Abstract
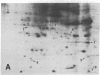
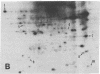
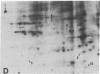
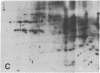
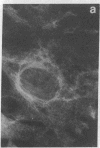
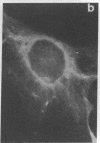
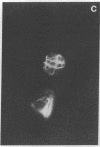
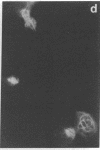
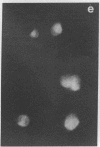
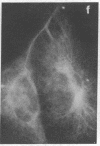
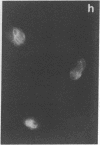
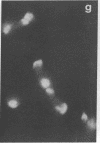
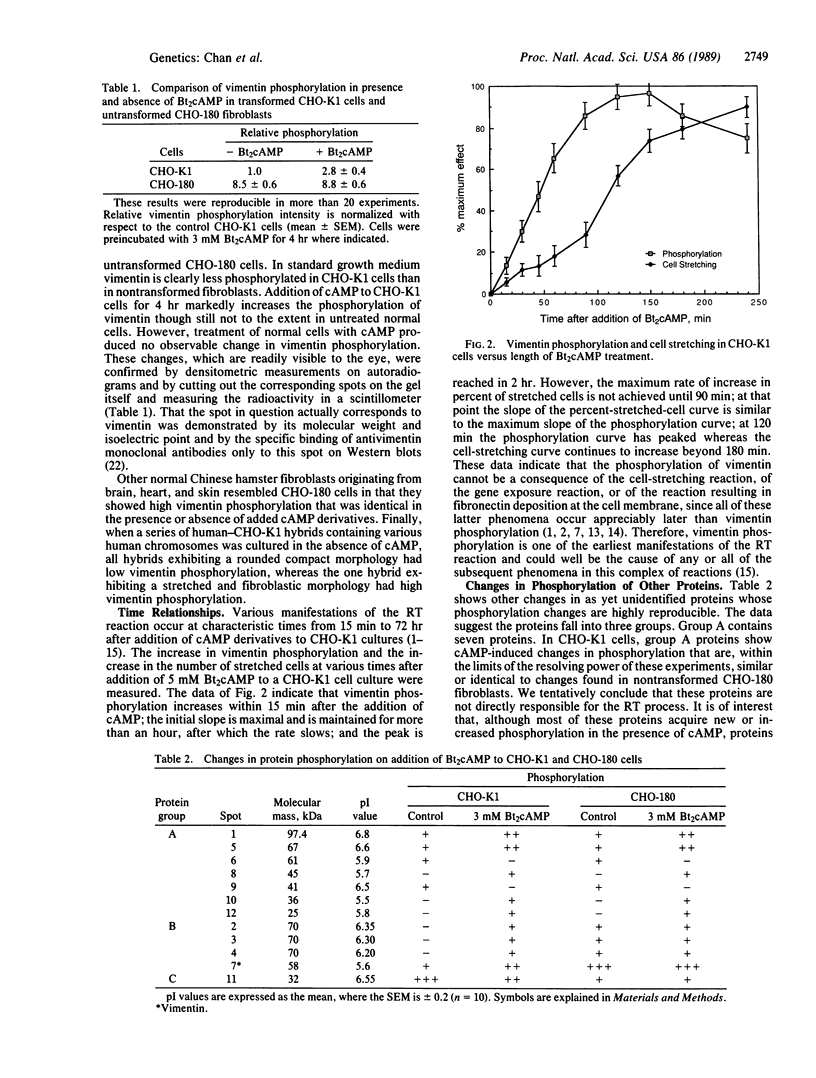
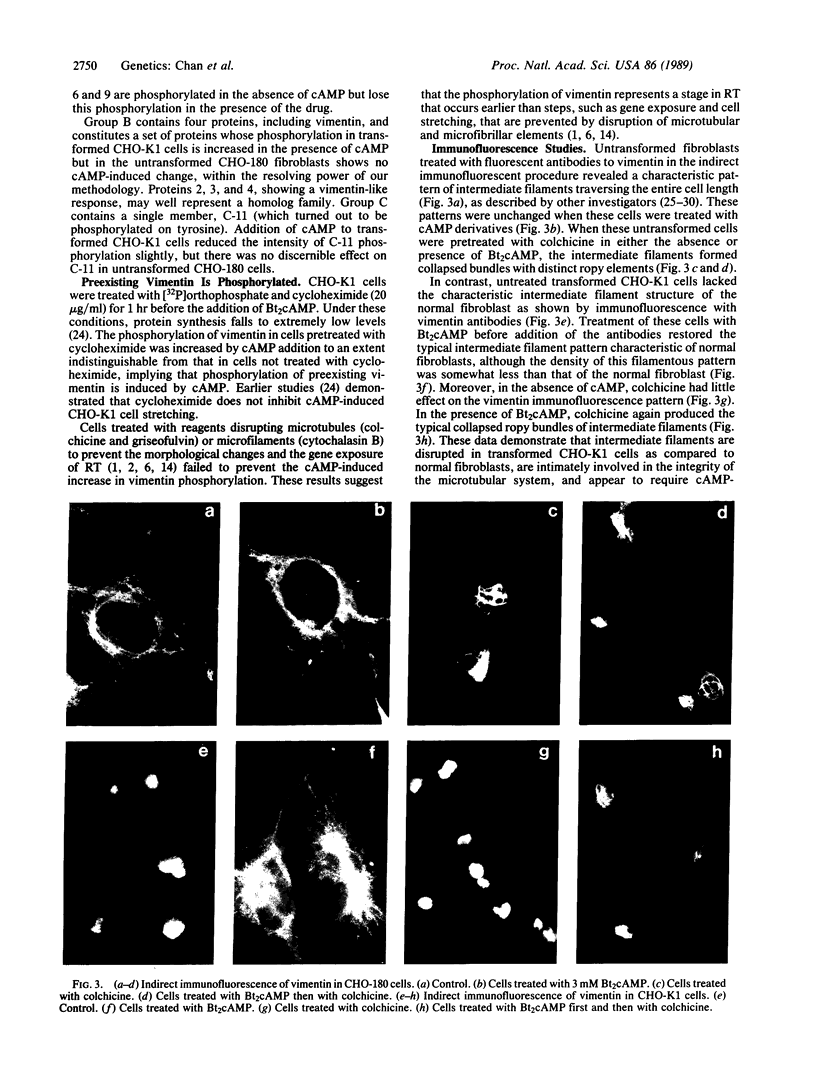
An organized cytoskeleton is required for the cAMP-induced reverse transformation reaction in CHO-K1 cells. In the course of the reaction a considerable fraction of the genome changes its nuclease sensitivity. The current paper presents the following evidence that cAMP-induced phosphorylation of vimentin is an early step in this reaction complex. (i) Vimentin is only slightly phosphorylated in transformed CHO-K1 cells but is heavily phosphorylated in normal fibroblasts. (ii) cAMP addition almost triples the vimentin phosphorylation of CHO-K1 cells but does not change that of normal cells. (iii) Vimentin phosphorylation is one of the earliest phenomena to occur after addition of cAMP to CHO-K1 cells, preceding the cell-stretching reaction and other manifestations of reverse transformation. (iv) Indirect immunofluorescence experiments demonstrate that vimentin appears as a condensed mass in transformed CHO-K1 cells but cAMP addition restores the filamentous structure characteristic of the normal fibroblast. (v) Other transformed cells unresponsive to reverse transformation by cAMP failed to demonstrate increased phosphorylation of vimentin on treatment with cAMP. These results support the proposed scheme that phosphorylation of cytoskeletal elements initiates a large-scale genetic regulatory action in which a substantial change in the spectrum of genome exposure and sequestration occurs. A function for intermediate filaments in reverse transformation is implied.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashall F., Puck T. T. Cytoskeletal involvement in cAMP-induced sensitization of chromatin to nuclease digestion in transformed Chinese hamster ovary K1 cells. