Abstract
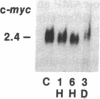
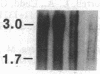
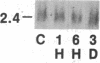
Two classes (site 1- and site 2-selective) of cAMP analogs, which either alone or in combination demonstrate a preference for binding to type II rather than type I cAMP-dependent protein kinase isozyme, potently inhibit growth in a spectrum of human cancer cell lines in culture. Treatment of K-562 human leukemic cells for 3 days with 30 and 10 microM 8-chloroadenosine 3',5'-cyclic monophosphate (8-Cl-cAMP) (site 1-selective) resulted in 60% and 20% growth inhibition, respectively (with over 90% viability). N6-Benzyl-cAMP (site 2-selective) (30 microM) treatment resulted in 20% growth inhibition by day 3. When 8-Cl-cAMP (10 microM) and N6-benzyl-cAMP (30 microM) were both added, growth was almost completely arrested. The growth inhibition was accompanied by megakaryocytic differentiation in K-562 cells. The untreated control cells expressed little or no detectable levels of glycoprotein IIb-IIIa surface antigen complex. 8-Cl-cAMP (30 microM) treatment for 3 days substantially increased the antigen expression, while N6-benzyl-cAMP caused little or no change in the antigen expression. When cells were treated with 8-Cl-cAMP in combination with N6-benzyl-cAMP, antigen expression was synergistically enhanced, and cells demonstrated megakaryocyte morphology. By Northern blotting, we examined the mRNA levels of the type I and type II protein kinase regulatory subunits (RI alpha and RII beta), the catalytic subunit, and c-myc during 8-Cl-cAMP treatment. The steady-state level of RII beta cAMP receptor mRNA sharply increased within 1 hr of treatment and remained elevated for 3 days, while that of the RI alpha receptor markedly decreased to below control level within 6 hr and remained low during treatment. However, 8-Cl-cAMP did not affect the mRNA level of the catalytic subunit. 8-Cl-cAMP treatment also brought about a rapid decrease in c-myc mRNA. Thus, differential regulation of cAMP receptor genes is an early event in cAMP-induced differentiation and growth control of K-562 leukemia cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ally S., Tortora G., Clair T., Grieco D., Merlo G., Katsaros D., Ogreid D., Døskeland S. O., Jahnsen T., Cho-Chung Y. S. Selective modulation of protein kinase isozymes by the site-selective analog 8-chloroadenosine 3',5'-cyclic monophosphate provides a biological means for control of human colon cancer cell growth. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6319–6322. doi: 10.1073/pnas.85.17.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson L. C., Nilsson K., Gahmberg C. G. K562--a human erythroleukemic cell line. Int J Cancer. 1979 Feb;23(2):143–147. doi: 10.1002/ijc.2910230202. [DOI] [PubMed] [Google Scholar]
- Cho-Chung Y. S. Cyclic AMP and mammary tumor regression. Cell Mol Biol Incl Cyto Enzymol. 1980;26(4):395–403. [PubMed] [Google Scholar]
- Cho-Chung Y. S. Hypothesis. Cyclic AMP and its receptor protein in tumor growth regulation in vivo. J Cyclic Nucleotide Res. 1980;6(3):163–177. [PubMed] [Google Scholar]
- Clair T., Ally S., Tagliaferri P., Robins R. K., Cho-Chung Y. S. Site-selective cAMP analogs induce nuclear translocation of the RII cAMP receptor protein in Ha-MuSV-transformed NIH/3T3 cells. FEBS Lett. 1987 Nov 30;224(2):377–384. doi: 10.1016/0014-5793(87)80488-x. [DOI] [PubMed] [Google Scholar]
- Clegg C. H., Correll L. A., Cadd G. G., McKnight G. S. Inhibition of intracellular cAMP-dependent protein kinase using mutant genes of the regulatory type I subunit. J Biol Chem. 1987 Sep 25;262(27):13111–13119. [PubMed] [Google Scholar]
- Colamonici O. R., Trepel J. B., Vidal C. A., Neckers L. M. Phorbol ester induces c-sis gene transcription in stem cell line K-562. Mol Cell Biol. 1986 May;6(5):1847–1850. doi: 10.1128/mcb.6.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Døskeland S. O. Evidence that rabbit muscle protein kinase has two kinetically distinct binding sites for adenosine 3' ; 5'-cyclic monophosphate. Biochem Biophys Res Commun. 1978 Jul 28;83(2):542–549. doi: 10.1016/0006-291x(78)91024-0. [DOI] [PubMed] [Google Scholar]
