Abstract
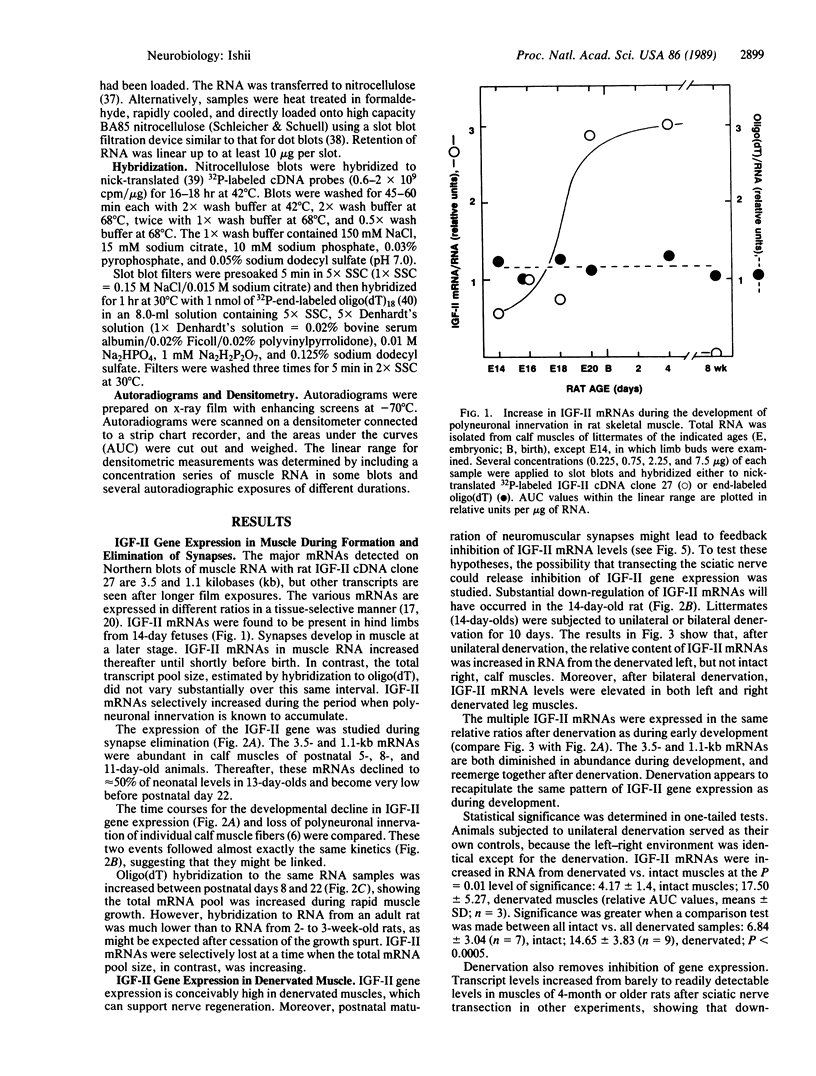
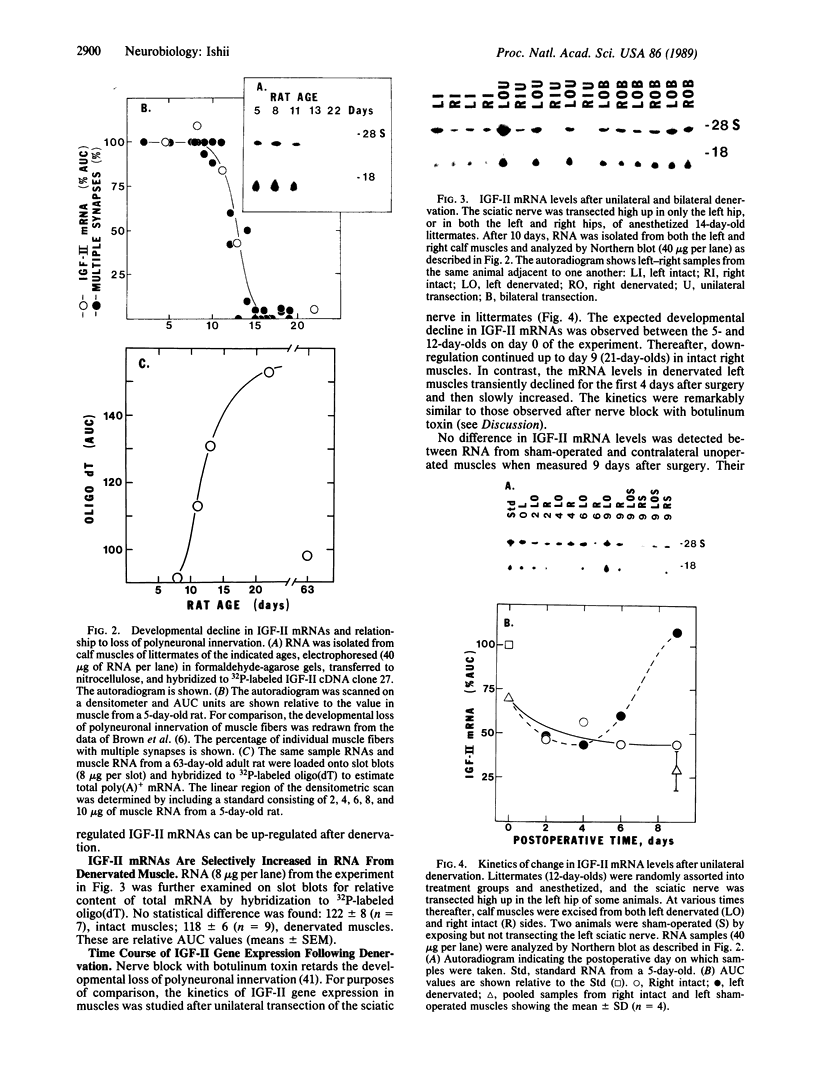
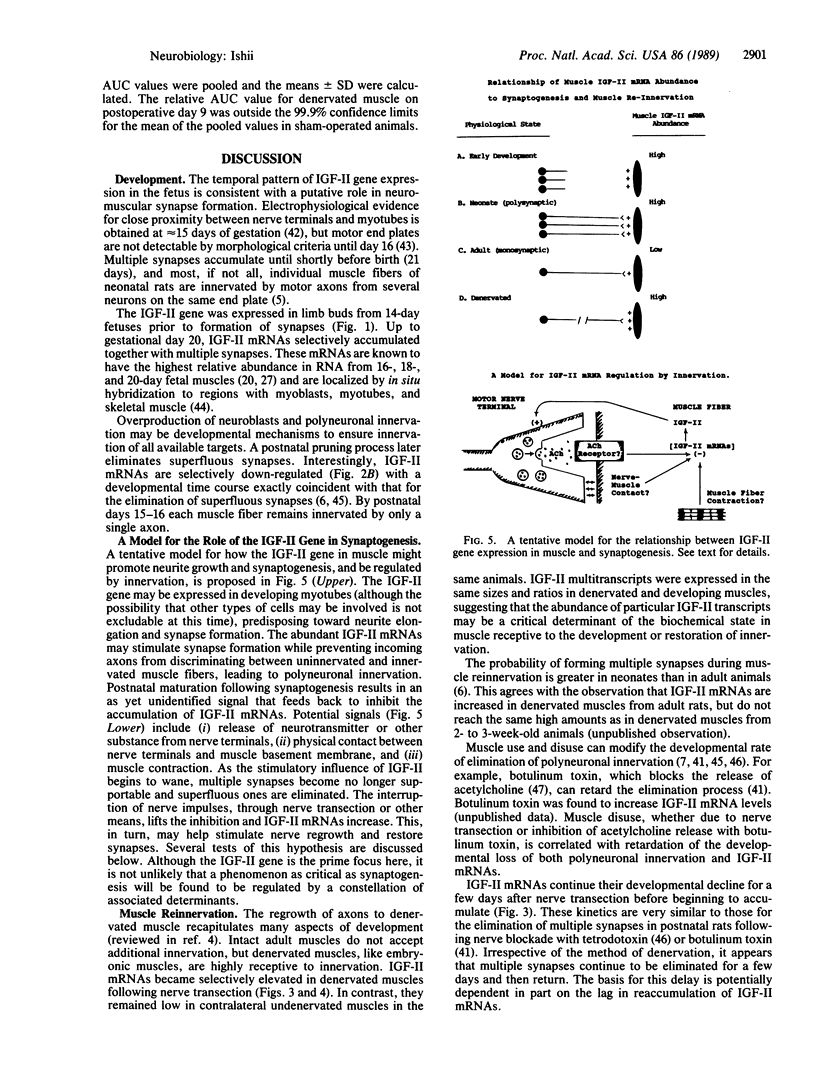
A striking correlation between insulin-like growth factor II (IGF-II) gene expression and turnover of neuromuscular synapses was observed. The IGF-II gene was expressed at a high level in fetal rat hind limb muscles prior to the developmental formation of synapses and increased while polyneuronal innervation accumulated. Thereafter, there was a selective down-regulation of IGF-II mRNAs that was exactly coincident with the postnatal time course for elimination of superfluous synapses. The hypothesis that innervation might provide a signal suppressing IGF-II gene expression was tested. Upon transection of the sciatic nerve, there was up-regulation of IGF-II mRNA content in muscle. This up-regulation was selective and correlated with the capacity of denervated muscle to accept reinnervation. These results suggest that the IGF-II gene may play a role in the development and turnover of synapses.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenman Y., de Vellis J. Brain neurons develop in a serum and glial free environment: effects of transferrin, insulin, insulin-like growth factor-I and thyroid hormone on neuronal survival, growth and differentiation. Brain Res. 1987 Mar 17;406(1-2):32–42. doi: 10.1016/0006-8993(87)90766-9. [DOI] [PubMed] [Google Scholar]
- Ambache N. The peripheral action of Cl. botulinum toxin. J Physiol. 1949 Mar 15;108(2):127–141. [PMC free article] [PubMed] [Google Scholar]
- Beck F., Samani N. J., Penschow J. D., Thorley B., Tregear G. W., Coghlan J. P. Histochemical localization of IGF-I and -II mRNA in the developing rat embryo. Development. 1987 Sep;101(1):175–184. doi: 10.1242/dev.101.1.175. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in striated muscle during development. J Physiol. 1974 Sep;241(2):515–545. doi: 10.1113/jphysiol.1974.sp010670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell T. L., Humbel R. E. Hormone families: pancreatic hormones and homologous growth factors. Nature. 1980 Oct 30;287(5785):781–787. doi: 10.1038/287781a0. [DOI] [PubMed] [Google Scholar]
