Abstract
Muscle cells in vitro and in vivo are multinucleated and express acetylcholine receptors (AcChoRs). On innervated cells, the AcChoRs form clusters which lie under the nerve terminals. However, noninnervated cells in culture also express clusters of AcChoR. Both in vivo and in vitro the AcChoR clusters appear to be associated with clusters of nuclei. We have used in situ hybridization to determine whether all the nuclei in cultured chicken embryo myotubes are equally active in expressing the AcChoR alpha subunit message. Cells were hybridized with 35S-labeled probes that contained either both an exon and an intron region or only exon sequences. Control cultures were hybridized with a labeled actin DNA probe or poly(U). The hybrids were detected by emulsion autoradiography; simultaneously, the nuclei were visualized with bisbenzamide. Cells hybridized with the intron/exon probe showed a striking preferential silver grain localization in and around some of the myotube nuclei, whereas those hybridized with the exon probe gave a rather homogeneous grain distribution in the cytoplasm. These results show that myotube nuclei possess differential activation capacities for the expression of AcChoR alpha subunit mRNA and that this difference is due to differential rates of transcription.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Cohen M. W. Nerve-induced and spontaneous redistribution of acetylcholine receptors on cultured muscle cells. J Physiol. 1977 Jul;268(3):757–773. doi: 10.1113/jphysiol.1977.sp011880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt-Jovin D. J., Jovin T. M. Analysis and sorting of living cells according to deoxyribonucleic acid content. J Histochem Cytochem. 1977 Jul;25(7):585–589. doi: 10.1177/25.7.70450. [DOI] [PubMed] [Google Scholar]
- Bruner J. M., Bursztajn S. Acetylcholine receptor clusters are associated with nuclei in rat myotubes. Dev Biol. 1986 May;115(1):35–43. doi: 10.1016/0012-1606(86)90225-3. [DOI] [PubMed] [Google Scholar]
