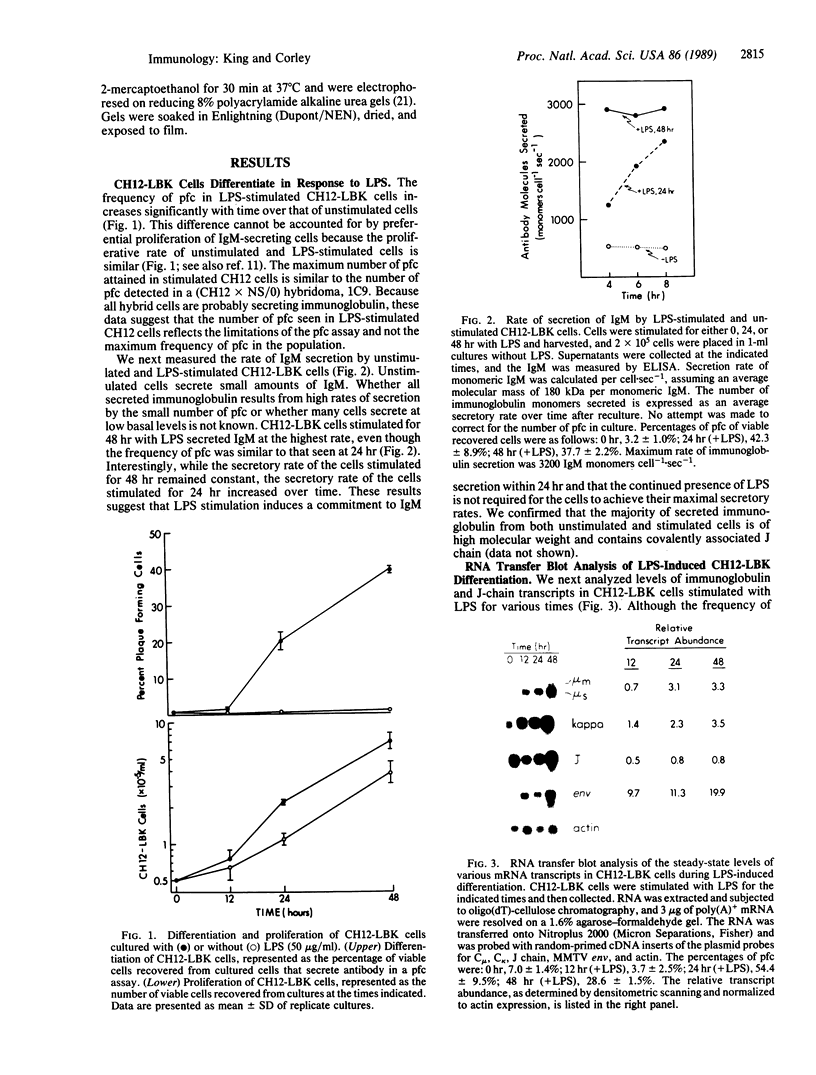
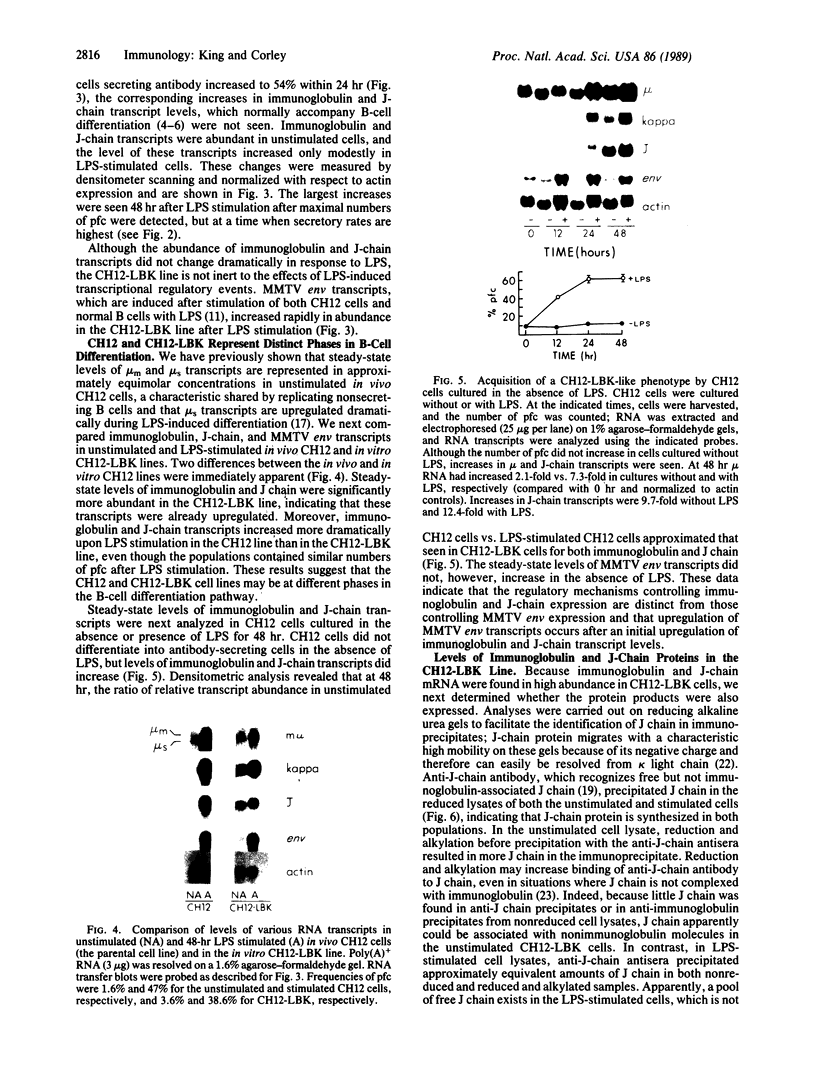
Abstract
We have identified and characterized an inducible in vitro subclone of the CH12 B-cell lymphoma, CH12-LBK, which appears to represent a transitional phase in the B-cell differentiation pathway. This phase, which we call the "presecretory" phase, falls between replicating B cells that are not secreting antibodies and B cells that secrete antibody at a high rate. Presecretory cells are characterized by abundant steady-state levels of immunoglobulin and joining (J) chain transcripts and of protein but low levels of mouse mammary tumor virus envelope transcripts and low rates of immunoglobulin secretion. Additional stimulation is required for presecretory cells to differentiate into cells that secrete antibodies at a high rate. The existence of cells with this phenotype suggests that high-level expression of immunoglobulin and J-chain protein does not necessarily commit a B cell to polymerize and secrete multimeric immunoglobulin. Rather, other gene products, expressed after immunoglobulin and J-chain transcripts have been upregulated late in B-cell differentiation, appear responsible for inducing high rates of antibody secretion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Buxbaum J., Citronbaum R., Douglas S., Forni L., Melchers F., Pernis B., Stott D. IgM-producing tumors in the BALB-c mouse: a model for B-cell maturation. J Exp Med. 1974 Sep 1;140(3):742–763. doi: 10.1084/jem.140.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman M. A., Tigges M. A., Minie M. E., Koshland M. E. A model system for peptide hormone action in differentiation: interleukin 2 induces a B lymphoma to transcribe the J chain gene. Cell. 1986 Nov 21;47(4):609–617. doi: 10.1016/0092-8674(86)90625-2. [DOI] [PubMed] [Google Scholar]
- Cann G. M., Zaritsky A., Koshland M. E. Primary structure of the immunoglobulin J chain from the mouse. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6656–6660. doi: 10.1073/pnas.79.21.6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Bettecken U., Wecker E., Schimpl A. Transcriptional control of mu- and kappa-gene expression in resting and bacterial lipopolysaccharide-activated normal B cells. Immunobiology. 1987 Mar;174(2):162–176. doi: 10.1016/s0171-2985(87)80036-0. [DOI] [PubMed] [Google Scholar]
