Abstract
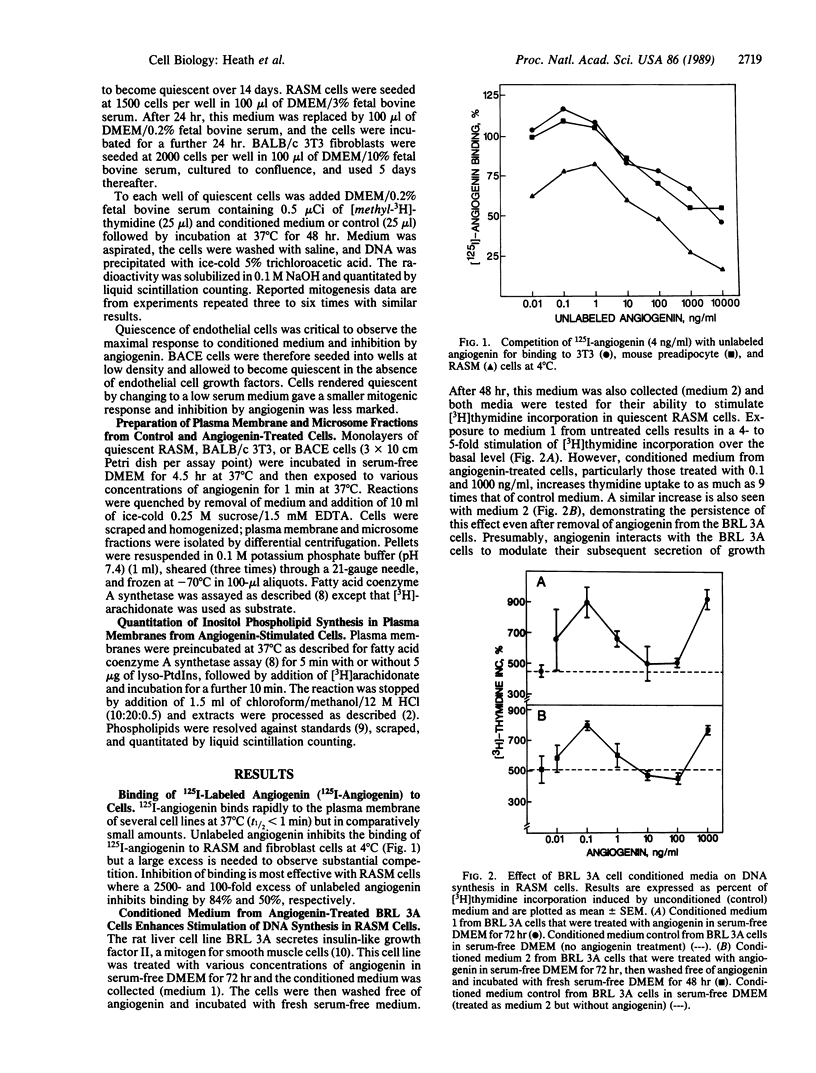
125I-labeled angiogenin binds rapidly to the plasma membrane of several cell lines at 37 degrees C (t1/2 less than 1 min) but in comparatively small amounts. Competition with unlabeled angiogenin varies markedly with different cell lines, being most effective in vascular smooth muscle and fibroblast cells. Angiogenin modulates mitogenic stimuli in bovine adrenal capillary endothelial (BACE), rat aortic smooth muscle (RASM), and fibroblast (3T3) cells. Thus, it enhances the mitogenic effect of certain conditioned media on RASM and 3T3 cells, but it inhibits the mitogenic effect on BACE cells. In RASM and 3T3 cells, mitogenesis is increased at low (less than 5 ng/ml) and high (greater than 100 ng/ml) but not at intermediate concentrations of angiogenin. Plasma membranes from RASM and 3T3 cells that have been treated with angiogenin show an enhanced in vitro synthesis of phosphatidylinositol bisphosphate (PtdInsP2) with an angiogenin concentration dependence similar to that of enhanced mitogenesis. PtdInsP2 synthesis arises by activation of a fatty acid (arachidonyl) coenzyme A synthetase and either a plasma membrane fatty acid acyltransferase or phosphatidylinositol kinase(s), or both. Increased PtdInsP2 or the derived second messengers (e.g., diacylglycerol) may mediate modulation of the mitogenic stimulus. The differential mitogenic interaction of angiogenin with several cell types, either stimulation or inhibition, probably reflects the multistep nature of angiogenesis.
