Abstract
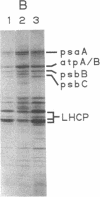
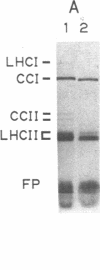
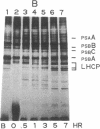
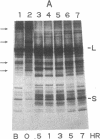
Nitrogen-limited Chlamydomonas reinhardtii is chlorotic and very deficient in chlorophyll a/b light-harvesting complexes (LHC). Rates of synthesis of photosynthetic proteins, but especially the LHC apoproteins, are reduced 10- to 40-fold. Moderately high levels of chloroplast transcripts accumulate in nitrogen-limited cells, and there is a correlation between chloroplast DNA levels and chloroplast mRNA abundance. In contrast, nuclear transcripts encoding LHCII and ribulose 1,5-bisphosphate carboxylase small subunits are markedly reduced. Thus, nitrogen availability affects chloroplast protein synthesis by inhibition of translation and, to a lesser extent, chloroplast DNA amplification. Regulation of nuclear-encoded photosynthetic proteins by nitrogen is achieved through mechanisms affecting transcription and/or mRNA stability.
Keywords: chlorophyll; light-harvesting antenna; chloroplast; ribulose 1,5-bisphosphate carboxylase; thylakoids
Full text
PDF
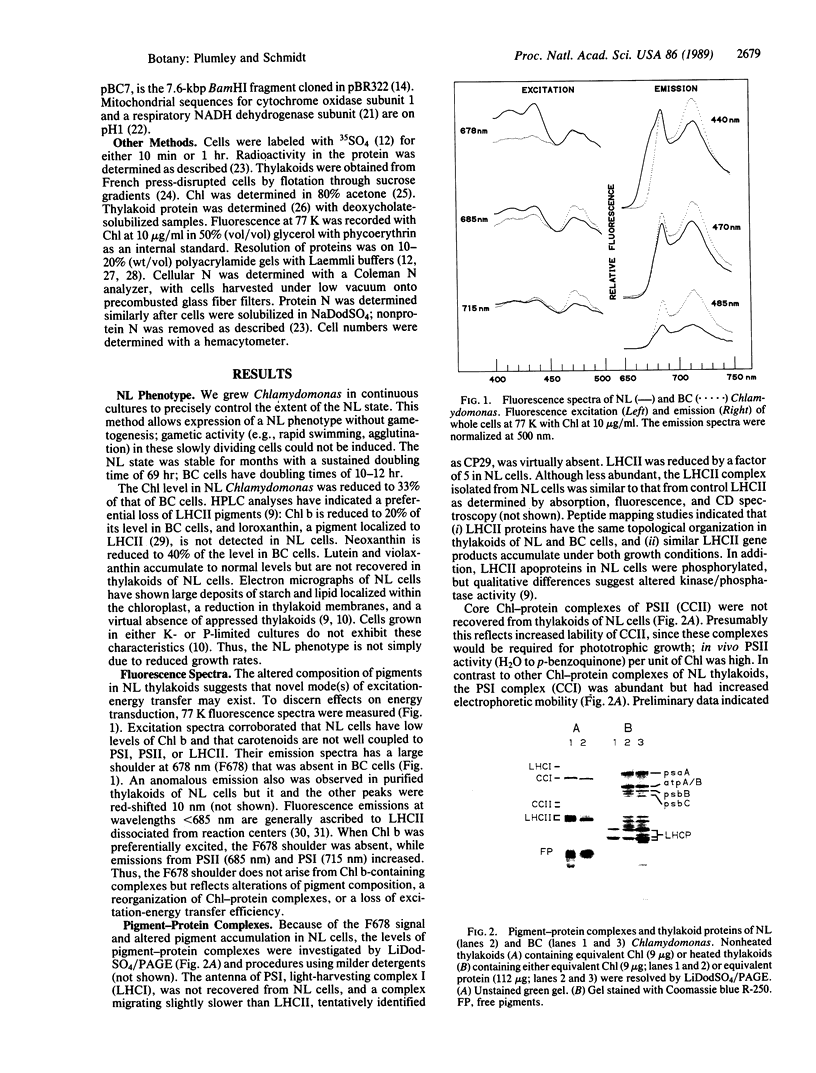
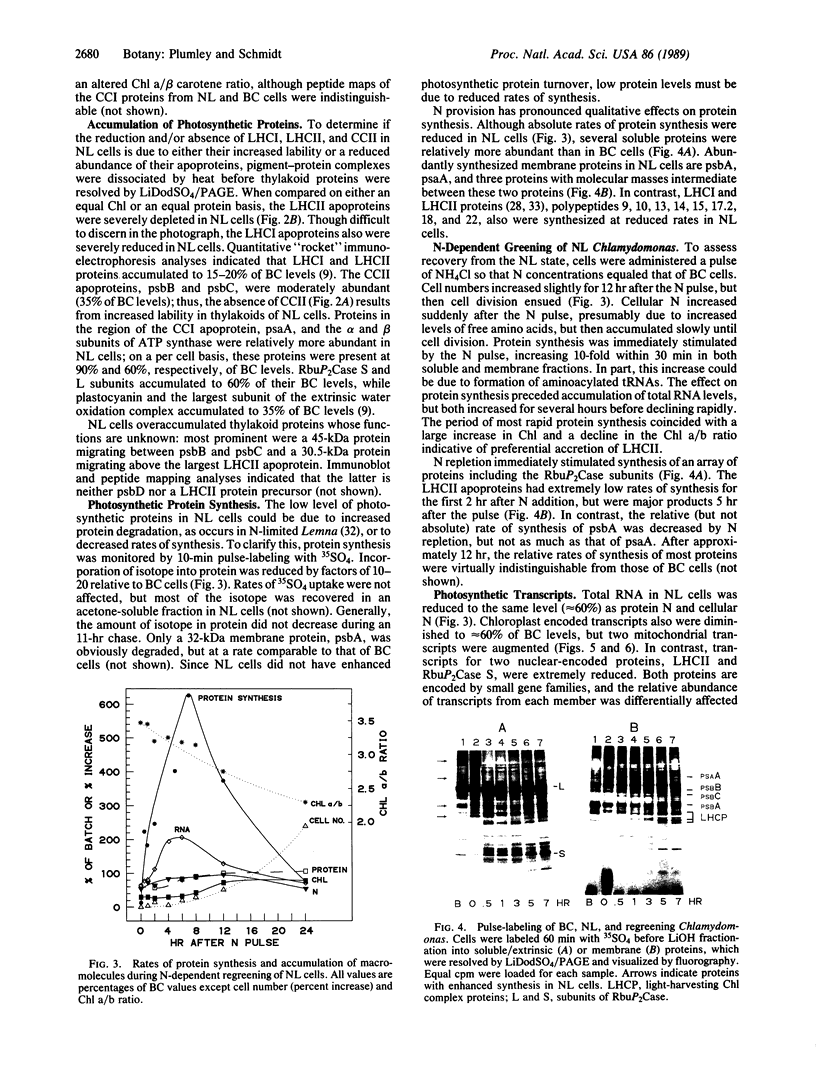

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendich A. J. Why do chloroplasts and mitochondria contain so many copies of their genome? Bioessays. 1987 Jun;6(6):279–282. doi: 10.1002/bies.950060608. [DOI] [PubMed] [Google Scholar]
- Boer P. H., Bonen L., Lee R. W., Gray M. W. Genes for respiratory chain proteins and ribosomal RNAs are present on a 16-kilobase-pair DNA species from Chlamydomonas reinhardtii mitochondria. Proc Natl Acad Sci U S A. 1985 May;82(10):3340–3344. doi: 10.1073/pnas.82.10.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer P. H., Gray M. W. The URF 5 gene of Chlamydomonas reinhardtii mitochondria: DNA sequence and mode of transcription. EMBO J. 1986 Jan;5(1):21–28. doi: 10.1002/j.1460-2075.1986.tb04172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia C. P., Duesing J. H., Arntzen C. J. Developmental Loss of Photosystem II Activity and Structure in a Chloroplast-Encoded Tobacco Mutant, Lutescens-1. Plant Physiol. 