- Conger J. D., Pike B. L., Nossal G. J. Clonal analysis of the anti-DNA repertoire of murine B lymphocytes. Proc Natl Acad Sci U S A. 1987 May;84(9):2931–2935. doi: 10.1073/pnas.84.9.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corley R. B., LoCascio N. J., Ovnic M., Haughton G. Two separate functions of class II (Ia) molecules: T-cell stimulation and B-cell excitation. Proc Natl Acad Sci U S A. 1985 Jan;82(2):516–520. doi: 10.1073/pnas.82.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdu I., Moldoveanu Z., Cooper M. D., Mestecky J. Ultrastructural studies of human lymphoid cells. mu and J chain expression as a function of B cell differentiation. J Exp Med. 1983 Dec 1;158(6):1993–2006. doi: 10.1084/jem.158.6.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland M. E. The coming of age of the immunoglobulin J chain. Annu Rev Immunol. 1985;3:425–453. doi: 10.1146/annurev.iy.03.040185.002233. [DOI] [PubMed] [Google Scholar]
- Lamson G., Koshland M. E. Changes in J chain and mu chain RNA expression as a function of B cell differentiation. J Exp Med. 1984 Sep 1;160(3):877–892. doi: 10.1084/jem.160.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Bonifacino J. S., Yuan L. C., Klausner R. D. Degradation from the endoplasmic reticulum: disposing of newly synthesized proteins. Cell. 1988 Jul 15;54(2):209–220. doi: 10.1016/0092-8674(88)90553-3. [DOI] [PubMed] [Google Scholar]
- Machamer C. E., Cresswell P. Biosynthesis and glycosylation of the invariant chain associated with HLA-DR antigens. J Immunol. 1982 Dec;129(6):2564–2569. [PubMed] [Google Scholar]
- Matthyssens G., Rabbitts T. H. The sequence at the 3' terminus of mouse immunoglobulin secreted mu chain messenger RNA determined from cloned cDNA. Nucleic Acids Res. 1980 Feb 25;8(4):703–713. [PMC free article] [PubMed] [Google Scholar]
- Max E. E., Korsmeyer S. J. Human J chain gene. Structure and expression in B lymphoid cells. J Exp Med. 1985 Apr 1;161(4):832–849. doi: 10.1084/jem.161.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M., Fu S. M., Kunkel H. G. J chain biosynthesis in pre-B cells and other possible precursor B cells. J Exp Med. 1981 Jul 1;154(1):138–145. doi: 10.1084/jem.154.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers F., Andersson J., Corbel C., Leptin M., Lernhardt W., Gerhard W., Zeuthen J. Regulation of B lymphocyte replication and maturation. J Cell Biochem. 1982;19(4):315–332. doi: 10.1002/jcb.240190403. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Gravel Y., Williamson A. R., Baumal R. Modification and fate of J chain in myeloma cells in the presence and absence of polymeric immunoglobulin secretion. Eur J Immunol. 1978 Feb;8(2):94–101. doi: 10.1002/eji.1830080205. [DOI] [PubMed] [Google Scholar]