- Gharrett A. J., Malkinson A. M., Sheppard J. R. Cyclic AMP-dependent protein kinases from normal and SV40-transformed 3T3 cells. Nature. 1976 Dec 16;264(5587):673–675. doi: 10.1038/264673a0. [DOI] [PubMed] [Google Scholar]
- Jungmann R. A., Lee S., DeAngelo A. B. Translocation of cytoplasmic protein kinase and cyclic adenosine monophosphate-binding protein to intracellular acceptor sites. Adv Cyclic Nucleotide Res. 1975;5:281–306. [PubMed] [Google Scholar]
- Katsaros D., Tortora G., Tagliaferri P., Clair T., Ally S., Neckers L., Robins R. K., Cho-Chung Y. S. Site-selective cyclic AMP analogs provide a new approach in the control of cancer cell growth. FEBS Lett. 1987 Oct 19;223(1):97–103. doi: 10.1016/0014-5793(87)80517-3. [DOI] [PubMed] [Google Scholar]
- Krebs E. G. Protein kinases. Curr Top Cell Regul. 1972;5:99–133. [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levene R. B., Lamaziere J. M., Broxmeyer H. E., Lu L., Rabellino E. M. Human megakaryocytes. V. Changes in the phenotypic profile of differentiating megakaryocytes. J Exp Med. 1985 Mar 1;161(3):457–474. doi: 10.1084/jem.161.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy F. O., Oyen O., Sandberg M., Taskén K., Eskild W., Hansson V., Jahnsen T. Molecular cloning, complementary deoxyribonucleic acid structure and predicted full-length amino acid sequence of the hormone-inducible regulatory subunit of 3'-5'-cyclic adenosine monophosphate-dependent protein kinase from human testis. Mol Endocrinol. 1988 Dec;2(12):1364–1373. doi: 10.1210/mend-2-12-1364. [DOI] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Marie J. P., Izaguirre C. A., Civin C. I., Mirro J., McCulloch E. A. The presence within single K-562 cells of erythropoietic and granulopoietic differentiation markers. Blood. 1981 Oct;58(4):708–711. [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Ogreid D., Ekanger R., Suva R. H., Miller J. P., Sturm P., Corbin J. D., Døskeland S. O. Activation of protein kinase isozymes by cyclic nucleotide analogs used singly or in combination. Principles for optimizing the isozyme specificity of analog combinations. Eur J Biochem. 1985 Jul 1;150(1):219–227. doi: 10.1111/j.1432-1033.1985.tb09010.x. [DOI] [PubMed] [Google Scholar]
- Olah E., Natsumeda Y., Ikegami T., Kote Z., Horanyi M., Szelenyi J., Paulik E., Kremmer T., Hollan S. R., Sugar J. Induction of erythroid differentiation and modulation of gene expression by tiazofurin in K-562 leukemia cells. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6533–6537. doi: 10.1073/pnas.85.17.6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyen O., Scott J. D., Cadd G. G., McKnight G. S., Krebs E. G., Hansson V., Jahnsen T. A unique mRNA species for a regulatory subunit of cAMP-dependent protein kinase is specifically induced in haploid germ cells. FEBS Lett. 1988 Mar 14;229(2):391–394. doi: 10.1016/0014-5793(88)81163-3. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Johnson G. S., Anderson W. B. Role of cyclic nucleotides in growth control. Annu Rev Biochem. 1975;44:491–522. doi: 10.1146/annurev.bi.44.070175.002423. [DOI] [PubMed] [Google Scholar]
- Potter V. R. Phenotypic diversity in experimental hepatomas: the concept of partially blocked ontogeny. The 10th Walter Hubert Lecture. Br J Cancer. 1978 Jul;38(1):1–23. doi: 10.1038/bjc.1978.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K. N. Differentiation of neuroblastoma cells in culture. Biol Rev Camb Philos Soc. 1975 May;50(2):129–165. doi: 10.1111/j.1469-185x.1975.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Rannels S. R., Corbin J. D. Two different intrachain cAMP binding sites of cAMP-dependent protein kinases. J Biol Chem. 1980 Aug 10;255(15):7085–7088. [PubMed] [Google Scholar]
- Ratoosh S. L., Lifka J., Hedin L., Jahsen T., Richards J. S. Hormonal regulation of the synthesis and mRNA content of the regulatory subunit of cyclic AMP-dependent protein kinase type II in cultured rat ovarian granulosa cells. J Biol Chem. 1987 May 25;262(15):7306–7313. [PubMed] [Google Scholar]