1986 Sep;82(1):19–27. doi: 10.1104/pp.82.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang K. S., Sueoka N. Replication of chloroplast DNA in Chlamydomonas reinhardi during vegetative cell cycle: its mode and regulation. Proc Natl Acad Sci U S A. 1967 May;57(5):1506–1513. doi: 10.1073/pnas.57.5.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Bennoun P. Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: wild-type and mutant strains deficient in photosystem II reaction center. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2175–2179. doi: 10.1073/pnas.72.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira R. B., Davies D. D. Protein Degradation in Lemna with Particular Reference to Ribulose Bisphosphate Carboxylase: II. The Effect of Nutrient Starvation. Plant Physiol. 1987 Apr;83(4):878–883. doi: 10.1104/pp.83.4.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M., Rahire M. Sequence, evolution and differential expression of the two genes encoding variant small subunits of ribulose bisphosphate carboxylase/oxygenase in Chlamydomonas reinhardtii. J Mol Biol. 1986 Oct 5;191(3):421–432. doi: 10.1016/0022-2836(86)90137-3. [DOI] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrin D. L., Michaels A. S., Paul A. L. Regulation of genes encoding the large subunit of ribulose-1,5-bisphosphate carboxylase and the photosystem II polypeptides D-1 and D-2 during the cell cycle of Chlamydomonas reinhardtii. J Cell Biol. 1986 Nov;103(5):1837–1845. doi: 10.1083/jcb.103.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrin D. L., Plumley F. G., Ikeuchi M., Michaels A. S., Schmidt G. W. Chlorophyll antenna proteins of photosystem I: topology, synthesis, and regulation of the 20-kDa subunit of Chlamydomonas light-harvesting complex of photosystem I. Arch Biochem Biophys. 1987 May 1;254(2):397–408. doi: 10.1016/0003-9861(87)90117-2. [DOI] [PubMed] [Google Scholar]