Full text
PDF
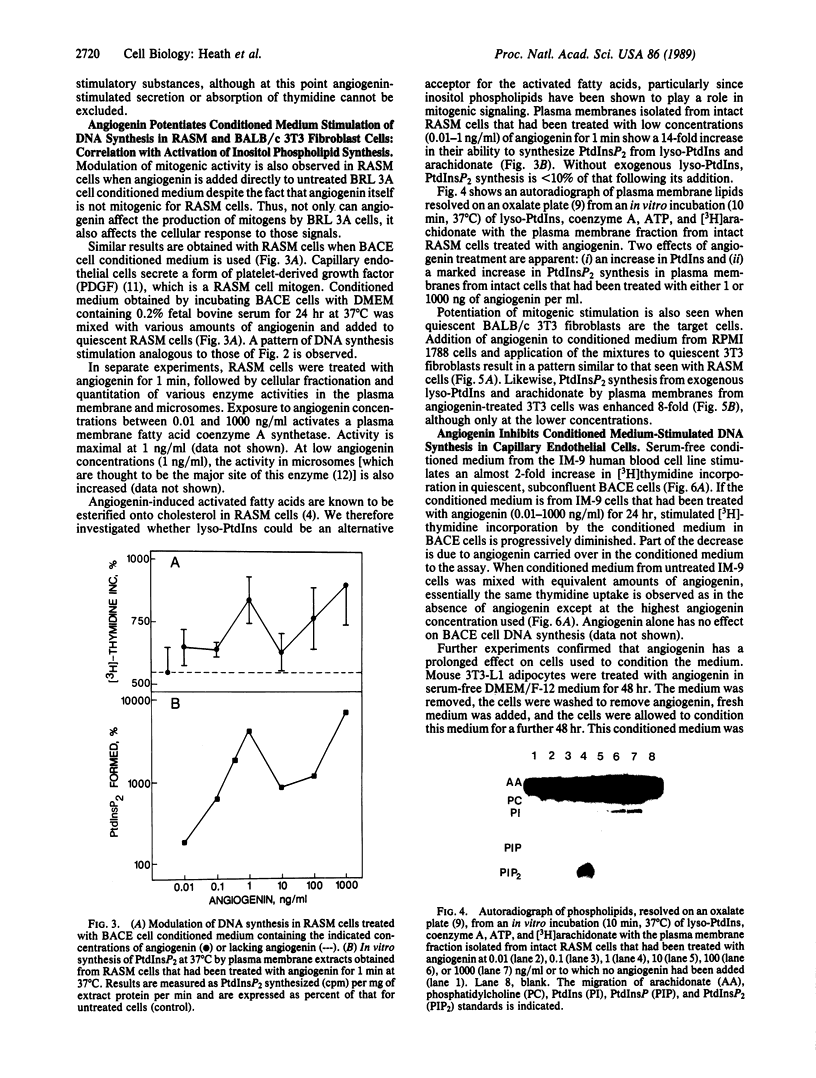

Images in this article
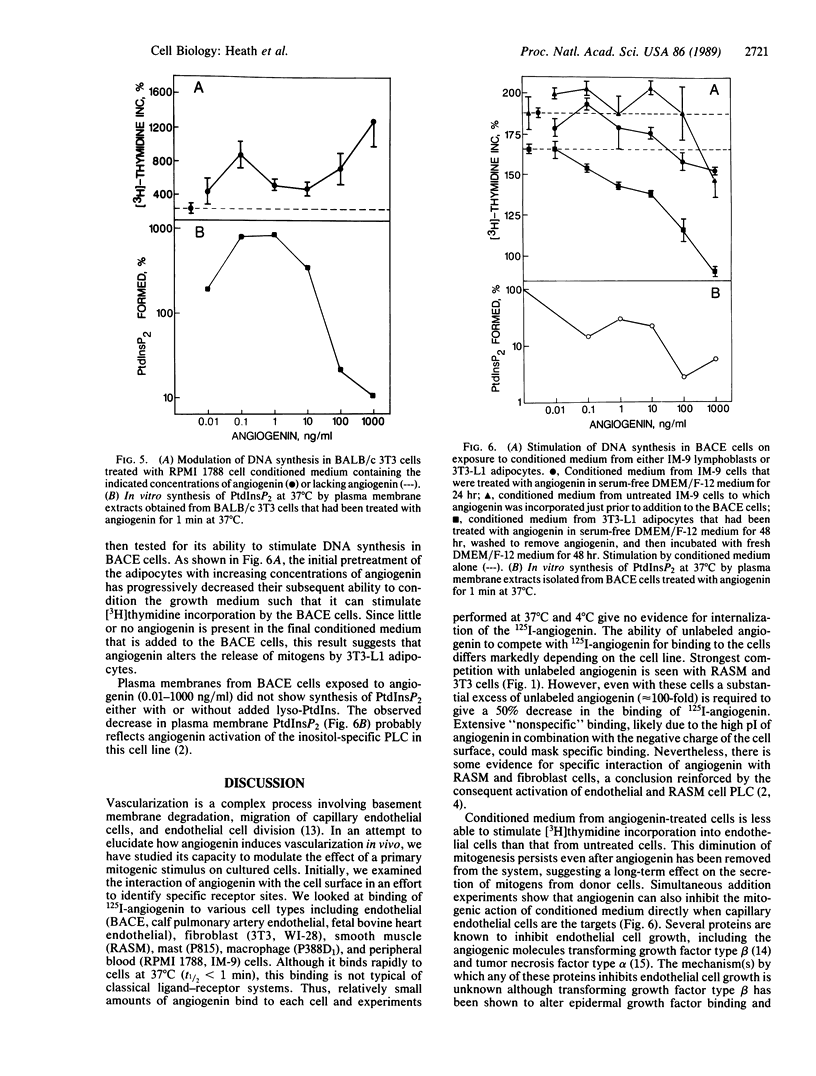
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bicknell R., Vallee B. L. Angiogenin activates endothelial cell phospholipase C. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5961–5965. doi: 10.1073/pnas.85.16.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell R., Vallee B. L. Angiogenin stimulates endothelial cell prostacyclin secretion by activation of phospholipase A2. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1573–1577. doi: 10.1073/pnas.86.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin S. R., Lee W. M., Williams P. W., Giels G. M., Williams L. T. c-myc gene expression is stimulated by agents that activate protein kinase C and does not account for the mitogenic effect of PDGF. Cell. 1985 Nov;43(1):243–251. doi: 10.1016/0092-8674(85)90029-7. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Heber A. An 81 kd protein complexed with middle T antigen and pp60c-src: a possible phosphatidylinositol kinase. Cell. 1987 Sep 25;50(7):1031–1037. doi: 10.1016/0092-8674(87)90169-3. [DOI] [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farstad M., Bremer J., Norum K. R. Long-chain acyl-CoA synthetase in rat liver. A new assay procedure for the enzyme, and studies on its intracellular localization. Biochim Biophys Acta. 1967 Mar 15;132(2):492–502. doi: 10.1016/0005-2744(67)90167-2. [DOI] [PubMed] [Google Scholar]
- Ferber E., Resch K. Phospholipid metabolism of stimulated lymphocytes: activation of acyl-CoA:lysolecithin acyltransferases in microsomal membranes. Biochim Biophys Acta. 1973 Feb 14;296(2):335–349. doi: 10.1016/0005-2760(73)90092-1. [DOI] [PubMed] [Google Scholar]