- Rimmer E. F., Horton M. A. Expression of myeloid-specific antigens on two human erythroleukaemia cell lines, HEL and K562. Leuk Res. 1984;8(2):207–211. doi: 10.1016/0145-2126(84)90144-9. [DOI] [PubMed] [Google Scholar]
- Robinson-Steiner A. M., Corbin J. D. Probable involvement of both intrachain cAMP binding sites in activation of protein kinase. J Biol Chem. 1983 Jan 25;258(2):1032–1040. [PubMed] [Google Scholar]
- Russell D. H. Type I cyclic AMP-dependent protein kinase as a positive effector of growth. Adv Cyclic Nucleotide Res. 1978;9:493–506. [PubMed] [Google Scholar]
- Rutherford T. R., Clegg J. B., Weatherall D. J. K562 human leukaemic cells synthesise embryonic haemoglobin in response to haemin. Nature. 1979 Jul 12;280(5718):164–165. doi: 10.1038/280164a0. [DOI] [PubMed] [Google Scholar]
- Sachs L. Constitutive uncoupling of pathways of gene expression that control growth and differentiation in myeloid leukemia: a model for the origin and progression of malignancy. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6152–6156. doi: 10.1073/pnas.77.10.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. A., Rubin C. S. Identification and differential expression of two forms of regulatory subunits (RII) of cAMP-dependent protein kinase II in Friend erythroleukemic cells. Differentiation and 8-bromo-cAMP elicit a large and selective increase in the rate of biosynthesis of only one type of RII. J Biol Chem. 1985 May 25;260(10):6296–6303. [PubMed] [Google Scholar]
- Strife A., Clarkson B. Biology of chronic myelogenous leukemia: is discordant maturation the primary defect? Semin Hematol. 1988 Jan;25(1):1–19. [PubMed] [Google Scholar]
- Tabilio A., Pelicci P. G., Vinci G., Mannoni P., Civin C. I., Vainchenker W., Testa U., Lipinski M., Rochant H., Breton-Gorius J. Myeloid and megakaryocytic properties of K-562 cell lines. Cancer Res. 1983 Oct;43(10):4569–4574. [PubMed] [Google Scholar]
- Tagliaferri P., Katsaros D., Clair T., Neckers L., Robins R. K., Cho-Chung Y. S. Reverse transformation of Harvey murine sarcoma virus-transformed NIH/3T3 cells by site-selective cyclic AMP analogs. J Biol Chem. 1988 Jan 5;263(1):409–416. [PubMed] [Google Scholar]
- Tetteroo P. A., Massaro F., Mulder A., Schreuder-van Gelder R., von dem Borne A. E. Megakaryoblastic differentiation of proerythroblastic K562 cell-line cells. Leuk Res. 1984;8(2):197–206. doi: 10.1016/0145-2126(84)90143-7. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortora G., Tagliaferri P., Clair T., Colamonici O., Neckers L. M., Robins R. K., Cho-Chung Y. S. Site-selective cAMP analogs at micromolar concentrations induce growth arrest and differentiation of acute promyelocytic, chronic myelocytic, and acute lymphocytic human leukemia cell lines. Blood. 1988 Jan;71(1):230–233. [PubMed] [Google Scholar]
- Trepel J. B., Colamonici O. R., Kelly K., Schwab G., Watt R. A., Sausville E. A., Jaffe E. S., Neckers L. M. Transcriptional inactivation of c-myc and the transferrin receptor in dibutyryl cyclic AMP-treated HL-60 cells. Mol Cell Biol. 1987 Jul;7(7):2644–2648. doi: 10.1128/mcb.7.7.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G., Perussia B. Human natural killer cells: biologic and pathologic aspects. Lab Invest. 1984 May;50(5):489–513. [PubMed] [Google Scholar]
- Uhler M. D., McKnight G. S. Expression of cDNAs for two isoforms of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1987 Nov 5;262(31):15202–15207. [PubMed] [Google Scholar]
- Vinci G., Tabilio A., Deschamps J. F., Van Haeke D., Henri A., Guichard J., Tetteroo P., Lansdorp P. M., Hercend T., Vainchenker W. Immunological study of in vitro maturation of human megakaryocytes. Br J Haematol. 1984 Apr;56(4):589–605. doi: 10.1111/j.1365-2141.1984.tb02184.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Biggs W. H., 3rd, Montminy M. R. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988 Aug 11;334(6182):494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]
- ar-Rushdi A., Nishikura K., Erikson J., Watt R., Rovera G., Croce C. M. Differential expression of the translocated and the untranslocated c-myc oncogene in Burkitt lymphoma. Science. 1983 Oct 28;222(4622):390–393. doi: 10.1126/science.6414084. [DOI] [PubMed] [Google Scholar]