Abstract
A rapid nonradioactive approach to the diagnosis of sickle cell anemia is described based on an allele-specific polymerase chain reaction (ASPCR). This method allows direct detection of the normal or the sickle cell beta-globin allele in genomic DNA without additional steps of probe hybridization, ligation, or restriction enzyme cleavage. Two allele-specific oligonucleotide primers, one specific for the sickle cell allele and one specific for the normal allele, together with another primer complementary to both alleles were used in the polymerase chain reaction with genomic DNA templates. The allele-specific primers differed from each other in their terminal 3' nucleotide. Under the proper annealing temperature and polymerase chain reaction conditions, these primers only directed amplification on their complementary allele. In a single blind study of DNA samples from 12 individuals, this method correctly and unambiguously allowed for the determination of the genotypes with no false negatives or positives. If ASPCR is able to discriminate all allelic variation (both transition and transversion mutations), this method has the potential to be a powerful approach for genetic disease diagnosis, carrier screening, HLA typing, human gene mapping, forensics, and paternity testing.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson M. R., Deutscher M. P., Kornberg A., Russell A. F., Moffatt J. G. Enzymatic synthesis of deoxyribonucleic acid. XXXIV. Termination of chain growth by a 2',3'-dideoxyribonucleotide. Biochemistry. 1969 Dec;8(12):4897–4904. doi: 10.1021/bi00840a037. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Karam J. H., Rutter W. J. Polymorphic DNA region adjacent to the 5' end of the human insulin gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. C., Kan Y. W. A sensitive new prenatal test for sickle-cell anemia. N Engl J Med. 1982 Jul 1;307(1):30–32. doi: 10.1056/NEJM198207013070105. [DOI] [PubMed] [Google Scholar]
- Chehab F. F., Doherty M., Cai S. P., Kan Y. W., Cooper S., Rubin E. M. Detection of sickle cell anaemia and thalassaemias. Nature. 1987 Sep 24;329(6137):293–294. doi: 10.1038/329293b0. [DOI] [PubMed] [Google Scholar]
- Conner B. J., Reyes A. A., Morin C., Itakura K., Teplitz R. L., Wallace R. B. Detection of sickle cell beta S-globin allele by hybridization with synthetic oligonucleotides. Proc Natl Acad Sci U S A. 1983 Jan;80(1):278–282. doi: 10.1073/pnas.80.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geever R. F., Wilson L. B., Nallaseth F. S., Milner P. F., Bittner M., Wilson J. T. Direct identification of sickle cell anemia by blot hybridization. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5081–5085. doi: 10.1073/pnas.78.8.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y. W., Dozy A. M. Antenatal diagnosis of sickle-cell anaemia by D.N.A. analysis of amniotic-fluid cells. Lancet. 1978 Oct 28;2(8096):910–912. doi: 10.1016/s0140-6736(78)91629-x. [DOI] [PubMed] [Google Scholar]
- Kidd V. J., Wallace R. B., Itakura K., Woo S. L. alpha 1-antitrypsin deficiency detection by direct analysis of the mutation in the gene. Nature. 1983 Jul 21;304(5923):230–234. doi: 10.1038/304230a0. [DOI] [PubMed] [Google Scholar]
- Landegren U., Kaiser R., Sanders J., Hood L. A ligase-mediated gene detection technique. Science. 1988 Aug 26;241(4869):1077–1080. doi: 10.1126/science.3413476. [DOI] [PubMed] [Google Scholar]
- Morel P. A., Dorman J. S., Todd J. A., McDevitt H. O., Trucco M. Aspartic acid at position 57 of the HLA-DQ beta chain protects against type I diabetes: a family study. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8111–8115. doi: 10.1073/pnas.85.21.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozari G., Rahbar S., Wallace R. B. Discrimination among the transcripts of the allelic human beta-globin genes beta A, beta S and beta C using oligodeoxynucleotide hybridization probes. Gene. 1986;43(1-2):23–28. doi: 10.1016/0378-1119(86)90004-1. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
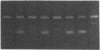
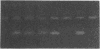
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Selden R. F., Howie K. B., Rowe M. E., Goodman H. M., Moore D. D. Human growth hormone as a reporter gene in regulation studies employing transient gene expression. Mol Cell Biol. 1986 Sep;6(9):3173–3179. doi: 10.1128/mcb.6.9.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick M. H., Wallace R. B. Simultaneous analysis of multiple polymorphic loci using amplified sequence polymorphisms (ASPs). Genomics. 1988 May;2(4):273–279. doi: 10.1016/0888-7543(88)90014-6. [DOI] [PubMed] [Google Scholar]
- Tindall K. R., Kunkel T. A. Fidelity of DNA synthesis by the Thermus aquaticus DNA polymerase. Biochemistry. 1988 Aug 9;27(16):6008–6013. doi: 10.1021/bi00416a027. [DOI] [PubMed] [Google Scholar]