Abstract
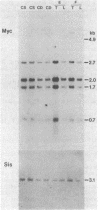
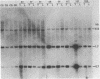
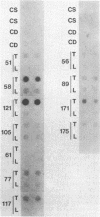
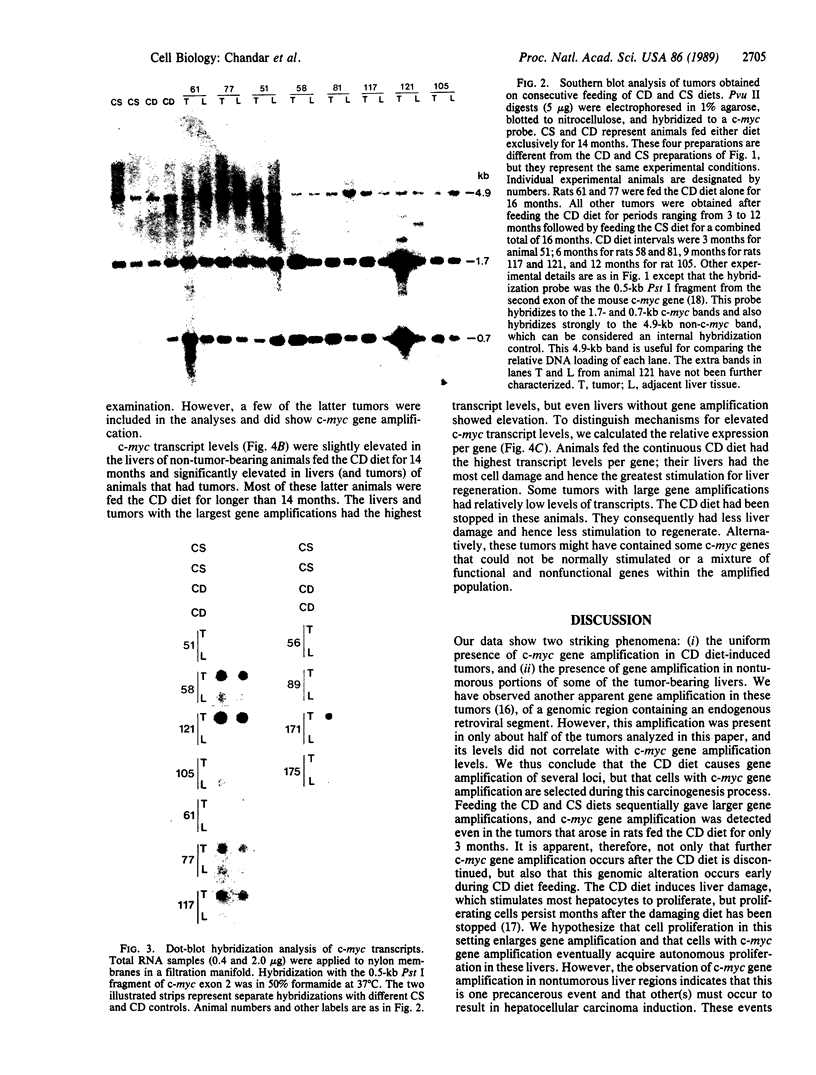
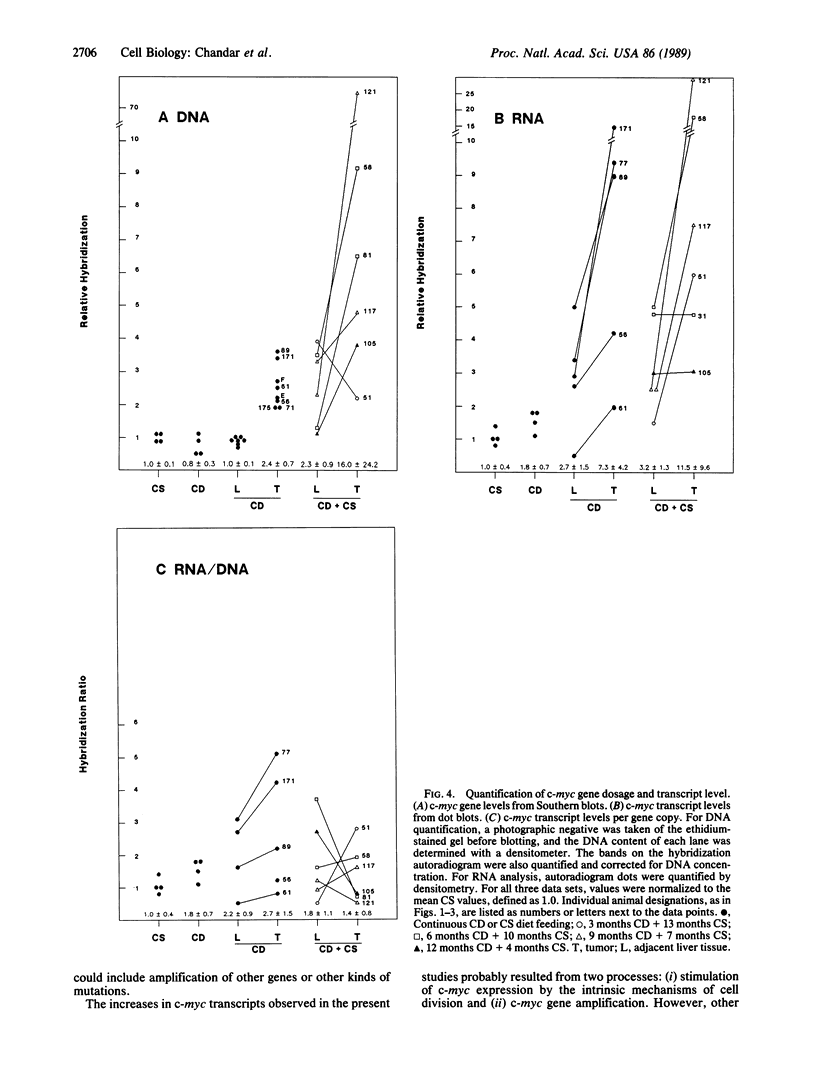
Liver tumors arise in rats fed a choline-devoid diet without added carcinogens. We found amplification of the c-myc gene in 13/13 of these tumors. The amplification ranged from 2- to 70-fold and was accompanied by an increase in c-myc gene expression. Amplification of c-myc was larger in tumors of rats fed a choline-devoid diet followed by a choline-supplemented diet than in tumors from animals fed a choline-devoid diet exclusively. In the former animals, low levels of c-myc gene amplification were also detected in nontumorous regions of tumor-bearing livers. The choline-devoid diet provides an in vivo experimental model for the induction of gene amplification in the rat liver. In this setting, amplification of the c-myc gene may be an early and critical event in carcinogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandar N., Amenta J., Kandala J. C., Lombardi B. Liver cell turnover in rats fed a choline-devoid diet. Carcinogenesis. 1987 May;8(5):669–673. doi: 10.1093/carcin/8.5.669. [DOI] [PubMed] [Google Scholar]
- Chandar N., Lombardi B. Liver cell proliferation and incidence of hepatocellular carcinomas in rats fed consecutively a choline-devoid and a choline-supplemented diet. Carcinogenesis. 1988 Feb;9(2):259–263. doi: 10.1093/carcin/9.2.259. [DOI] [PubMed] [Google Scholar]
- Chandar N., Lombardi B., Schulz W., Locker J. Analysis of ras genes and linked viral sequences in rat hepatocarcinogenesis. Am J Pathol. 1987 Nov;129(2):232–241. [PMC free article] [PubMed] [Google Scholar]
- Cole M. D. The myc oncogene: its role in transformation and differentiation. Annu Rev Genet. 1986;20:361–384. doi: 10.1146/annurev.ge.20.120186.002045. [DOI] [PubMed] [Google Scholar]
- Cote G. J., Chiu J. F. The expressions of oncogenes and liver-specific genes in Morris hepatomas. Biochem Biophys Res Commun. 1987 Mar 13;143(2):624–629. doi: 10.1016/0006-291x(87)91399-4. [DOI] [PubMed] [Google Scholar]
- Cote G. J., Lastra B. A., Cook J. R., Huang D. P., Chiu J. F. Oncogene expression in rat hepatomas and during hepatocarcinogenesis. Cancer Lett. 1985 Mar;26(2):121–127. doi: 10.1016/0304-3835(85)90017-5. [DOI] [PubMed] [Google Scholar]