- Brown A. L., Graham D. E., Nissley S. P., Hill D. J., Strain A. J., Rechler M. M. Developmental regulation of insulin-like growth factor II mRNA in different rat tissues. J Biol Chem. 1986 Oct 5;261(28):13144–13150. [PubMed] [Google Scholar]
- Brown M. C., Holland R. L., Hopkins W. G. Restoration of focal multiple innervation in rat muscles by transmission block during a critical stage of development. J Physiol. 1981 Sep;318:355–364. doi: 10.1113/jphysiol.1981.sp013869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIAKULAS J. J., PAULY J. E. A STUDY OF POSTNATAL GROWTH OF SKELETAL MUSCLE IN THE RAT. Anat Rec. 1965 May;152:55–61. doi: 10.1002/ar.1091520107. [DOI] [PubMed] [Google Scholar]
- Dennis M. J., Ziskind-Conhaim L., Harris A. J. Development of neuromuscular junctions in rat embryos. Dev Biol. 1981 Jan 30;81(2):266–279. doi: 10.1016/0012-1606(81)90290-6. [DOI] [PubMed] [Google Scholar]
- Dull T. J., Gray A., Hayflick J. S., Ullrich A. Insulin-like growth factor II precursor gene organization in relation to insulin gene family. 1984 Aug 30-Sep 5Nature. 310(5980):777–781. doi: 10.1038/310777a0. [DOI] [PubMed] [Google Scholar]
- Elsberg C. A. EXPERIMENTS ON MOTOR NERVE REGENERATION AND THE DIRECT NEUROTIZATION OF PARALYZED MUSCLES BY THEIR OWN AND BY FOREIGN NERVES. Science. 1917 Mar 30;45(1161):318–320. doi: 10.1126/science.45.1161.318. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Goodyer C. G., De Stéphano L., Lai W. H., Guyda H. J., Posner B. I. Characterization of insulin-like growth factor receptors in rat anterior pituitary, hypothalamus, and brain. Endocrinology. 1984 Apr;114(4):1187–1195. doi: 10.1210/endo-114-4-1187. [DOI] [PubMed] [Google Scholar]
- HOFFMAN H. A study of the factors influencing innervation of muscles by implanted nerves. Aust J Exp Biol Med Sci. 1951 Jul;29(4):289–308. doi: 10.1038/icb.1951.35. [DOI] [PubMed] [Google Scholar]
- Harley C. B. Hybridization of oligo(dT) to RNA on nitrocellulose. Gene Anal Tech. 1987 Mar-Apr;4(2):17–22. doi: 10.1016/0735-0651(87)90013-6. [DOI] [PubMed] [Google Scholar]
