Abstract
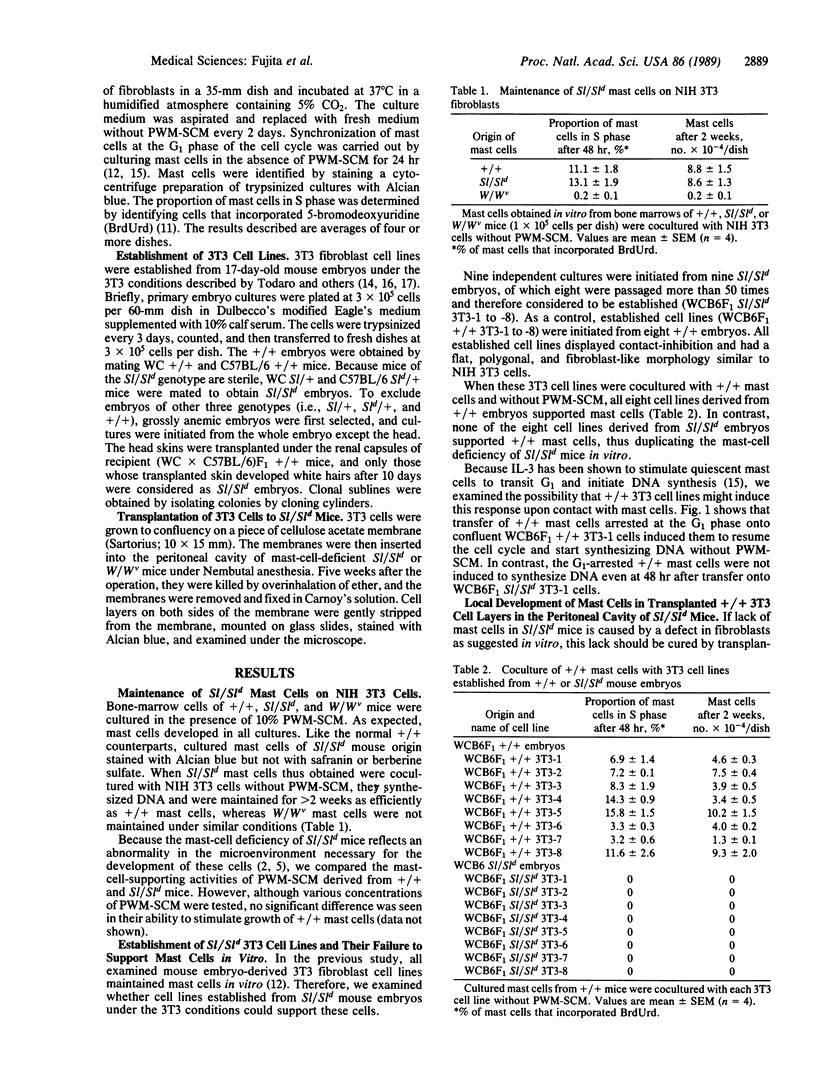
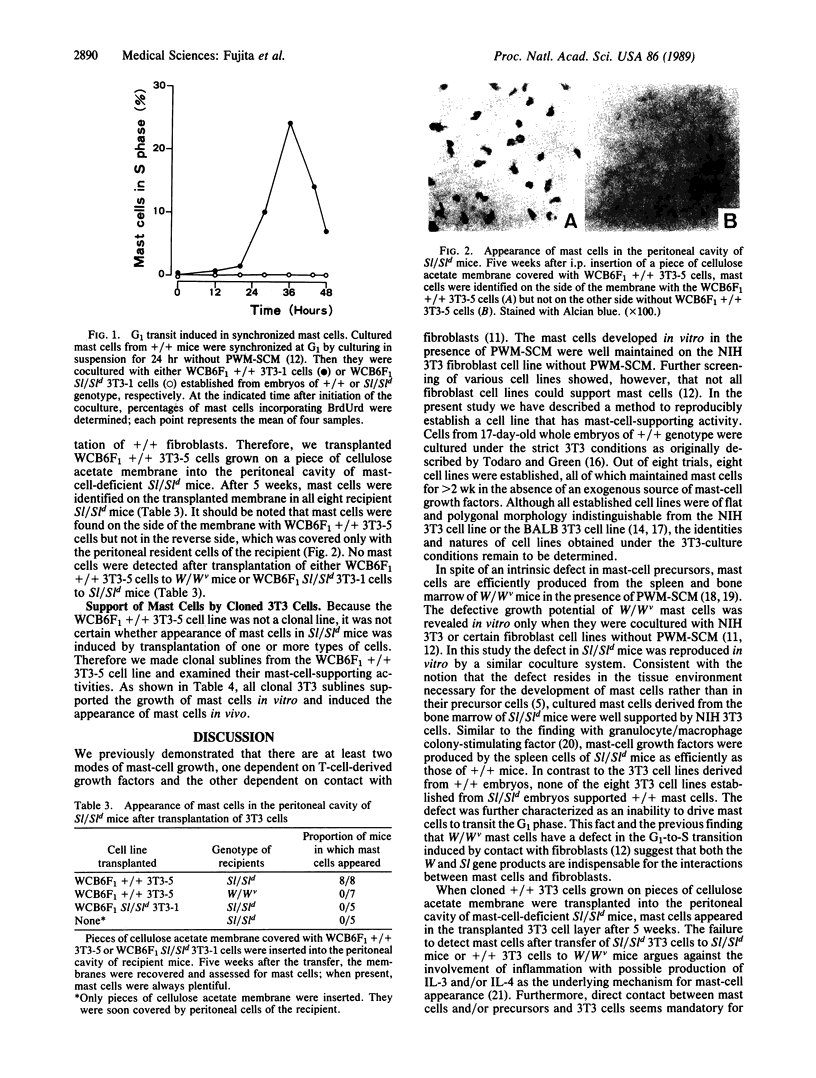
Sl/Sld mutant mice are profoundly deficient in tissue mast cells as a result of a defect in the microenvironment promoting the development of these cells. To facilitate the analysis of the Sl mutation, we attempted to establish an in vitro system in which the in vivo defect of Sl/Sld mice could be reproduced. 3T3 cell lines were established from 17-day-old embryos of Sl/Sld and congenic +/+ genotypes and were cocultured with mast cells obtained in vitro from the bone marrow of +/+ mice. All eight 3T3 cell lines derived from +/+ of T-cell-derived growth factors. By contrast, none of eight 3T3 cell lines from Sl/Sld embryos supported mast cells under similar conditions. The defect in Sl/Sld 3T3 cells was further characterized as a failure to induce the G1-to-S transition in synchronized mast cells upon contact, suggesting that the Sl gene product is indispensable for this activity. When 3T3 cells of +/+ genotype, grown on pieces of cellulose acetate membrane, were transplanted into the peritoneal cavity of Sl/Sld mice, mast cells appeared locally in the transplanted 3T3 cell layers. These results suggested an essential role of fibroblasts in vivo as the tissue microenvironment promoting the development of mast cells and that they are defective in Sl/Sld mice. The present coculture system duplicated mast-cell deficiency of Sl/Sld mice in vitro and should prove useful for analysis of the Sl gene product.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Moore M. A. In vitro duplication and "cure" of haemopoietic defects in genetically anaemic mice. Nature. 1977 Sep 29;269(5627):412–414. doi: 10.1038/269412a0. [DOI] [PubMed] [Google Scholar]
- Fujita J., Nakayama H., Onoue H., Ebi Y., Kanakura Y., Kuriu A., Kitamura Y. Failure of W/Wv mouse-derived cultured mast cells to enter S phase upon contact with NIH/3T3 fibroblasts. Blood. 1988 Aug;72(2):463–468. [PubMed] [Google Scholar]