- Dandekar S., Sukumar S., Zarbl H., Young L. J., Cardiff R. D. Specific activation of the cellular Harvey-ras oncogene in dimethylbenzanthracene-induced mouse mammary tumors. Mol Cell Biol. 1986 Nov;6(11):4104–4108. doi: 10.1128/mcb.6.11.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devare S. G., Reddy E. P., Law J. D., Robbins K. C., Aaronson S. A. Nucleotide sequence of the simian sarcoma virus genome: demonstration that its acquired cellular sequences encode the transforming gene product p28sis. Proc Natl Acad Sci U S A. 1983 Feb;80(3):731–735. doi: 10.1073/pnas.80.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ghoshal A. K., Farber E. The induction of liver cancer by dietary deficiency of choline and methionine without added carcinogens. Carcinogenesis. 1984 Oct;5(10):1367–1370. doi: 10.1093/carcin/5.10.1367. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Earley K., Locker J., Lombardi B. 32P-postlabeling analysis of liver DNA adducts in rats chronically fed a choline-devoid diet. Carcinogenesis. 1987 Jan;8(1):187–189. doi: 10.1093/carcin/8.1.187. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Makino R., Kawamura H., Arisawa A., Yoneda K. Characterization of rat c-myc and adjacent regions. Nucleic Acids Res. 1987 Aug 25;15(16):6419–6436. doi: 10.1093/nar/15.16.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Makino R., Sugimura T. Amplification and over-expression of the c-myc gene in Morris hepatomas. Gan. 1984 Jun;75(6):475–478. [PubMed] [Google Scholar]
- Huber B. E., Thorgeirsson S. S. Analysis of c-myc expression in a human hepatoma cell line. Cancer Res. 1987 Jul 1;47(13):3414–3420. [PubMed] [Google Scholar]
- Ishikawa F., Takaku F., Hayashi K., Nagao M., Sugimura T. Activation of rat c-raf during transfection of hepatocellular carcinoma DNA. Proc Natl Acad Sci U S A. 1986 May;83(10):3209–3212. doi: 10.1073/pnas.83.10.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnath L., Locker J. Variable methylation of the ribosomal RNA genes of the rat. Nucleic Acids Res. 1982 Jul 10;10(13):3877–3892. doi: 10.1093/nar/10.13.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker J., Reddy T. V., Lombardi B. DNA methylation and hepatocarcinogenesis in rats fed a choline-devoid diet. Carcinogenesis. 1986 Aug;7(8):1309–1312. doi: 10.1093/carcin/7.8.1309. [DOI] [PubMed] [Google Scholar]
- McMahon G., Hanson L., Lee J. J., Wogan G. N. Identification of an activated c-Ki-ras oncogene in rat liver tumors induced by aflatoxin B1. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9418–9422. doi: 10.1073/pnas.83.24.9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikol Y. B., Hoover K. L., Creasia D., Poirier L. A. Hepatocarcinogenesis in rats fed methyl-deficient, amino acid-defined diets. Carcinogenesis. 1983 Dec;4(12):1619–1629. doi: 10.1093/carcin/4.12.1619. [DOI] [PubMed] [Google Scholar]