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5145–5149. doi: 10.1073/pnas.81.16.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashall F., Sullivan N., Puck T. T. Specificity of the cAMP-induced gene exposure reaction in CHO cells. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3908–3912. doi: 10.1073/pnas.85.11.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A. Cell configuration-related control of vimentin biosynthesis and phosphorylation in cultured mammalian cells. J Cell Biol. 1983 Sep;97(3):858–865. doi: 10.1083/jcb.97.3.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G. S., Lockwood A. H. Redistribution of myosin during morphological reversion of Chinese hamster ovary cells induced by db-cAMP. Exp Cell Res. 1980 Sep;129(1):31–45. doi: 10.1016/0014-4827(80)90328-6. [DOI] [PubMed] [Google Scholar]
- Bloom G. S., Lockwood A. H. Specific protein phosphorylation during cyclic AMP-mediated morphological reversion of transformed cells. J Supramol Struct. 1980;14(2):241–254. doi: 10.1002/jss.400140213. [DOI] [PubMed] [Google Scholar]
- Fey S. J., Larsen P. M., Celis J. E. Evidence for coordinated phosphorylation of keratins and vimentin during mitosis in transformed human amnion cells. Phosphate turnover of modified proteins. FEBS Lett. 1983 Jun 27;157(1):165–169. doi: 10.1016/0014-5793(83)81138-7. [DOI] [PubMed] [Google Scholar]
- Gabrielson E. G., Scoggin C., Puck T. T. Phosphorylation changes induced by cAMP derivatives in the CHO cell and selected mutants. Exp Cell Res. 1982 Nov;142(1):63–68. doi: 10.1016/0014-4827(82)90409-8. [DOI] [PubMed] [Google Scholar]
- Garrels J. I. Two dimensional gel electrophoresis and computer analysis of proteins synthesized by clonal cell lines. J Biol Chem. 1979 Aug 25;254(16):7961–7977. [PubMed] [Google Scholar]
- Gawlitta W., Osborn M., Weber K. Coiling of intermediate filaments induced by microinjection of a vimentin-specific antibody does not interfere with locomotion and mitosis. Eur J Cell Biol. 1981 Dec;26(1):83–90. [PubMed] [Google Scholar]
