Abstract
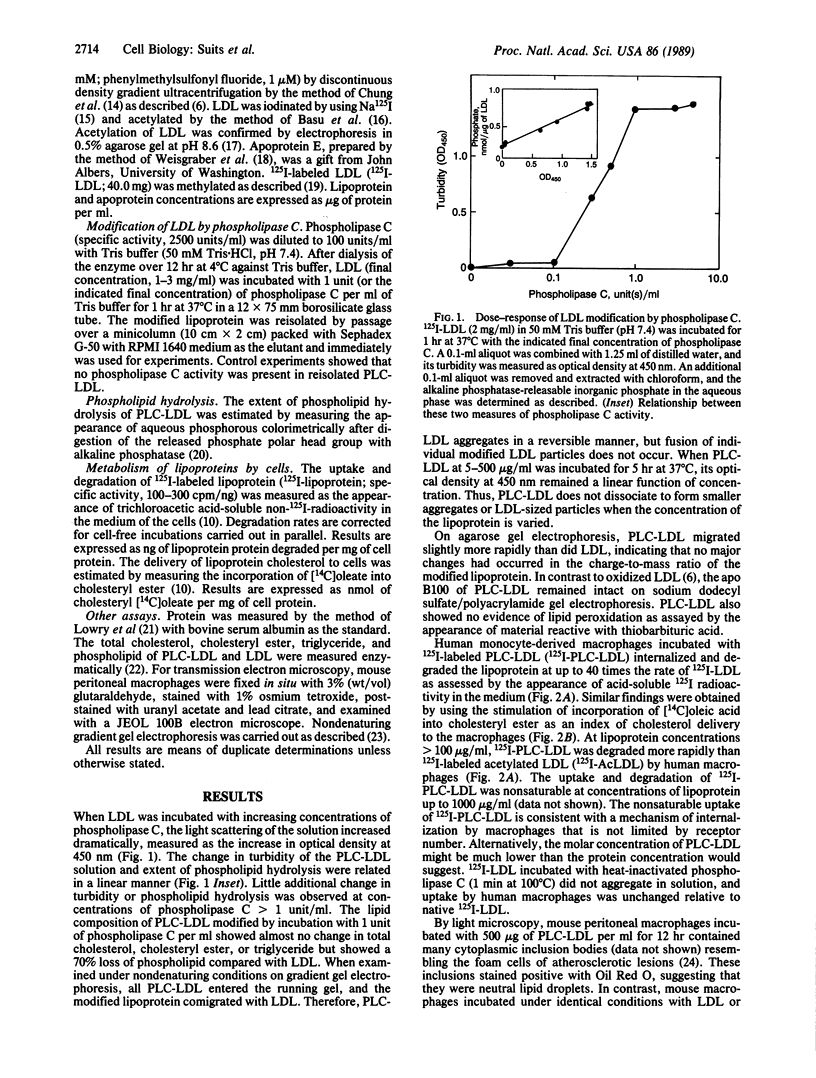
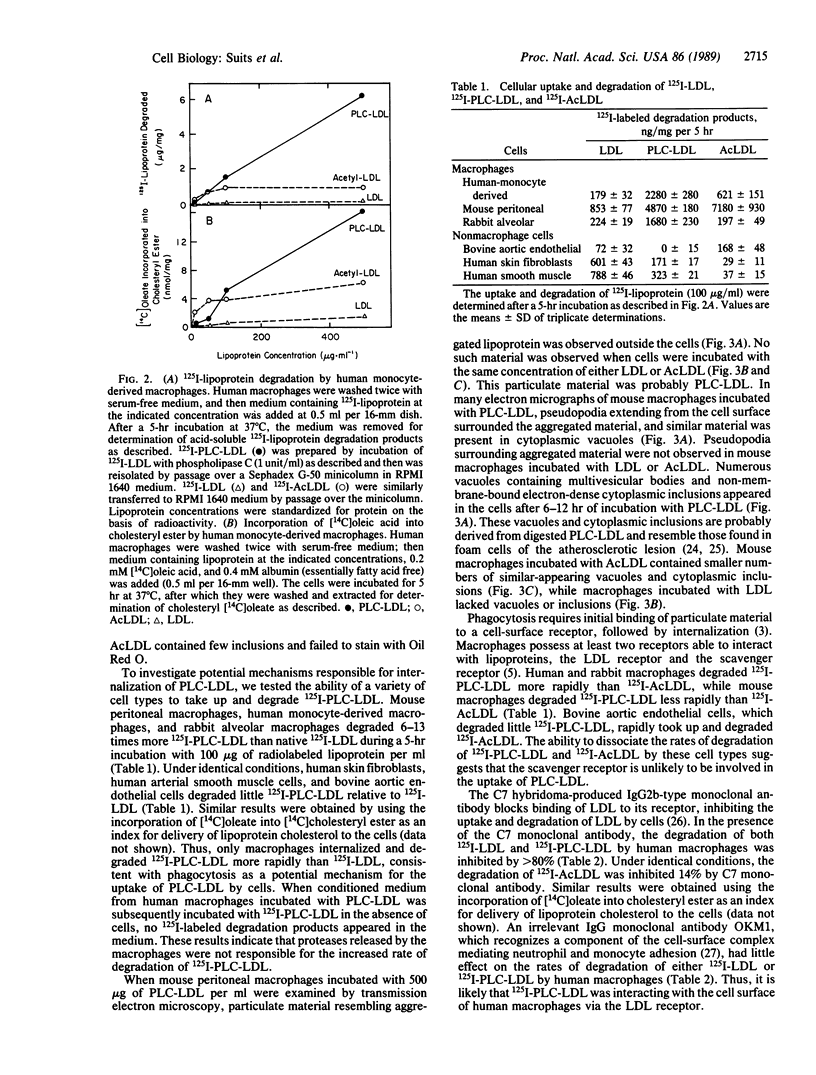
Low density lipoprotein (LDL) modified by incubation with phospholipase C (PLC-LDL) aggregates in solution and is rapidly taken up and degraded by human and mouse macrophages, producing foam cells in vitro. Human, mouse, and rabbit macrophages degraded 125I-labeled PLC-LDL (125I-PLC-LDL) more rapidly than native 125I-labeled LDL (125I-LDL), while nonphagocytic cells such as human fibroblasts and bovine aortic endothelial cells degraded 125I-PLC-LDL more slowly than 125I-LDL. This suggested the mechanism for internalization of PLC-LDL was phagocytosis. When examined by electron microscopy, mouse peritoneal macrophages appeared to be phagocytosing PLC-LDL. The uptake and degradation of 125I-PLC-LDL by human macrophages was inhibited greater than 80% by the monoclonal antibody C7 (IgG2b) produced by hybridoma C7, which blocks the ligand binding domain of the LDL receptor. Similarly, methylation of 125I-LDL (125I-MeLDL) prior to treatment with phospholipase C decreased its subsequent uptake and degradation by human macrophages by greater than 90%. The uptake and degradation of phospholipase C-modified 125I-MeLDL by macrophages could be restored by incubation of the methylated lipoprotein with apoprotein E, a ligand recognized by the LDL receptor. These results indicate that macrophages internalize PLC-LDL by LDL receptor-dependent phagocytosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviram M. Platelet-modified low-density lipoproteins: studies in normal subjects and in patients with homozygous familial hypercholesterolemia. Clin Biochem. 1987 Apr;20(2):91–95. doi: 10.1016/s0009-9120(87)80106-6. [DOI] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson G. W., Brown M. S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisiegel U., Schneider W. J., Goldstein J. L., Anderson R. G., Brown M. S. Monoclonal antibodies to the low density lipoprotein receptor as probes for study of receptor-mediated endocytosis and the genetics of familial hypercholesterolemia. J Biol Chem. 1981 Nov 25;256(22):11923–11931. [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Regulation of the activity of the low density lipoprotein receptor in human fibroblasts. Cell. 1975 Nov;6(3):307–316. doi: 10.1016/0092-8674(75)90182-8. [DOI] [PubMed] [Google Scholar]
- Chait A., Bierman E. L., Albers J. J. Low-density lipoprotein receptor activity in cultured human skin fibroblasts. Mechanism of insulin-induced stimulation. J Clin Invest. 1979 Nov;64(5):1309–1319. doi: 10.1172/JCI109587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung B. H., Wilkinson T., Geer J. C., Segrest J. P. Preparative and quantitative isolation of plasma lipoproteins: rapid, single discontinuous density gradient ultracentrifugation in a vertical rotor. J Lipid Res. 1980 Mar;21(3):284–291. [PubMed] [Google Scholar]
