Abstract
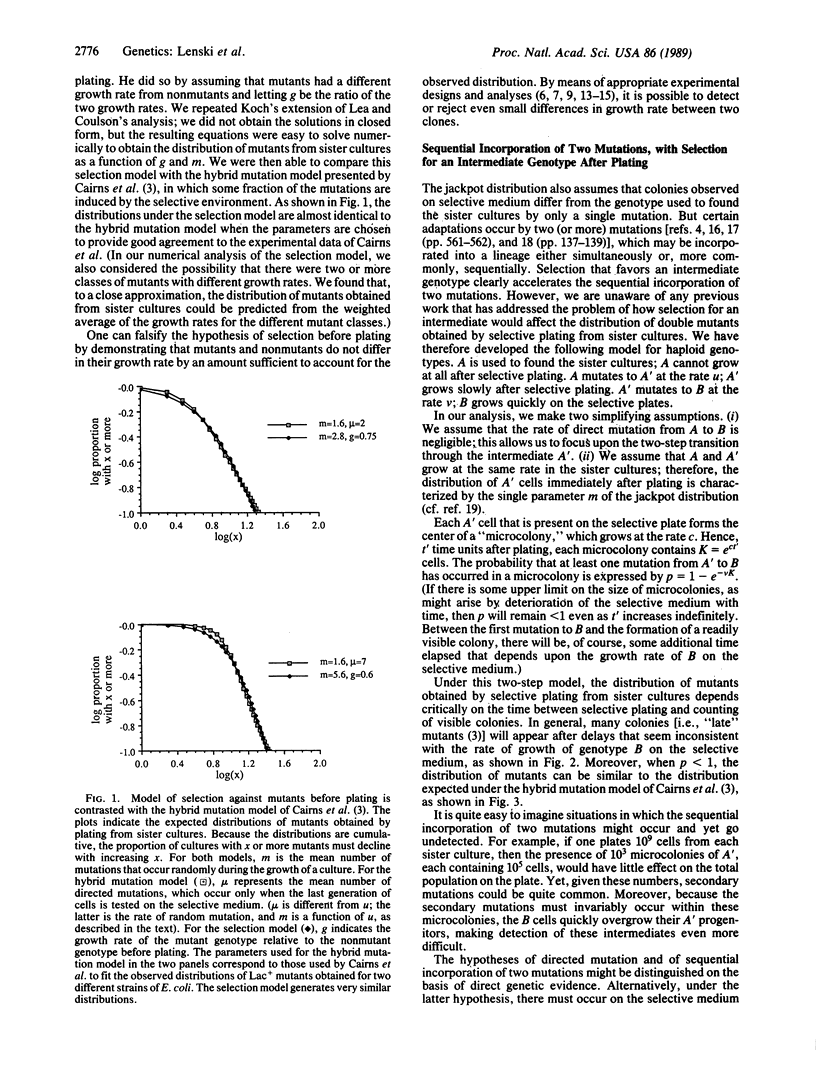
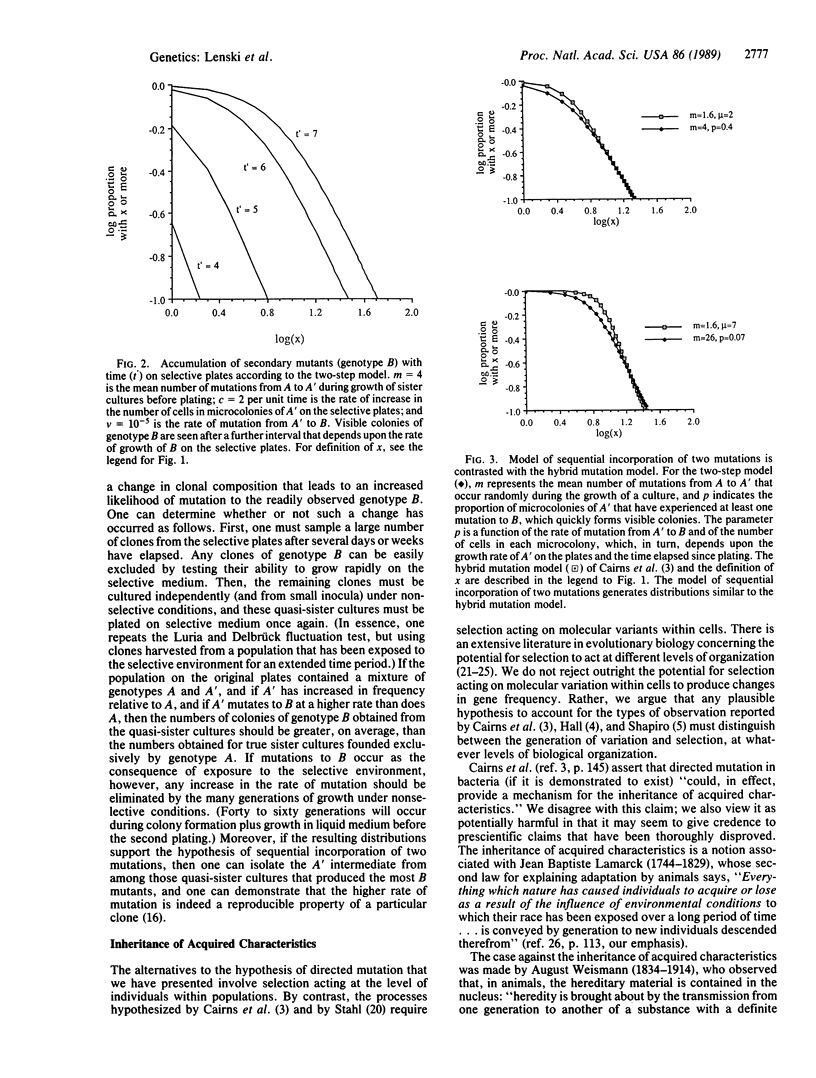
Bacterial populations have served as model systems for studying evolutionary processes ever since the classic experiments of Luria and Delbrück, which demonstrated the occurrence of mutations prior to selection for the traits they conferred. However, several authors have recently presented experiments suggesting that bacteria may have mechanisms for directing which mutations occur, such that the rate of adaptive mutations is enhanced. Before the hypothesis of directed mutation is accepted, it is imperative to consider alternative hypotheses that might account for the same observations. To this end, we expand upon existing mathematical theory of the dynamics of mutation and selection in clonal populations for two cases of particular interest. The first case concerns selection against mutants before plating; this selection occurs as the result of differences in growth rate between mutants and nonmutants. We demonstrate that this selection model gives rise to distributions of mutants, obtained by plating from sister cultures, that are very similar to those expected when some mutations are induced by the selective environment. The second case concerns the sequential incorporation of two mutations as the result of selection for an intermediate genotype after plating. We demonstrate that this two-step mutation model also yields distributions that are similar to those expected when some mutations are induced by the selective environment. These two cases therefore provide alternatives to the hypothesis of directed mutation. We suggest experiments that might be used to examine our alternative hypotheses. We also contrast the hypothesis of directed mutation with the notion of inheritance of acquired characteristics.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouma J. E., Lenski R. E. Evolution of a bacteria/plasmid association. Nature. 1988 Sep 22;335(6188):351–352. doi: 10.1038/335351a0. [DOI] [PubMed] [Google Scholar]
- Cairns J., Overbaugh J., Miller S. The origin of mutants. Nature. 1988 Sep 8;335(6186):142–145. doi: 10.1038/335142a0. [DOI] [PubMed] [Google Scholar]
