Abstract
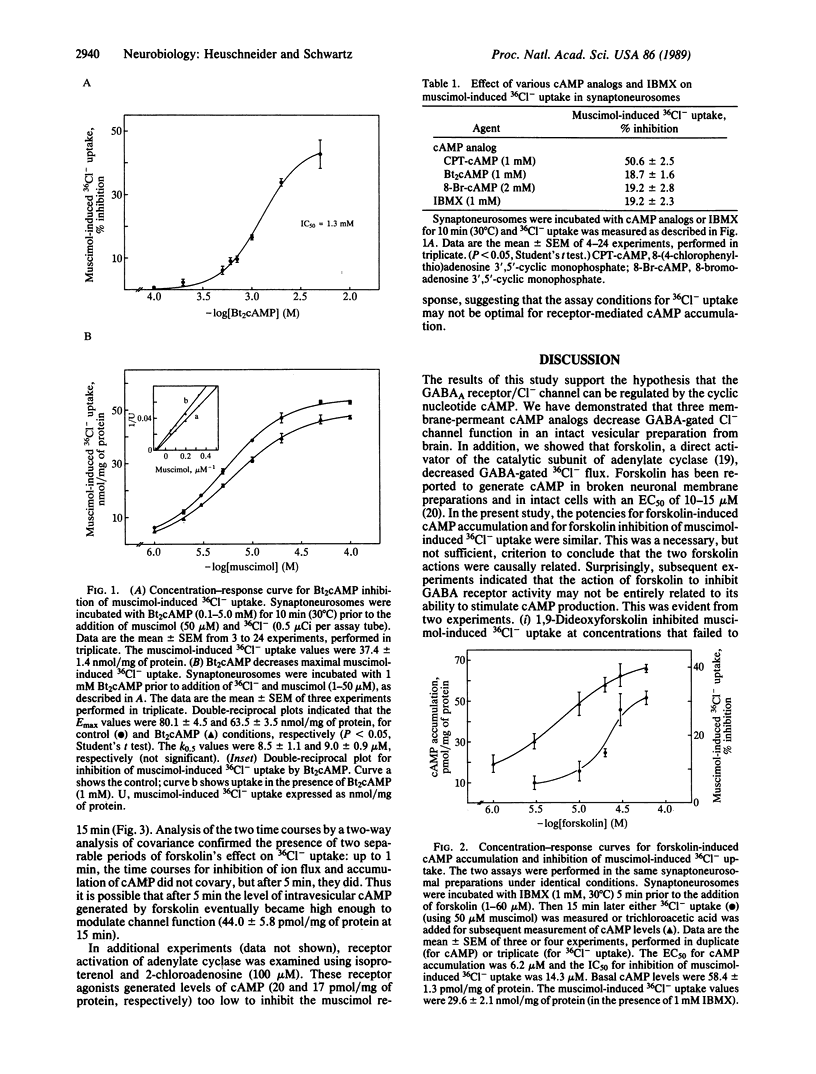
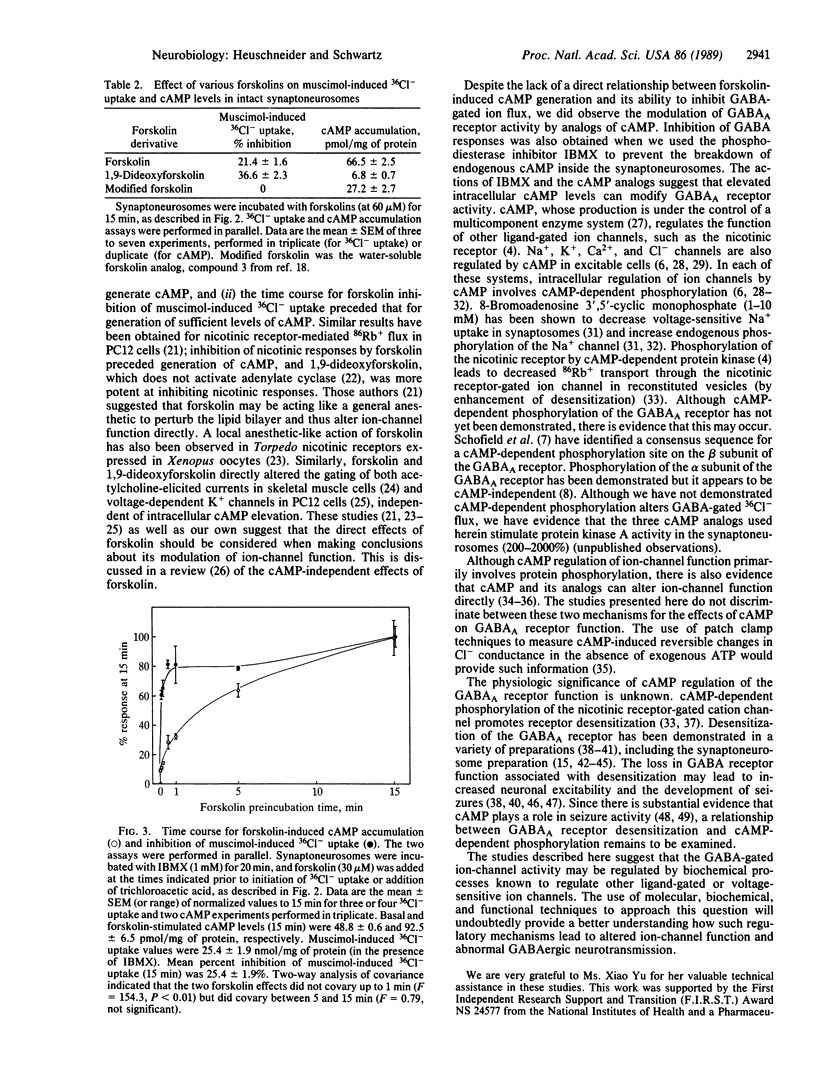
The effects of the cyclic nucleotide cAMP on gamma-aminobutyric acid-gated chloride channel function were investigated. The membrane-permeant cAMP analog N6,O2'-dibutyryladenosine 3',5'-cyclic monophosphate inhibited muscimol-induced 36Cl- uptake into rat cerebral cortical synaptoneurosomes in a concentration-dependent manner (IC50 = 1.3 mM). The inhibition was due to a decrease in the maximal effect of muscimol, with no change in potency. Similar effects were observed with 8-(4-chlorophenylthio)adenosine 3',5'-cyclic monophosphate, 8-bromoadenosine 3',5'-cyclic monophosphate, and the phosphodiesterase inhibitor isobutylmethylxanthine. The effect of endogenous cAMP accumulation on the gamma-aminobutyric acid-gated Cl- channel was studied with forskolin, an activator of adenylate cyclase. Under identical conditions, in the intact synaptoneurosomes, forskolin inhibited muscimol-induced 36Cl- uptake and generated cAMP with similar potencies (IC50 = 14.3 microM; EC50 = 6.2 microM, respectively). Surprisingly, 1,9-dideoxyforskolin, which does not activate adenylate cyclase, also inhibited the muscimol response, suggesting that forskolin and its lipophilic derivatives may interact with the Cl- channel directly. Indeed, forskolin inhibition of muscimol-induced 36Cl- uptake was extremely rapid (within 5 sec), preceding the accumulation of sufficient levels of cAMP. After 5 min, a slower phase of inhibition was seen, similar to the time course for cAMP accumulation. The data suggest that gamma-aminobutyric acid (GABAA) receptor function in brain can be regulated by cAMP-dependent phosphorylation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Deshpande S. S., Aracava Y., Alkondon M., Daly J. W. A possible involvement of cyclic AMP in the expression of desensitization of the nicotinic acetylcholine receptor. A study with forskolin and its analogs. FEBS Lett. 1986 Apr 7;199(1):113–120. doi: 10.1016/0014-5793(86)81235-2. [DOI] [PubMed] [Google Scholar]
- Allan A. M., Harris R. A. gamma-Aminobutyric acid agonists and antagonists alter chloride flux across brain membranes. Mol Pharmacol. 1986 May;29(5):497–505. [PubMed] [Google Scholar]
- Armstrong D., Eckert R. Voltage-activated calcium channels that must be phosphorylated to respond to membrane depolarization. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2518–2522. doi: 10.1073/pnas.84.8.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash D. J., Subbarao K. Desensitization of gamma-aminobutyric acid receptor from rat brain: two distinguishable receptors on the same membrane. Biochemistry. 1987 Dec 1;26(24):7556–7562. doi: 10.1021/bi00398a004. [DOI] [PubMed] [Google Scholar]
