Abstract
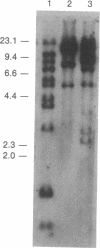
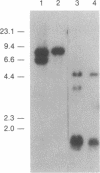
The bcl-2 gene has been identified as a gene directly involved in the consistent chromosome translocation t(14;18), which is found in approximately 90% of human follicular lymphoma cases, and is a prime candidate for the oncogene playing a crucial role in follicular lymphomagenesis. In this paper, we describe a case of chronic lymphocytic leukemia showing the juxtaposition of the bcl-2 gene on chromosome 18 to immunoglobulin lambda light chain (Ig lambda) gene on chromosome 22 in a head-to-head configuration. Sequencing analysis of the joining site of the bcl-2 gene and Ig lambda gene has shown that the breakpoint is within the 5' flanking region of the bcl-2 gene and about 2.2 kilobases 5' to the joining segment of Ig lambda locus in a germ-line configuration. The extranucleotide, commonly appearing at the joining site of the t(14;18) translocation involving the IgH locus, is absent from the joining site of bcl-2 and Ig lambda. The lack of extranucleotide suggests that the juxtaposition of the bcl-2 and Ig lambda genes occurred during physiological rearrangement of the Ig lambda gene since it has been shown that the rearrangement of the Ig lambda locus is not accompanied by extranucleotides.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakhshi A., Jensen J. P., Goldman P., Wright J. J., McBride O. W., Epstein A. L., Korsmeyer S. J. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985 Jul;41(3):899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- Cleary M. L., Sklar J. Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7439–7443. doi: 10.1073/pnas.82.21.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossman J., Neckers L. M., Hsu S., Longo D., Jaffe E. S. Low-grade lymphomas. Expression of developmentally regulated B-cell antigens. Am J Pathol. 1984 Apr;115(1):117–124. [PMC free article] [PubMed] [Google Scholar]
- Croce C. M., Erikson J., Haluska F. G., Finger L. R., Showe L. C., Tsujimoto Y. Molecular genetics of human B- and T-cell neoplasia. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):891–898. doi: 10.1101/sqb.1986.051.01.102. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Nowell P. C. Molecular basis of human B cell neoplasia. Blood. 1985 Jan;65(1):1–7. [PubMed] [Google Scholar]
- Desiderio S. V., Yancopoulos G. D., Paskind M., Thomas E., Boss M. A., Landau N., Alt F. W., Baltimore D. Insertion of N regions into heavy-chain genes is correlated with expression of terminal deoxytransferase in B cells. Nature. 1984 Oct 25;311(5988):752–755. doi: 10.1038/311752a0. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauwerky C. E., Hoxie J., Nowell P. C., Croce C. M. Pre-B-cell leukemia with a t(8; 14) and a t(14; 18) translocation is preceded by follicular lymphoma. Oncogene. 1988 May;2(5):431–435. [PubMed] [Google Scholar]
- Hieter P. A., Hollis G. F., Korsmeyer S. J., Waldmann T. A., Leder P. Clustered arrangement of immunoglobulin lambda constant region genes in man. Nature. 1981 Dec 10;294(5841):536–540. doi: 10.1038/294536a0. [DOI] [PubMed] [Google Scholar]
- Lenoir G. M., Preud'homme J. L., Bernheim A., Berger R. Correlation between immunoglobulin light chain expression and variant translocation in Burkitt's lymphoma. Nature. 1982 Jul 29;298(5873):474–476. doi: 10.1038/298474a0. [DOI] [PubMed] [Google Scholar]
- Magrath I., Erikson J., Whang-Peng J., Sieverts H., Armstrong G., Benjamin D., Triche T., Alabaster O., Croce C. M. Synthesis of kappa light chains by cell lines containing an 8;22 chromosomal translocation derived from a male homosexual with Burkitt's lymphoma. Science. 1983 Dec 9;222(4628):1094–1098. doi: 10.1126/science.6316501. [DOI] [PubMed] [Google Scholar]
- Pegoraro L., Palumbo A., Erikson J., Falda M., Giovanazzo B., Emanuel B. S., Rovera G., Nowell P. C., Croce C. M. A 14;18 and an 8;14 chromosome translocation in a cell line derived from an acute B-cell leukemia. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7166–7170. doi: 10.1073/pnas.81.22.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto M., Jaeger U., Hockett R. D., Graninger W., Bennett S., Goldman P., Korsmeyer S. J. Alternative promoters and exons, somatic mutation and deregulation of the Bcl-2-Ig fusion gene in lymphoma. EMBO J. 1988 Jan;7(1):123–131. doi: 10.1002/j.1460-2075.1988.tb02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Bashir M. M., Givol I., Cossman J., Jaffe E., Croce C. M. DNA rearrangements in human follicular lymphoma can involve the 5' or the 3' region of the bcl-2 gene. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1329–1331. doi: 10.1073/pnas.84.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Cossman J., Jaffe E., Croce C. M. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985 Jun 21;228(4706):1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Croce C. M. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5214–5218. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Finger L. R., Yunis J., Nowell P. C., Croce C. M. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984 Nov 30;226(4678):1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Gorham J., Cossman J., Jaffe E., Croce C. M. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science. 1985 Sep 27;229(4720):1390–1393. doi: 10.1126/science.3929382. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Warnke R. A., Sklar J., Cleary M. L. Molecular analysis of the t(14;18) chromosomal translocation in malignant lymphomas. N Engl J Med. 1987 Nov 5;317(19):1185–1189. doi: 10.1056/NEJM198711053171904. [DOI] [PubMed] [Google Scholar]
- Yunis J. J. The chromosomal basis of human neoplasia. Science. 1983 Jul 15;221(4607):227–236. doi: 10.1126/science.6336310. [DOI] [PubMed] [Google Scholar]