- James R., Niall H., Kwok S., Bryand-Greenwood G. Primary structure of porcine relaxin: homology with insulin and related growth factors. Nature. 1977 Jun 9;267(5611):544–546. doi: 10.1038/267544a0. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A. M., Zacks S. I. The fine structure of motor endplate morphogenesis. J Cell Biol. 1969 Jul;42(1):154–169. doi: 10.1083/jcb.42.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lømo T. Requirements for the formation and maintenance of neuromuscular connections. Curr Top Dev Biol. 1980;16:253–281. [PubMed] [Google Scholar]
- Miledi R., Potter L. T. Acetylcholine receptors in muscle fibres. Nature. 1971 Oct 29;233(5322):599–603. doi: 10.1038/233599a0. [DOI] [PubMed] [Google Scholar]
- O'Brien R. A., Ostberg A. J., Vrbová G. Observations on the elimination of polyneuronal innervation in developing mammalian skeletal muscle. J Physiol. 1978 Sep;282:571–582. doi: 10.1113/jphysiol.1978.sp012482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puro D. G., Agardh E. Insulin-mediated regulation of neuronal maturation. Science. 1984 Sep 14;225(4667):1170–1172. doi: 10.1126/science.6089343. [DOI] [PubMed] [Google Scholar]
- Recio-Pinto E., Ishii D. N. Effects of insulin, insulin-like growth factor-II and nerve growth factor on neurite outgrowth in cultured human neuroblastoma cells. Brain Res. 1984 Jun 8;302(2):323–334. doi: 10.1016/0006-8993(84)90246-4. [DOI] [PubMed] [Google Scholar]
- Recio-Pinto E., Rechler M. M., Ishii D. N. Effects of insulin, insulin-like growth factor-II, and nerve growth factor on neurite formation and survival in cultured sympathetic and sensory neurons. J Neurosci. 1986 May;6(5):1211–1219. doi: 10.1523/JNEUROSCI.06-05-01211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rinderknecht E., Humbel R. E. Primary structure of human insulin-like growth factor II. FEBS Lett. 1978 May 15;89(2):283–286. doi: 10.1016/0014-5793(78)80237-3. [DOI] [PubMed] [Google Scholar]
- Rinderknecht E., Humbel R. E. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. 1978 Apr 25;253(8):2769–2776. [PubMed] [Google Scholar]
- Rotwein P., Burgess S. K., Milbrandt J. D., Krause J. E. Differential expression of insulin-like growth factor genes in rat central nervous system. Proc Natl Acad Sci U S A. 1988 Jan;85(1):265–269. doi: 10.1073/pnas.85.