- Fett J. W., Strydom D. J., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985 Sep 24;24(20):5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Fràter-Schröder M., Müller G., Birchmeier W., Böhlen P. Transforming growth factor-beta inhibits endothelial cell proliferation. Biochem Biophys Res Commun. 1986 May 29;137(1):295–302. doi: 10.1016/0006-291x(86)91209-x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sastre F., Folch-Pi J. Thin-layer chromatography of the phosphoinositides. J Lipid Res. 1968 Jul;9(4):532–533. [PubMed] [Google Scholar]
- Gunther S., Alexander R. W., Atkinson W. J., Gimbrone M. A., Jr Functional angiotensin II receptors in cultured vascular smooth muscle cells. J Cell Biol. 1982 Feb;92(2):289–298. doi: 10.1083/jcb.92.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Whitman M., Schaffhausen B., Pallas D. C., White M., Cantley L., Roberts T. M. Common elements in growth factor stimulation and oncogenic transformation: 85 kd phosphoprotein and phosphatidylinositol kinase activity. Cell. 1987 Sep 25;50(7):1021–1029. doi: 10.1016/0092-8674(87)90168-1. [DOI] [PubMed] [Google Scholar]
- Matuoka K., Fukami K., Nakanishi O., Kawai S., Takenawa T. Mitogenesis in response to PDGF and bombesin abolished by microinjection of antibody to PIP2. Science. 1988 Feb 5;239(4840):640–643. doi: 10.1126/science.2829356. [DOI] [PubMed] [Google Scholar]
- Moses A. C., Nissley S. P., Short P. A., Rechler M. M., Podskalny J. M. Purification and characterization of multiplication-stimulating activity. Insulin-like growth factors purified from rat-liver-cell-conditioned medium. Eur J Biochem. 1980 Jan;103(2):387–400. doi: 10.1111/j.1432-1033.1980.tb04325.x. [DOI] [PubMed] [Google Scholar]