- Fujita J., Nakayama H., Onoue H., Kanakura Y., Nakano T., Asai H., Takeda S., Honjo T., Kitamura Y. Fibroblast-dependent growth of mouse mast cells in vitro: duplication of mast cell depletion in mutant mice of W/Wv genotype. J Cell Physiol. 1988 Jan;134(1):78–84. doi: 10.1002/jcp.1041340109. [DOI] [PubMed] [Google Scholar]
- Galli S. J., Arizono N., Murakami T., Dvorak A. M., Fox J. G. Development of large numbers of mast cells at sites of idiopathic chronic dermatitis in genetically mast cell-deficient WBB6F1-W/Wv mice. Blood. 1987 Jun;69(6):1661–1666. [PubMed] [Google Scholar]
- Galli S. J., Dvorak A. M., Dvorak H. F. Basophils and mast cells: morphologic insights into their biology, secretory patterns, and function. Prog Allergy. 1984;34:1–141. [PubMed] [Google Scholar]
- Galli S. J., Kitamura Y. Genetically mast-cell-deficient W/Wv and Sl/Sld mice. Their value for the analysis of the roles of mast cells in biologic responses in vivo. Am J Pathol. 1987 Apr;127(1):191–198. [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi Y., Kanakura Y., Fujita J., Takeda S., Nakano T., Tarui S., Honjo T., Kitamura Y. Interleukin 4 as an essential factor for in vitro clonal growth of murine connective tissue-type mast cells. J Exp Med. 1987 Jan 1;165(1):268–273. doi: 10.1084/jem.165.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G. M., Phillips R. A. Maintenance of hemopoiesis in long-term bone marrow cultures from S1/S1d and W/Wv mice. Exp Hematol. 1984 Dec;12(11):822–824. [PubMed] [Google Scholar]
- Kelvin D. J., Chance S., Shreeve M., Axelrad A. A., Connolly J. A., McLeod D. Interleukin 3 and cell cycle progression. J Cell Physiol. 1986 Jun;127(3):403–409. doi: 10.1002/jcp.1041270308. [DOI] [PubMed] [Google Scholar]
- Kitamura Y., Go S. Decreased production of mast cells in S1/S1d anemic mice. Blood. 1979 Mar;53(3):492–497. [PubMed] [Google Scholar]
- Knospe W. H., Hinrichs B., Fried W., Robinson W., Trobaugh F. E., Jr Normal colony stimulating factor (CSF) production by bone marrow stromal cells and abnormal granulopoiesis with decreased CFUc in S1/S1d mice. Exp Hematol. 1976 May;4(3):125–130. [PubMed] [Google Scholar]