- Chang K. S., Rothblum K. N., Schwartz R. J. The complete sequence of the chicken alpha-cardiac actin gene: a highly conserved vertebrate gene. Nucleic Acids Res. 1985 Feb 25;13(4):1223–1237. doi: 10.1093/nar/13.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander L. L., Rubin L. L. Acetylcholine receptor clustering and nuclear movement in muscle fibers in culture. J Cell Biol. 1987 Jan;104(1):87–95. doi: 10.1083/jcb.104.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S., Goldman D., Heinemann S., Patrick J. Muscle acetylcholine receptor biosynthesis. Regulation by transcript availability. J Biol Chem. 1987 Apr 5;262(10):4911–4916. [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D., Cohen S. A. The distribution of acetylcholine sensitivity over uninnervated and innervated muscle fibers grown in cell culture. Dev Biol. 1973 Mar;31(1):147–162. doi: 10.1016/0012-1606(73)90326-6. [DOI] [PubMed] [Google Scholar]
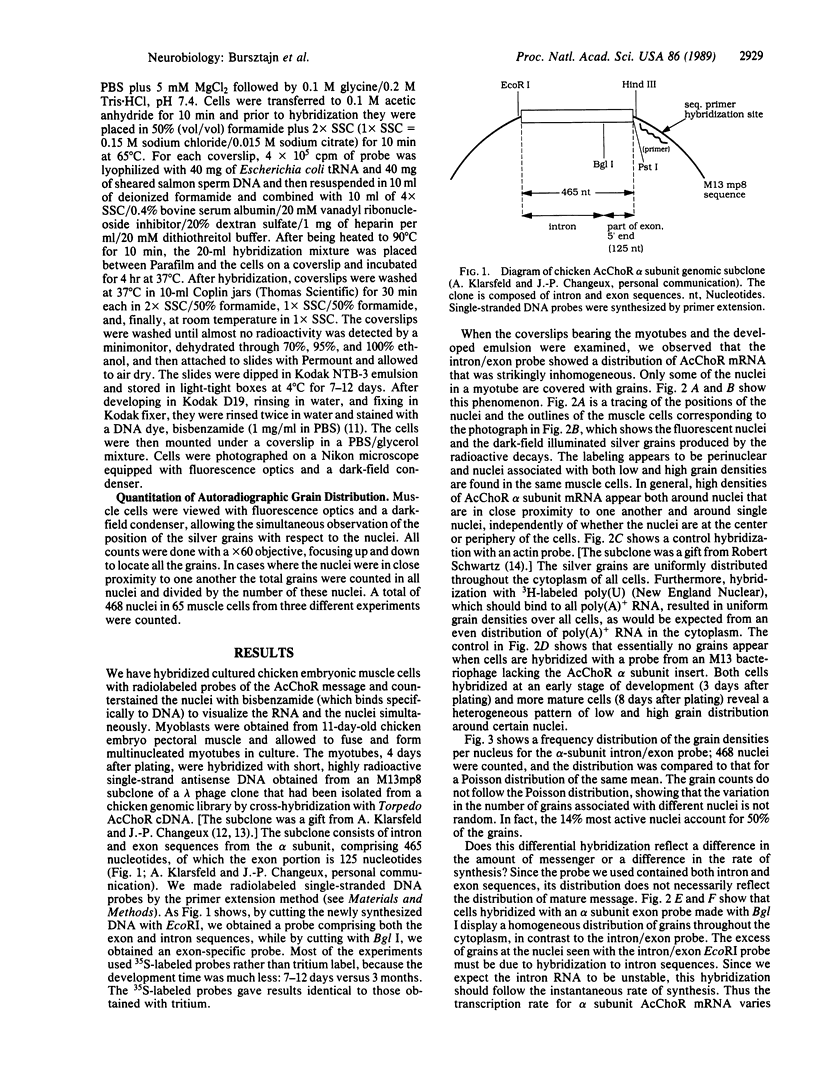
- Fontaine B., Sassoon D., Buckingham M., Changeux J. P. Detection of the nicotinic acetylcholine receptor alpha-subunit mRNA by in situ hybridization at neuromuscular junctions of 15-day-old chick striated muscles. EMBO J. 1988 Mar;7(3):603–609. doi: 10.1002/j.1460-2075.1988.tb02853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E., Fischbach G. D. Early events in neuromuscular junction formation in vitro: induction of acetylcholine receptor clusters in the postsynaptic membrane and morphology of newly formed synapses. J Cell Biol. 1979 Oct;83(1):143–158. doi: 10.1083/jcb.83.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D., Boulter J., Heinemann S., Patrick J. Muscle denervation increases the levels of two mRNAs coding for the acetylcholine receptor alpha-subunit. J Neurosci. 1985 Sep;5(9):2553–2558. doi: 10.1523/JNEUROSCI.05-09-02553.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D., Brenner H. R., Heinemann S. Acetylcholine receptor alpha-, beta-, gamma-, and delta-subunit mRNA levels are regulated by muscle activity. Neuron. 1988 Jun;1(4):329–333. doi: 10.1016/0896-6273(88)90081-5. [DOI] [PubMed] [Google Scholar]
- Harris D. A., Falls D. L., Fischbach G. D. Differential activation of myotube nuclei following exposure to an acetylcholine receptor-inducing factor. Nature. 1989 Jan 12;337(6203):173–176. doi: 10.1038/337173a0. [DOI] [PubMed] [Google Scholar]