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGER F., THOMPSON E. O., KITAI R. The amide groups of insulin. Biochem J. 1955 Mar;59(3):509–518. doi: 10.1042/bj0590509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara V. R., Hall K., Misaki M., Fryklund L., Christensen N., Wetterberg L. Ontogenesis of somatomedin and insulin receptors in the human fetus. J Clin Invest. 1983 May;71(5):1084–1094. doi: 10.1172/JCI110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara V. R., Uvnäs-Moberg K., Uvnäs B., Hall K., Wetterberg L., Posloncec B., Goiny M. The distribution of somatomedins in the nervous system of the cat and their release following neural stimulation. Acta Physiol Scand. 1982 Aug;115(4):467–470. doi: 10.1111/j.1748-1716.1982.tb07105.x. [DOI] [PubMed] [Google Scholar]
- Schwabe C., McDonald J. K. Primary structure of the B-chain of porcine relaxin. Biochem Biophys Res Commun. 1977 Mar 21;75(2):503–510. doi: 10.1016/0006-291x(77)91070-1. [DOI] [PubMed] [Google Scholar]
- Soares M. B., Ishii D. N., Efstratiadis A. Developmental and tissue-specific expression of a family of transcripts related to rat insulin-like growth factor II mRNA. Nucleic Acids Res. 1985 Feb 25;13(4):1119–1134. doi: 10.1093/nar/13.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares M. B., Turken A., Ishii D., Mills L., Episkopou V., Cotter S., Zeitlin S., Efstratiadis A. Rat insulin-like growth factor II gene. A single gene with two promoters expressing a multitranscript family. J Mol Biol. 1986 Dec 20;192(4):737–752. doi: 10.1016/0022-2836(86)90025-2. [DOI] [PubMed] [Google Scholar]
- Stylianopoulou F., Herbert J., Soares M. B., Efstratiadis A. Expression of the insulin-like growth factor II gene in the choroid plexus and the leptomeninges of the adult rat central nervous system. Proc Natl Acad Sci U S A. 1988 Jan;85(1):141–145. doi: 10.1073/pnas.85.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W., Kuffler D. P., Jansen J. K. The effect of prolonged, reversible block of nerve impulses on the elimination of polyneuronal innervation of new-born rat skeletal muscle fibers. Neuroscience. 1979;4(2):271–281. doi: 10.1016/0306-4522(79)90088-5. [DOI] [PubMed] [Google Scholar]
- Thompson W. Synapse elimination in neonatal rat muscle is sensitive to pattern of muscle use. Nature. 1983 Apr 14;302(5909):614–616. doi: 10.1038/302614a0. [DOI] [PubMed] [Google Scholar]