- Doolittle W. F., Sapienza C. Selfish genes, the phenotype paradigm and genome evolution. Nature. 1980 Apr 17;284(5757):601–603. doi: 10.1038/284601a0. [DOI] [PubMed] [Google Scholar]
- Dykhuizen D. E., Hartl D. L. Selection in chemostats. Microbiol Rev. 1983 Jun;47(2):150–168. doi: 10.1128/mr.47.2.150-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykhuizen D., Hartl D. L. Selective neutrality of 6PGD allozymes in E. coli and the effects of genetic background. Genetics. 1980 Dec;96(4):801–817. doi: 10.1093/genetics/96.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G. Adaptive evolution that requires multiple spontaneous mutations. I. Mutations involving an insertion sequence. Genetics. 1988 Dec;120(4):887–897. doi: 10.1093/genetics/120.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey D. A. Selfish DNA: a sexually-transmitted nuclear parasite. Genetics. 1982 Jul-Aug;101(3-4):519–531. doi: 10.1093/genetics/101.3-4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenski R. E., Slatkin M., Ayala F. J. Another alternative to directed mutation. Nature. 1989 Jan 12;337(6203):123–124. doi: 10.1038/337123b0. [DOI] [PubMed] [Google Scholar]
- Lenski R. E. Two-step resistance by Escherichia coli B to bacteriophage T2. Genetics. 1984 May;107(1):1–7. doi: 10.1093/genetics/107.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyed H. S., Bertrand K. P. Mutations in multicopy Tn10 tet plasmids that confer resistance to inhibitory effects of inducers of tet gene expression. J Bacteriol. 1983 Aug;155(2):557–564. doi: 10.1128/jb.155.2.557-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Origin of mutants disputed. Nature. 1988 Dec 8;336(6199):525–528. doi: 10.1038/336525a0. [DOI] [PubMed] [Google Scholar]
- Paquin C. E., Adams J. Relative fitness can decrease in evolving asexual populations of S. cerevisiae. Nature. 1983 Nov 24;306(5941):368–370. doi: 10.1038/306368a0. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A. Observations on the formation of clones containing araB-lacZ cistron fusions. Mol Gen Genet. 1984;194(1-2):79–90. doi: 10.1007/BF00383501. [DOI] [PubMed] [Google Scholar]
- Stahl F. W. Bacterial genetics. A unicorn in the garden. Nature. 1988 Sep 8;335(6186):112–113. doi: 10.1038/335112a0. [DOI] [PubMed] [Google Scholar]