- Matsuda H., Kitamura Y., Sonoda T., Imori T. Precursor of mast cells fixed in the skin of mice. J Cell Physiol. 1981 Sep;108(3):409–415. doi: 10.1002/jcp.1041080315. [DOI] [PubMed] [Google Scholar]
- McCulloch E. A., Siminovitch L., Till J. E., Russell E. S., Bernstein S. E. The cellular basis of the genetically determined hemopoietic defect in anemic mice of genotype Sl-Sld. Blood. 1965 Oct;26(4):399–410. [PubMed] [Google Scholar]
- Nakahata T., Spicer S. S., Cantey J. R., Ogawa M. Clonal assay of mouse mast cell colonies in methylcellulose culture. Blood. 1982 Aug;60(2):352–361. [PubMed] [Google Scholar]
- RUSSELL E. S., BERNSTEIN S. E., LAWSON F. A., SMITH L. J. Long-continued function of normal blood-forming tissue transplanted into genetically anemic hosts. J Natl Cancer Inst. 1959 Sep;23:557–566. [PubMed] [Google Scholar]
- Russell E. S. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- Schrader J. W., Lewis S. J., Clark-Lewis I., Culvenor J. G. The persisting (P) cell: histamine content, regulation by a T cell-derived factor, origin from a bone marrow precursor, and relationship to mast cells. Proc Natl Acad Sci U S A. 1981 Jan;78(1):323–327. doi: 10.1073/pnas.78.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. W. The panspecific hemopoietin of activated T lymphocytes (interleukin-3). Annu Rev Immunol. 1986;4:205–230. doi: 10.1146/annurev.iy.04.040186.001225. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Rennick D. M. Characterization of a murine lymphokine distinct from interleukin 2 and interleukin 3 (IL-3) possessing a T-cell growth factor activity and a mast-cell growth factor activity that synergizes with IL-3. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1857–1861. doi: 10.1073/pnas.83.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung Y. P., Moore M. A. Long-term in vitro culture of murine mast cells. III. Discrimination of mast cells growth factor and granulocyte-CSF. J Immunol. 1982 Sep;129(3):1256–1261. [PubMed] [Google Scholar]