- Reynolds S. H., Stowers S. J., Maronpot R. R., Anderson M. W., Aaronson S. A. Detection and identification of activated oncogenes in spontaneously occurring benign and malignant hepatocellular tumors of the B6C3F1 mouse. Proc Natl Acad Sci U S A. 1986 Jan;83(1):33–37. doi: 10.1073/pnas.83.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds S. H., Stowers S. J., Patterson R. M., Maronpot R. R., Aaronson S. A., Anderson M. W. Activated oncogenes in B6C3F1 mouse liver tumors: implications for risk assessment. Science. 1987 Sep 11;237(4820):1309–1316. doi: 10.1126/science.3629242. [DOI] [PubMed] [Google Scholar]
- Stanton L. W., Fahrlander P. D., Tesser P. M., Marcu K. B. Nucleotide sequence comparison of normal and translocated murine c-myc genes. Nature. 1984 Aug 2;310(5976):423–425. doi: 10.1038/310423a0. [DOI] [PubMed] [Google Scholar]
- Sümegi J., Spira J., Bazin H., Szpirer J., Levan G., Klein G. Rat c-myc oncogene is located on chromosome 7 and rearranges in immunocytomas with t(6:7) chromosomal translocation. Nature. 1983 Dec 1;306(5942):497–498. doi: 10.1038/306497a0. [DOI] [PubMed] [Google Scholar]
- Tashiro F., Morimura S., Hayashi K., Makino R., Kawamura H., Horikoshi N., Nemoto K., Ohtsubo K., Sugimura T., Ueno Y. Expression of the c-Ha-ras and c-myc genes in aflatoxin B1-induced hepatocellular carcinomas. Biochem Biophys Res Commun. 1986 Jul 31;138(2):858–864. doi: 10.1016/s0006-291x(86)80575-7. [DOI] [PubMed] [Google Scholar]
- Thompson N. L., Mead J. E., Braun L., Goyette M., Shank P. R., Fausto N. Sequential protooncogene expression during rat liver regeneration. Cancer Res. 1986 Jun;46(6):3111–3117. [PubMed] [Google Scholar]
- Wilson M. J., Shivapurkar N., Poirier L. A. Hypomethylation of hepatic nuclear DNA in rats fed with a carcinogenic methyl-deficient diet. Biochem J. 1984 Mar 15;218(3):987–990. doi: 10.1042/bj2180987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S., Sells M. A., Reddy T. V., Lombardi B. Hepatocarcinogenic and promoting action of a choline-devoid diet in the rat. Cancer Res. 1985 Jun;45(6):2834–2842. [PubMed] [Google Scholar]
- Zhang X. K., Huang D. P., Chiu D. K., Chiu J. F. The expression of oncogenes in human developing liver and hepatomas. Biochem Biophys Res Commun. 1987 Feb 13;142(3):932–938. doi: 10.1016/0006-291x(87)91503-8. [DOI] [PubMed] [Google Scholar]