- Georgatos S. D., Blobel G. Lamin B constitutes an intermediate filament attachment site at the nuclear envelope. J Cell Biol. 1987 Jul;105(1):117–125. doi: 10.1083/jcb.105.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsie A. W., Jones C., Puck T. T. Further changes in differentiation state accompanying the conversion of Chinese hamster cells of fibroblastic form by dibutyryl adenosine cyclic 3':5'-monophosphate and hormones. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1648–1652. doi: 10.1073/pnas.68.7.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsie A. W., Puck T. T. Morphological transformation of Chinese hamster cells by dibutyryl adenosine cyclic 3':5'-monophosphate and testosterone. Proc Natl Acad Sci U S A. 1971 Feb;68(2):358–361. doi: 10.1073/pnas.68.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klymkowsky M. W. Intermediate filaments in 3T3 cells collapse after intracellular injection of a monoclonal anti-intermediate filament antibody. Nature. 1981 May 21;291(5812):249–251. doi: 10.1038/291249a0. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments: a chemically heterogeneous, developmentally regulated class of proteins. Annu Rev Biochem. 1982;51:219–250. doi: 10.1146/annurev.bi.51.070182.001251. [DOI] [PubMed] [Google Scholar]
- LeCam A., Gottesman M. M., Pastan I. Mechanism of cyclic AMP effect on nutrient transport in Chinese hamster ovary cells. A genetic approach. J Biol Chem. 1980 Sep 10;255(17):8103–8108. [PubMed] [Google Scholar]
- LeCam A., Nicolas J. C., Singh T. J., Cabral F., Pastan I., Gottesman M. M. Cyclic AMP-dependent phosphorylation in intact cells and in cell-free extracts from Chinese hamster ovary cells. Studies with wild type and cyclic AMP-resistant mutants. J Biol Chem. 1981 Jan 25;256(2):933–941. [PubMed] [Google Scholar]
- Lin J. J., Feramisco J. R. Disruption of the in vivo distribution of the intermediate filaments in fibroblasts through the microinjection of a specific monoclonal antibody. Cell. 1981 Apr;24(1):185–193. doi: 10.1016/0092-8674(81)90514-6. [DOI] [PubMed] [Google Scholar]
- Lincoln T. M., Corbin J. D. On the role of the cAMP and cGMP-dependent protein kinases in cell function. J Cyclic Nucleotide Res. 1978 Feb;4(1):3–14. [PubMed] [Google Scholar]
- Lockwood A. H., Trivette D. D., Pendergast M. Molecular events in cAMP-mediated reverse transformation. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):909–919. doi: 10.1101/sqb.1982.046.01.085. [DOI] [PubMed] [Google Scholar]
- Meek W. D., Puck T. T. Role of the microfibrillar system in knob action of transformed cells. J Supramol Struct. 1979;12(3):335–354. doi: 10.1002/jss.400120306. [DOI] [PubMed] [Google Scholar]