- Jackson P. D., Felsenfeld G. A method for mapping intranuclear protein-DNA interactions and its application to a nuclease hypersensitive site. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2296–2300. doi: 10.1073/pnas.82.8.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
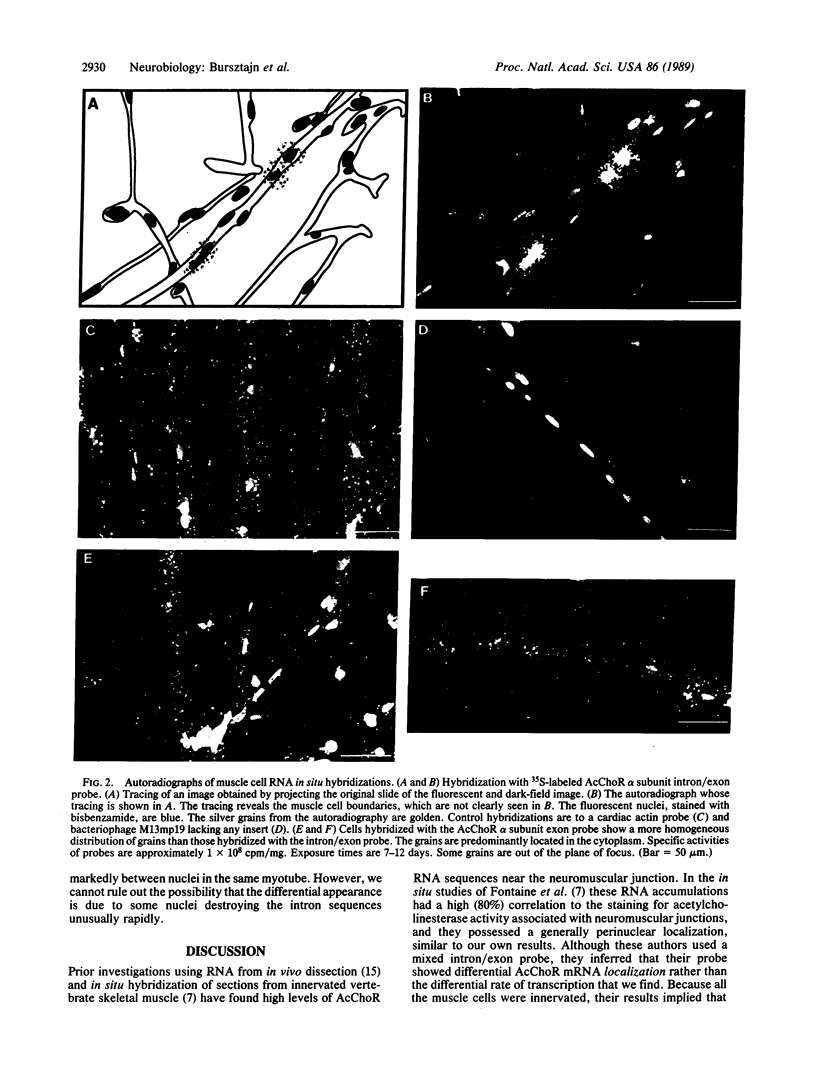
- Klarsfeld A., Changeux J. P. Activity regulates the levels of acetylcholine receptor alpha-subunit mRNA in cultured chicken myotubes. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4558–4562. doi: 10.1073/pnas.82.13.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarsfeld A., Daubas P., Bourachot B., Changeux J. P. A 5'-flanking region of the chicken acetylcholine receptor alpha-subunit gene confers tissue specificity and developmental control of expression in transfected cells. Mol Cell Biol. 1987 Feb;7(2):951–955. doi: 10.1128/mcb.7.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
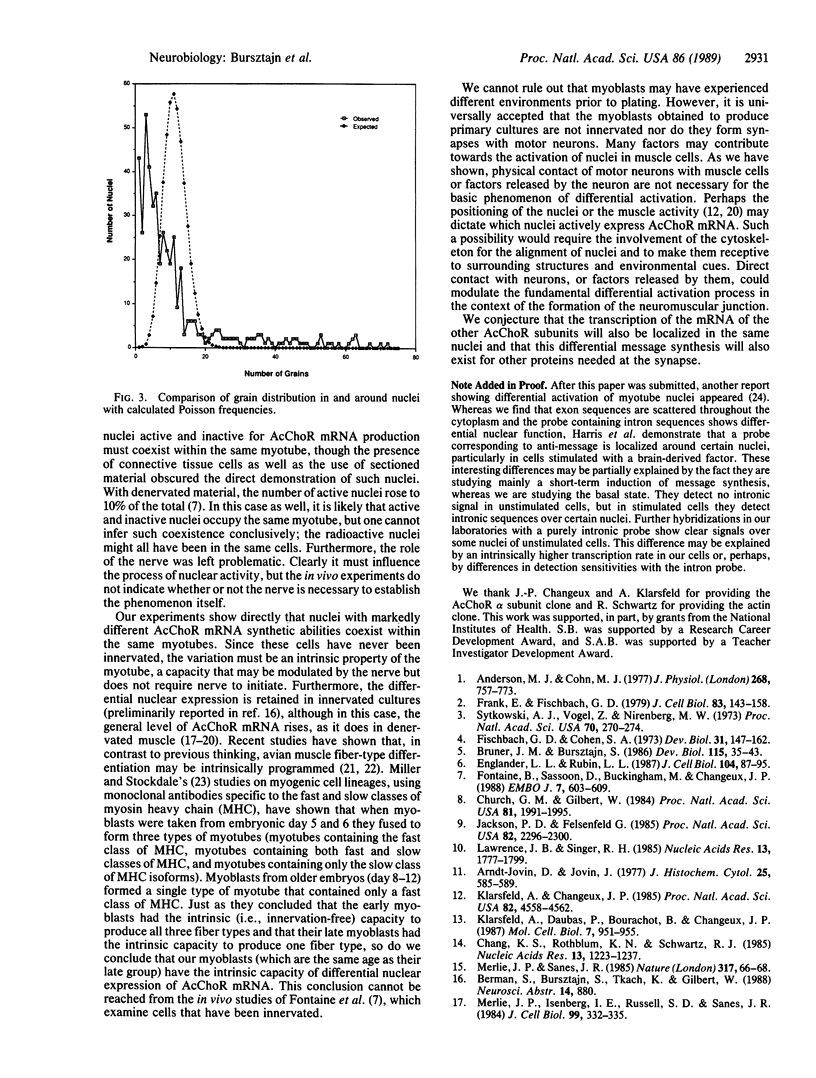
- Lawrence J. B., Singer R. H. Quantitative analysis of in situ hybridization methods for the detection of actin gene expression. Nucleic Acids Res. 1985 Mar 11;13(5):1777–1799. doi: 10.1093/nar/13.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P., Isenberg K. E., Russell S. D., Sanes J. R. Denervation supersensitivity in skeletal muscle: analysis with a cloned cDNA probe. J Cell Biol. 1984 Jul;99(1 Pt 1):332–335. doi: 10.1083/jcb.99.1.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P., Sanes J. R. Concentration of acetylcholine receptor mRNA in synaptic regions of adult muscle fibres. Nature. 1985 Sep 5;317(6032):66–68. doi: 10.1038/317066a0. [DOI] [PubMed] [Google Scholar]
- Miller J. B., Stockdale F. E. Developmental regulation of the multiple myogenic cell lineages of the avian embryo. J Cell Biol. 1986 Dec;103(6 Pt 1):2197–2208. doi: 10.1083/jcb.103.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytkowski A. J., Vogel Z., Nirenberg M. W. Development of acetylcholine receptor clusters on cultured muscle cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):270–274. doi: 10.1073/pnas.70.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]