- Costa M. R., Catterall W. A. Cyclic AMP-dependent phosphorylation of the alpha subunit of the sodium channel in synaptic nerve ending particles. J Biol Chem. 1984 Jul 10;259(13):8210–8218. [PubMed] [Google Scholar]
- Faingold C. L., Gehlbach G., Caspary D. M. Decreased effectiveness of GABA-mediated inhibition in the inferior colliculus of the genetically epilepsy-prone rat. Exp Neurol. 1986 Jul;93(1):145–159. doi: 10.1016/0014-4886(86)90154-8. [DOI] [PubMed] [Google Scholar]
- Ferrendelli J. A. Roles of biogenic amines and cyclic nucleotides in seizure mechanisms. Adv Neurol. 1986;44:393–400. [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Gyenes M., Farrant M., Farb D. H. "Run-down" of gamma-aminobutyric acidA receptor function during whole-cell recording: a possible role for phosphorylation. Mol Pharmacol. 1988 Dec;34(6):719–723. [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Hollingsworth E. B., McNeal E. T., Burton J. L., Williams R. J., Daly J. W., Creveling C. R. Biochemical characterization of a filtered synaptoneurosome preparation from guinea pig cerebral cortex: cyclic adenosine 3':5'-monophosphate-generating systems, receptors, and enzymes. J Neurosci. 1985 Aug;5(8):2240–2253. doi: 10.1523/JNEUROSCI.05-08-02240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Garber S. S., Aldrich R. W. Effect of forskolin on voltage-gated K+ channels is independent of adenylate cyclase activation. Science. 1988 Jun 17;240(4859):1652–1655. doi: 10.1126/science.2454506. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Delcour A. H., Greengard P., Hess G. P. Phosphorylation of the nicotinic acetylcholine receptor regulates its rate of desensitization. Nature. 1986 Jun 19;321(6072):774–776. doi: 10.1038/321774a0. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Greengard P. cAMP-dependent protein kinase phosphorylates the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1130–1134. doi: 10.1073/pnas.80.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard J. R., Alger B. E. Whole-cell voltage-clamp study of the fading of GABA-activated currents in acutely dissociated hippocampal neurons. J Neurophysiol. 1986 Jul;56(1):1–18. doi: 10.1152/jn.1986.56.1.1. [DOI] [PubMed] [Google Scholar]
- Krnjević K. Desensitization of GABA receptors. Adv Biochem Psychopharmacol. 1981;26:111–120. [PubMed] [Google Scholar]