- Nielson S. E., Puck T. T. Deposition of fibronectin in the course of reverse transformation of Chinese hamster ovary cells by cyclic AMP. Proc Natl Acad Sci U S A. 1980 Feb;77(2):985–989. doi: 10.1073/pnas.77.2.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983 Apr;48(4):372–394. [PubMed] [Google Scholar]
- Pastan I., Willingham M. Cellular transformation and the 'morphologic phenotype' of transformed cells. Nature. 1978 Aug 17;274(5672):645–650. doi: 10.1038/274645a0. [DOI] [PubMed] [Google Scholar]
- Patterson D., Waldren C. A. The effect of inhibitors of RNA and protein synthesis on dibutyryl cyclic AMP mediated morphological transformations of Chinese hamster ovary cells in vitro. Biochem Biophys Res Commun. 1973 Jan 23;50(2):566–573. doi: 10.1016/0006-291x(73)90877-2. [DOI] [PubMed] [Google Scholar]
- Platt J. L., Michael A. F. Retardation of fading and enhancement of intensity of immunofluorescence by p-phenylenediamine. J Histochem Cytochem. 1983 Jun;31(6):840–842. doi: 10.1177/31.6.6341464. [DOI] [PubMed] [Google Scholar]
- Porter K. R., Puck T. T., Hsie A. W., Kelley D. An electron microscopy study of the effects on dibutyryl cyclic AMP on Chinese hamster ovary cells. Cell. 1974 Jul;2(3):145–162. doi: 10.1016/0092-8674(74)90089-0. [DOI] [PubMed] [Google Scholar]
- Puck T. T. Cyclic AMP, the microtubule-microfilament system, and cancer. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4491–4495. doi: 10.1073/pnas.74.10.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumsby G., Puck T. T. Ornithine decarboxylase induction and the cytoskeleton in normal and transformed cells. J Cell Physiol. 1982 May;111(2):133–139. doi: 10.1002/jcp.1041110203. [DOI] [PubMed] [Google Scholar]
- Schonberg S., Patterson D., Puck T. T. Resistance of Chinese hamster ovary cell chromatin to endonuclease digestion. I. Reversal by cAMP. Exp Cell Res. 1983 Apr 15;145(1):57–62. doi: 10.1016/s0014-4827(83)80007-x. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- TJIO J. H., PUCK T. T. Genetics of somatic mammalian cells. II. Chromosomal constitution of cells in tissue culture. J Exp Med. 1958 Aug 1;108(2):259–268. doi: 10.1084/jem.108.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]