Abstract
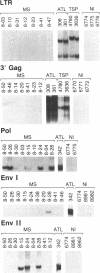
Twenty-one patients with multiple sclerosis, chronic progressive type, were examined for DNA sequences homologous to a human retrovirus. Genomic DNA from peripheral blood mononuclear cells was analyzed for the presence of homologous sequences to the human T-cell leukemia/lymphoma virus type I (HTLV-I) long terminal repeat, 3' gag, pol, and env domains by the enzymatic in vitro gene amplification technique, polymerase chain reaction. Positive identification of homologous pol sequences was made in the amplified DNA from six of these patients (29%). Three of these six patients (14%) also tested positive for the env region, but not for the other regions tested. In contrast, none of the samples from 35 normal individuals studied was positive when amplified and tested with the same primers and probes. Comparison of patterns obtained from controls and from patients with adult T-cell leukemia or tropical spastic paraparesis suggests that the DNA sequences identified are exogenous to the human genome and may correspond to a human retroviral species. The data support the detection of a human retroviral agent in some patients with multiple sclerosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott M. A., Poiesz B. J., Byrne B. C., Kwok S., Sninsky J. J., Ehrlich G. D. Enzymatic gene amplification: qualitative and quantitative methods for detecting proviral DNA amplified in vitro. J Infect Dis. 1988 Dec;158(6):1158–1169. doi: 10.1093/infdis/158.6.1158. [DOI] [PubMed] [Google Scholar]
- Bhagavati S., Ehrlich G., Kula R. W., Kwok S., Sninsky J., Udani V., Poiesz B. J. Detection of human T-cell lymphoma/leukemia virus type I DNA and antigen in spinal fluid and blood of patients with chronic progressive myelopathy. N Engl J Med. 1988 May 5;318(18):1141–1147. doi: 10.1056/NEJM198805053181801. [DOI] [PubMed] [Google Scholar]
- Birnbaum G., Aubitz S., Kotilinek L. Search for autonomously proliferating spinal fluid lymphocytes in patients with multiple sclerosis. Neurology. 1988 Jan;38(1):28–30. doi: 10.1212/wnl.38.1.28. [DOI] [PubMed] [Google Scholar]
- Ehrlich G. D., Han T., Bettigole R., Merl S. A., Lehr B., Tomar R. H., Poiesz B. J. Human T-lymphotropic virus type I-associated benign transient immature T-cell lymphocytosis. Am J Hematol. 1988 Jan;27(1):49–55. doi: 10.1002/ajh.2830270112. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gessain A., Abel L., De-The G., Vernant J. C., Raverdy P., Guillard A. Lack of antibody to HTLV-I and HIV in patients with multiple sclerosis from France and French West Indies. Br Med J (Clin Res Ed) 1986 Aug 16;293(6544):424–425. doi: 10.1136/bmj.293.6544.424-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S. L., Aubert C., Burks J. S., Kerr C., Lyon-Caen O., de The G., Brahic M. Analysis of human T-lymphotrophic virus sequences in multiple sclerosis tissue. Nature. 1986 Jul 10;322(6075):176–177. doi: 10.1038/322176a0. [DOI] [PubMed] [Google Scholar]
- Karpas A., Kämpf U., Sidèn A., Koch M., Poser S. Lack of evidence for involvement of known human retroviruses in multiple sclerosis. Nature. 1986 Jul 10;322(6075):177–178. doi: 10.1038/322177a0. [DOI] [PubMed] [Google Scholar]
- Koprowski H., DeFreitas E. C., Harper M. E., Sandberg-Wollheim M., Sheremata W. A., Robert-Guroff M., Saxinger C. W., Feinberg M. B., Wong-Staal F., Gallo R. C. Multiple sclerosis and human T-cell lymphotropic retroviruses. Nature. 1985 Nov 14;318(6042):154–160. doi: 10.1038/318154a0. [DOI] [PubMed] [Google Scholar]
- Kwok S., Ehrlich G., Poiesz B., Kalish R., Sninsky J. J. Enzymatic amplification of HTLV-I viral sequences from peripheral blood mononuclear cells and infected tissues. Blood. 1988 Oct;72(4):1117–1123. [PubMed] [Google Scholar]
- Kwok S., Kellogg D., Ehrlich G., Poiesz B., Bhagavati S., Sninsky J. J. Characterization of a sequence of human T cell leukemia virus type I from a patient with chronic progressive myelopathy. J Infect Dis. 1988 Dec;158(6):1193–1197. doi: 10.1093/infdis/158.6.1193. [DOI] [PubMed] [Google Scholar]
- Madden D. L., Mundon F. K., Tzan N. R., Fuccillo D. A., Dalakas M. C., Calabrese V., Elizan T. S., Sever J. L. Serologic studies of MS patients, controls, and patients with other neurologic diseases: antibodies to HTLV-I, II, III. Neurology. 1988 Jan;38(1):81–84. doi: 10.1212/wnl.38.1.81. [DOI] [PubMed] [Google Scholar]
- Mager D. L., Freeman J. D. Human endogenous retroviruslike genome with type C pol sequences and gag sequences related to human T-cell lymphotropic viruses. J Virol. 1987 Dec;61(12):4060–4066. doi: 10.1128/jvi.61.12.4060-4066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Ohta M., Ohta K., Mori F., Nishitani H., Saida T. Sera from patients with multiple sclerosis react with human cell T lymphotropic virus-I gag proteins but not env proteins--Western blotting analysis. J Immunol. 1986 Dec 1;137(11):3440–3443. [PubMed] [Google Scholar]
- Osame M., Matsumoto M., Usuku K., Izumo S., Ijichi N., Amitani H., Tara M., Igata A. Chronic progressive myelopathy associated with elevated antibodies to human T-lymphotropic virus type I and adult T-cell leukemialike cells. Ann Neurol. 1987 Feb;21(2):117–122. doi: 10.1002/ana.410210203. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser C. M., Paty D. W., Scheinberg L., McDonald W. I., Davis F. A., Ebers G. C., Johnson K. P., Sibley W. A., Silberberg D. H., Tourtellotte W. W. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983 Mar;13(3):227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- Ranki A., Johansson E., Krohn K. Interpretation of antibodies reacting solely with human retroviral core proteins. N Engl J Med. 1988 Feb 18;318(7):448–449. doi: 10.1056/NEJM198802183180712. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. P., Armstrong H. A., Bulman D. E., Paty D. W., Ebers G. C. Absence of antibody to HTLV I and III in sera of Canadian patients with multiple sclerosis and chronic myelopathy. Ann Neurol. 1986 Oct;20(4):533–534. doi: 10.1002/ana.410200415. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román G. C., Schoenberg B. S., Madden D. L., Sever J. L., Hugon J., Ludolph A., Spencer P. S. Human T-lymphotropic virus type I antibodies in the serum of patients with tropical spastic paraparesis in the Seychelles. Arch Neurol. 1987 Jun;44(6):605–607. doi: 10.1001/archneur.1987.00520180029011. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]