- Krnjević K. Glutamate and gamma-aminobutyric acid in brain. Nature. 1970 Oct 10;228(5267):119–124. doi: 10.1038/228119a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurenza A., Khandelwal Y., De Souza N. J., Rupp R. H., Metzger H., Seamon K. B. Stimulation of adenylate cyclase by water-soluble analogues of forskolin. Mol Pharmacol. 1987 Jul;32(1):133–139. [PubMed] [Google Scholar]
- Levitan I. B. Phosphorylation of ion channels. J Membr Biol. 1985;87(3):177–190. doi: 10.1007/BF01871217. [DOI] [PubMed] [Google Scholar]
- Li M., McCann J. D., Liedtke C. M., Nairn A. C., Greengard P., Welsh M. J. Cyclic AMP-dependent protein kinase opens chloride channels in normal but not cystic fibrosis airway epithelium. Nature. 1988 Jan 28;331(6154):358–360. doi: 10.1038/331358a0. [DOI] [PubMed] [Google Scholar]
- Ludvig N., Moshé S. L. Cyclic AMP derivatives injected into the inferior colliculus induce audiogenic seizure-like phenomena in normal rats. Brain Res. 1987 Dec 22;437(1):193–196. doi: 10.1016/0006-8993(87)91545-9. [DOI] [PubMed] [Google Scholar]
- McHugh E. M., McGee R., Jr Direct anesthetic-like effects of forskolin on the nicotinic acetylcholine receptors of PC12 cells. J Biol Chem. 1986 Mar 5;261(7):3103–3106. [PubMed] [Google Scholar]
- Meldrum B. S. Epilepsy and gamma-aminobutyric acid-mediated inhibition. Int Rev Neurobiol. 1975;17:1–36. doi: 10.1016/s0074-7742(08)60205-6. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Gold G. H. A cyclic nucleotide-gated conductance in olfactory receptor cilia. 1987 Jan 29-Feb 4Nature. 325(6103):442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Rossie S., Catterall W. A. Cyclic-AMP-dependent phosphorylation of voltage-sensitive sodium channels in primary cultures of rat brain neurons. J Biol Chem. 1987 Sep 15;262(26):12735–12744. [PubMed] [Google Scholar]
- Schofield P. R., Darlison M. G., Fujita N., Burt D. R., Stephenson F. A., Rodriguez H., Rhee L. M., Ramachandran J., Reale V., Glencorse T. A. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987 Jul 16;328(6127):221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Schwartz R. D., Jackson J. A., Weigert D., Skolnick P., Paul S. M. Characterization of barbiturate-stimulated chloride efflux from rat brain synaptoneurosomes. J Neurosci. 1985 Nov;5(11):2963–2970. doi: 10.1523/JNEUROSCI.05-11-02963.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. D., Mindlin M. C. Inhibition of the GABA receptor-gated chloride ion channel in brain by noncompetitive inhibitors of the nicotinic receptor-gated cation channel. J Pharmacol Exp Ther. 1988 Mar;244(3):963–970. [PubMed] [Google Scholar]