- Herrin D. L., Schmidt G. W. Rapid, reversible staining of northern blots prior to hybridization. Biotechniques. 1988 Mar;6(3):196-7, 199-200. [PubMed] [Google Scholar]
- Herrin D. L., Schmidt G. W. trans-splicing of transcripts for the chloroplast psaA1 gene. In vivo requirement for nuclear gene products. J Biol Chem. 1988 Oct 15;263(29):14601–14604. [PubMed] [Google Scholar]
- Hunt E. R., Weber J. A., Gates D. M. Effects of Nitrate Application on Amaranthus powellii Wats. : I. Changes in Photosynthesis, Growth Rates, and Leaf Area. Plant Physiol. 1985 Nov;79(3):609–613. doi: 10.1104/pp.79.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K. H., Herrin D. L., Plumley F. G., Schmidt G. W. Biogenesis of photosystem II complexes: transcriptional, translational, and posttranslational regulation. J Cell Biol. 1986 Oct;103(4):1315–1325. doi: 10.1083/jcb.103.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanningmeier U., Howell S. H. Regulation of light-harvesting chlorophyll-binding protein mRNA accumulation in Chlamydomonas reinhardi. Possible involvement of chlorophyll synthesis precursors. J Biol Chem. 1984 Nov 10;259(21):13541–13549. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Saux C., Lemoine Y., Marion-Poll A., Valadier M. H., Deng M., Morot-Gaudry J. F. Consequence of Absence of Nitrate Reductase Activity on Photosynthesis in Nicotiana plumbaginifolia Plants. Plant Physiol. 1987 May;84(1):67–72. doi: 10.1104/pp.84.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd H. S., Ledoigt G., Howell S. H. Regulation of light-harvesting chlorophyll-binding protein (LHCP) mRNA accumulation during the cell cycle in Chlamydomonas reinhardi. Cell. 1983 Jan;32(1):99–107. doi: 10.1016/0092-8674(83)90500-7. [DOI] [PubMed] [Google Scholar]
- Williams B. D., Mitchell D. R., Rosenbaum J. L. Molecular cloning and expression of flagellar radial spoke and dynein genes of Chlamydomonas. J Cell Biol. 1986 Jul;103(1):1–11. doi: 10.1083/jcb.103.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]