- Müller G., Behrens J., Nussbaumer U., Böhlen P., Birchmeier W. Inhibitory action of transforming growth factor beta on endothelial cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5600–5604. doi: 10.1073/pnas.84.16.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike L. J., Eakes A. T. Epidermal growth factor stimulates the production of phosphatidylinositol monophosphate and the breakdown of polyphosphoinositides in A431 cells. J Biol Chem. 1987 Feb 5;262(4):1644–1651. [PubMed] [Google Scholar]
- Reed B. C., Lane M. D. Insulin receptor synthesis and turnover in differentiating 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):285–289. doi: 10.1073/pnas.77.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S., Russell R. A., Deykin D. Transfer of arachidonic acid to human platelet plasmalogen in response to thrombin. Biochem Biophys Res Commun. 1976 May 3;70(1):295–301. doi: 10.1016/0006-291x(76)91141-4. [DOI] [PubMed] [Google Scholar]
- Schweigerer L., Malerstein B., Gospodarowicz D. Tumor necrosis factor inhibits the proliferation of cultured capillary endothelial cells. Biochem Biophys Res Commun. 1987 Mar 30;143(3):997–1004. doi: 10.1016/0006-291x(87)90350-0. [DOI] [PubMed] [Google Scholar]
- Takehara K., LeRoy E. C., Grotendorst G. R. TGF-beta inhibition of endothelial cell proliferation: alteration of EGF binding and EGF-induced growth-regulatory (competence) gene expression. Cell. 1987 May 8;49(3):415–422. doi: 10.1016/0092-8674(87)90294-7. [DOI] [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., Pike L. J. Phosphatidylinositol kinase is activated in membranes derived from cells treated with epidermal growth factor. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7513–7517. doi: 10.1073/pnas.84.21.7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B., Prescott S. M., Majerus P. W. Discovery of an arachidonoyl coenzyme A synthetase in human platelets. J Biol Chem. 1982 Apr 10;257(7):3510–3515. [PubMed] [Google Scholar]