Z., Marmer B. L., Roswit W. T., Goldberg G. I. The activation of human skin fibroblast procollagenase. Sequence identification of the major conversion products. J Biol Chem. 1987 Apr 25;262(12):5886–5889. [PubMed] [Google Scholar]
- Harris E. D., Jr, Welgus H. G., Krane S. M. Regulation of the mammalian collagenases. Coll Relat Res. 1984 Dec;4(6):493–512. doi: 10.1016/s0174-173x(84)80015-1. [DOI] [PubMed] [Google Scholar]
- Hasty K. A., Jeffrey J. J., Hibbs M. S., Welgus H. G. The collagen substrate specificity of human neutrophil collagenase. J Biol Chem. 1987 Jul 25;262(21):10048–10052. [PubMed] [Google Scholar]
- Herron G. S., Banda M. J., Clark E. J., Gavrilovic J., Werb Z. Secretion of metalloproteinases by stimulated capillary endothelial cells. II. Expression of collagenase and stromelysin activities is regulated by endogenous inhibitors. J Biol Chem. 1986 Feb 25;261(6):2814–2818. [PubMed] [Google Scholar]
- Hibbs M. S., Hasty K. A., Seyer J. M., Kang A. H., Mainardi C. L. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem. 1985 Feb 25;260(4):2493–2500. [PubMed] [Google Scholar]
- Ishibashi M., Ito A., Sakyo K., Mori Y. Procollagenase activator produced by rabbit uterine cervical fibroblasts. Biochem J. 1987 Jan 15;241(2):527–534. doi: 10.1042/bj2410527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A., Nagase H. Evidence that human rheumatoid synovial matrix metalloproteinase 3 is an endogenous activator of procollagenase. Arch Biochem Biophys. 1988 Nov 15;267(1):211–216. doi: 10.1016/0003-9861(88)90025-2. [DOI] [PubMed] [Google Scholar]
- Kupper T. S., Chua A. O., Flood P., McGuire J., Gubler U. Interleukin 1 gene expression in cultured human keratinocytes is augmented by ultraviolet irradiation. J Clin Invest. 1987 Aug;80(2):430–436. doi: 10.1172/JCI113090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Wewer U. M. Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem. 1986;55:1037–1057. doi: 10.1146/annurev.bi.55.070186.005133. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M., Glaichenhaus N., Gesnel M. C., Breathnach R. Epidermal growth factor and oncogenes induce transcription of the same cellular mRNA in rat fibroblasts. EMBO J. 1985 Jun;4(6):1435–1440. doi: 10.1002/j.1460-2075.1985.tb03799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Morioka S., Jensen P. J., Lazarus G. S. Human epidermal plasminogen activator. Characterization, localization, and modulation. Exp Cell Res. 1985 Dec;161(2):364–372. doi: 10.1016/0014-4827(85)90093-x. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Stephens P. E., Smith B. J., Docherty A. J. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987 Nov 15;248(1):265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Nagase H., Harris E. D., Jr A metalloproteinase from human rheumatoid synovial fibroblasts that digests connective tissue matrix components. Purification and characterization. J Biol Chem. 1986 Oct 25;261(30):14245–14255. [PubMed] [Google Scholar]
- Paranjpe M., Engel L., Young N., Liotta L. A. Activation of human breast carcinoma collagenase through plasminogen activator. Life Sci. 1980 Apr 14;26(15):1223–1231. doi: 10.1016/0024-3205(80)90067-3. [DOI] [PubMed] [Google Scholar]
- Pentland A. P., Needleman P. Modulation of keratinocyte proliferation in vitro by endogenous prostaglandin synthesis. J Clin Invest. 1986 Jan;77(1):246–251. doi: 10.1172/JCI112283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Lachman L. B., Mainardi C. L., Kang A. H. Interleukin 1 stimulation of collagenase production by cultured fibroblasts. J Exp Med. 1983 Feb 1;157(2):801–806. doi: 10.1084/jem.157.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saus J., Quinones S., Otani Y., Nagase H., Harris E. D., Jr, Kurkinen M. The complete primary structure of human matrix metalloproteinase-3. Identity with stromelysin. J Biol Chem. 1988 May 15;263(14):6742–6745. [PubMed] [Google Scholar]
- Sellers A., Cartwright E., Murphy G., Reynolds J. J. Evidence that latent collagenases are enzyme-inhibitor complexes. Biochem J. 1977 May 1;163(2):303–307. doi: 10.1042/bj1630303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer J. L., Adams S. A., Grant G. A., Eisen A. Z. Purification and properties of a gelatin-specific neutral protease from human skin. J Biol Chem. 1981 May 10;256(9):4662–4668. [PubMed] [Google Scholar]
- Stricklin G. P., Bauer E. A., Jeffrey J. J., Eisen A. Z. Human skin collagenase: isolation of precursor and active forms from both fibroblast and organ cultures. Biochemistry. 1977 Apr 19;16(8):1607–1615. doi: 10.1021/bi00627a013. [DOI] [PubMed] [Google Scholar]
- Stricklin G. P., Eisen A. Z., Bauer E. A., Jeffrey J. J. Human skin fibroblast collagenase: chemical properties of precursor and active forms. Biochemistry. 1978 Jun 13;17(12):2331–2337. doi: 10.1021/bi00605a012. [DOI] [PubMed] [Google Scholar]
- Stricklin G. P., Jeffrey J. J., Roswit W. T., Eisen A. Z. Human skin fibroblast procollagenase: mechanisms of activation by organomercurials and trypsin. Biochemistry. 1983 Jan 4;22(1):61–68. doi: 10.1021/bi00270a009. [DOI] [PubMed] [Google Scholar]
- Vater C. A., Nagase H., Harris E. D., Jr Purification of an endogenous activator of procollagenase from rabbit synovial fibroblast culture medium. J Biol Chem. 1983 Aug 10;258(15):9374–9382. [PubMed] [Google Scholar]
- Welgus H. G., Kobayashi D. K., Jeffrey J. J. The collagen substrate specificity of rat uterus collagenase. J Biol Chem. 1983 Dec 10;258(23):14162–14165. [PubMed] [Google Scholar]
- Welgus H. G., Stricklin G. P., Eisen A. Z., Bauer E. A., Cooney R. V., Jeffrey J. J. A specific inhibitor of vertebrate collagenase produced by human skin fibroblasts. J Biol Chem. 1979 Mar 25;254(6):1938–1943. [PubMed] [Google Scholar]
- Werb Z., Mainardi C. L., Vater C. A., Harris E. D., Jr Endogenous activiation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N Engl J Med. 1977 May 5;296(18):1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]
- Whitham S. E., Murphy G., Angel P., Rahmsdorf H. J., Smith B. J., Lyons A., Harris T. J., Reynolds J. J., Herrlich P., Docherty A. J. Comparison of human stromelysin and collagenase by cloning and sequence analysis. Biochem J. 1986 Dec 15;240(3):913–916. doi: 10.1042/bj2400913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Kronberger A., Eisen A. Z., Marmer B. L., Grant G. A., Bauer E. A., Goldberg G. I. Human skin fibroblast stromelysin: structure, glycosylation, substrate specificity, and differential expression in normal and tumorigenic cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6725–6729. doi: 10.1073/pnas.84.19.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S. M., Eisen A. Z., Teter M., Clark S. D., Kronberger A., Goldberg G. Human fibroblast collagenase: glycosylation and tissue-specific levels of enzyme synthesis. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3756–3760. doi: 10.1073/pnas.83.11.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]