- Curtiss L. K., Black A. S., Takagi Y., Plow E. F. New mechanism for foam cell generation in atherosclerotic lesions. J Clin Invest. 1987 Aug;80(2):367–373. doi: 10.1172/JCI113081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone D. J., Mated N., Shio H., Minick C. R., Fowler S. D. Lipoprotein-heparin-fibronectin-denatured collagen complexes enhance cholesteryl ester accumulation in macrophages. J Cell Biol. 1984 Oct;99(4 Pt 1):1266–1274. doi: 10.1083/jcb.99.4.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine R. N., Fielding C. J. Comparison of cholesterol transport in pulmonary, peritoneal, and blood-derived macrophages from normo- and hypercholesterolemic rabbits. J Lipid Res. 1985 Aug;26(8):915–923. [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Griffin J. A., Silverstein S. C. Studies on the mechanism of phagocytosis. II. The interaction of macrophages with anti-immunoglobulin IgG-coated bone marrow-derived lymphocytes. J Exp Med. 1976 Sep 1;144(3):788–809. doi: 10.1084/jem.144.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinecke J. W., Rosen H., Chait A. Iron and copper promote modification of low density lipoprotein by human arterial smooth muscle cells in culture. J Clin Invest. 1984 Nov;74(5):1890–1894. doi: 10.1172/JCI111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinecke J. W., Rosen H., Suzuki L. A., Chait A. The role of sulfur-containing amino acids in superoxide production and modification of low density lipoprotein by arterial smooth muscle cells. J Biol Chem. 1987 Jul 25;262(21):10098–10103. [PubMed] [Google Scholar]
- Innerarity T. L., Friedlander E. J., Rall S. C., Jr, Weisgraber K. H., Mahley R. W. The receptor-binding domain of human apolipoprotein E. Binding of apolipoprotein E fragments. J Biol Chem. 1983 Oct 25;258(20):12341–12347. [PubMed] [Google Scholar]
- Kelley J. L., Rozek M. M., Suenram C. A., Schwartz C. J. Activation of human peripheral blood monocytes by lipoproteins. Am J Pathol. 1988 Feb;130(2):223–231. [PMC free article] [PubMed] [Google Scholar]
- Kokkonen J. O., Kovanen P. T. Stimulation of mast cells leads to cholesterol accumulation in macrophages in vitro by a mast cell granule-mediated uptake of low density lipoprotein. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2287–2291. doi: 10.1073/pnas.84.8.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss R. M., Burke D. J. Identification of multiple subclasses of plasma low density lipoproteins in normal humans. J Lipid Res. 1982 Jan;23(1):97–104. [PubMed] [Google Scholar]