- Schwartz R. D., Suzdak P. D., Paul S. M. gamma-Aminobutyric acid (GABA)- and barbiturate-mediated 36Cl- uptake in rat brain synaptoneurosomes: evidence for rapid desensitization of the GABA receptor-coupled chloride ion channel. Mol Pharmacol. 1986 Nov;30(5):419–426. [PubMed] [Google Scholar]
- Schwartz R. D. The GABAA receptor-gated ion channel: biochemical and pharmacological studies of structure and function. Biochem Pharmacol. 1988 Sep 15;37(18):3369–3375. doi: 10.1016/0006-2952(88)90684-3. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7(4):201–224. [PubMed] [Google Scholar]
- Seamon K. B., Vaillancourt R., Edwards M., Daly J. W. Binding of [3H]forskolin to rat brain membranes. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5081–5085. doi: 10.1073/pnas.81.16.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon K., Daly J. W. Activation of adenylate cyclase by the diterpene forskolin does not require the guanine nucleotide regulatory protein. J Biol Chem. 1981 Oct 10;256(19):9799–9801. [PubMed] [Google Scholar]
- Siegelbaum S. A., Camardo J. S., Kandel E. R. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982 Sep 30;299(5882):413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- Stelzer A., Kay A. R., Wong R. K. GABAA-receptor function in hippocampal cells is maintained by phosphorylation factors. Science. 1988 Jul 15;241(4863):339–341. doi: 10.1126/science.2455347. [DOI] [PubMed] [Google Scholar]
- Sweetnam P. M., Lloyd J., Gallombardo P., Malison R. T., Gallager D. W., Tallman J. F., Nestler E. J. Phosphorylation of the GABAa/benzodiazepine receptor alpha subunit by a receptor-associated protein kinase. J Neurochem. 1988 Oct;51(4):1274–1284. doi: 10.1111/j.1471-4159.1988.tb03097.x. [DOI] [PubMed] [Google Scholar]
- Thalmann R. H., Hershkowitz N. Some factors that influence the decrement in the response to GABA during its continuous iontophoretic application to hippocampal neurons. Brain Res. 1985 Sep 9;342(2):219–233. doi: 10.1016/0006-8993(85)91120-5. [DOI] [PubMed] [Google Scholar]
- Tonosaki K., Funakoshi M. Cyclic nucleotides may mediate taste transduction. Nature. 1988 Jan 28;331(6154):354–356. doi: 10.1038/331354a0. [DOI] [PubMed] [Google Scholar]
- Wagoner P. K., Pallotta B. S. Modulation of acetylcholine receptor desensitization by forskolin is independent of cAMP. Science. 1988 Jun 17;240(4859):1655–1657. doi: 10.1126/science.2454507. [DOI] [PubMed] [Google Scholar]
- White M. M. Forskolin alters acetylcholine receptor gating by a mechanism independent of adenylate cyclase activation. Mol Pharmacol. 1988 Oct;34(4):427–430. [PubMed] [Google Scholar]