- Mosmann T., Baumal R. Synthesis but not secretion of J chain by variant mouse myeloma cells which lose alpha-chain-synthesizing ability. J Immunol. 1975 Oct;115(4):955–962. [PubMed] [Google Scholar]
- Nakanishi K., Cohen D. I., Blackman M., Nielsen E., Ohara J., Hamaoka T., Koshland M. E., Paul W. E. Ig RNA expression in normal B cells stimulated with anti-IgM antibody and T cell-derived growth and differentiation factors. J Exp Med. 1984 Dec 1;160(6):1736–1751. doi: 10.1084/jem.160.6.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovnic M., Corley R. B. Quantitation of cell surface molecules on a differentiating, Ly-1+ B cell lymphoma. J Immunol. 1987 May 1;138(9):3075–3082. [PubMed] [Google Scholar]
- Parkhouse R. M., Askonas B. A. Immunoglobulin M biosynthesis. Intracellular accumulation of 7S subunits. Biochem J. 1969 Nov;115(2):163–169. doi: 10.1042/bj1150163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisfeld R. A., Small P. A., Jr Electrophoretic heterogeneity of polypeptide chains of specific antibodies. Science. 1966 May 27;152(3726):1253–1255. doi: 10.1126/science.152.3726.1253. [DOI] [PubMed] [Google Scholar]
- Roth R. A., Koshland M. E. Identification of a lymphocyte enzyme that catalyzes pentamer immunoglobulin M assembly. J Biol Chem. 1981 May 10;256(9):4633–4639. [PubMed] [Google Scholar]
- Schibler U., Marcu K. B., Perry R. P. The synthesis and processing of the messenger RNAs specifying heavy and light chain immunoglobulins in MPC-11 cells. Cell. 1978 Dec;15(4):1495–1509. doi: 10.1016/0092-8674(78)90072-7. [DOI] [PubMed] [Google Scholar]
- Sharma S., King L. B., Corley R. B. Molecular events during B lymphocyte differentiation. Induction of endogenous mouse mammary tumor proviral envelope transcripts after B cell stimulation. J Immunol. 1988 Oct 1;141(7):2510–2518. [PubMed] [Google Scholar]
- Sidman C. B lymphocyte differentiation and the control of IgM mu chain expression. Cell. 1981 Feb;23(2):379–389. doi: 10.1016/0092-8674(81)90133-1. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Old L. J., Boyse E. A. Surface alloantigens of plasma cells. J Exp Med. 1970 Jun 1;131(6):1325–1341. doi: 10.1084/jem.131.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B., Scher I., Schaefer M. E., Lindsten T., Finkelman F. D., Mond J. J. Size-dependent B lymphocyte subpopulations: relationship of cell volume to surface phenotype, cell cycle, proliferative response, and requirements for antibody production to TNP-Ficoll and TNP-BA. J Immunol. 1984 Nov;133(5):2333–2342. [PubMed] [Google Scholar]