- Krug E. L., Kent C. Assay for phospholipase C. Methods Enzymol. 1981;72:347–351. doi: 10.1016/s0076-6879(81)72025-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Low M. G., Prasad A. R. A phospholipase D specific for the phosphatidylinositol anchor of cell-surface proteins is abundant in plasma. Proc Natl Acad Sci U S A. 1988 Feb;85(4):980–984. doi: 10.1073/pnas.85.4.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Pitas R. E., Weisgraber K. H., Brown J. H., Gross E. Inhibition of lipoprotein binding to cell surface receptors of fibroblasts following selective modification of arginyl residues in arginine-rich and B apoproteins. J Biol Chem. 1977 Oct 25;252(20):7279–7287. [PubMed] [Google Scholar]
- Mendelsohn M. E., Loscalzo J. Role of platelets in cholesteryl ester formation by U-937 cells. J Clin Invest. 1988 Jan;81(1):62–68. doi: 10.1172/JCI113311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. E., Chait A., Bierman E. L., King W., Goodwin P., Walden C. E., Ross R. Lipid composition of aorta of Watanabe heritable hyperlipemic and comparably hypercholesterolemic fat-fed rabbits. Plasma lipid composition determines aortic lipid composition of hypercholesterolemic rabbits. Arteriosclerosis. 1988 Jul-Aug;8(4):338–347. doi: 10.1161/01.atv.8.4.338. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury B. G., Falcone D. J., Minick C. R. Insoluble low-density lipoprotein-proteoglycan complexes enhance cholesteryl ester accumulation in macrophages. Am J Pathol. 1985 Jul;120(1):6–11. [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. M. Selection and characterization of bovine aortic endothelial cells. In Vitro. 1978 Dec;14(12):966–980. doi: 10.1007/BF02616210. [DOI] [PubMed] [Google Scholar]
- Shio H., Farquhar M. G., de Duve C. Lysosomes of the arterial wall. IV. Cytochemical localization of acid phosphatase and catalase in smooth muscle cells and foam cells from rabbit atheromatous aorta. Am J Pathol. 1974 Jul;76(1):1–16. [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Vijayagopal P., Srinivasan S. R., Jones K. M., Radhakrishnamurthy B., Berenson G. S. Complexes of low-density lipoproteins and arterial proteoglycan aggregates promote cholesteryl ester accumulation in mouse macrophages. Biochim Biophys Acta. 1985 Dec 4;837(3):251–261. doi: 10.1016/0005-2760(85)90048-7. [DOI] [PubMed] [Google Scholar]
- Wallis W. J., Hickstein D. D., Schwartz B. R., June C. H., Ochs H. D., Beatty P. G., Klebanoff S. J., Harlan J. M. Monoclonal antibody-defined functional epitopes on the adhesion-promoting glycoprotein complex (CDw18) of human neutrophils. Blood. 1986 Apr;67(4):1007–1013. [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Role of lysine residues of plasma lipoproteins in high affinity binding to cell surface receptors on human fibroblasts. J Biol Chem. 1978 Dec 25;253(24):9053–9062. [PubMed] [Google Scholar]
- Weisgraber K. H., Rall S. C., Jr, Mahley R. W. Human E apoprotein heterogeneity. Cysteine-arginine interchanges in the amino acid sequence of the apo-E isoforms. J Biol Chem. 1981 Sep 10;256(17):9077–9083. [PubMed] [Google Scholar]




