SUMMARY
To estimate the frequency of infectious gastroenteritis across Australia, and to identify risk factors, we conducted a national telephone survey of 6087 randomly selected respondents in 2001–2002. The case definition was three or more loose stools and/or two or more vomits in a 24-hour period in the last 4 weeks, with adjustment to exclude non-infectious causes and symptoms secondary to a respiratory infection. Frequency data were weighted to the Australian population. Multivariate logistic regression was used to assess potential risk factors including season, region, demographic and socioeconomic status. Among contacted individuals, 67% responded. The case definition applied to 7% of respondents (450/6087) which extrapolates to 17·2 million (95% CI 14·5–19·9 million) cases of gastroenteritis in Australia in one year, or 0·92 (95% CI 0·77–1·06) cases/person per year. In the multivariate model, the odds of having gastroenteritis were increased in summer and in the warmest state, in young children, females, those with higher socioeconomic status and those without health insurance.
INTRODUCTION
Infectious gastroenteritis is a very common illness. It is a significant cause of morbidity and mortality in underdeveloped countries, especially in children [1, 2]. In developed countries, gastroenteritis is responsible for a high level of lost productivity, social disruption and use of health-care services [3–5]. In addition, there may be considerable unrecognized mortality following gastrointestinal infection [6]. Knowledge of the magnitude of the disease burden and how disease occurs by time, place and person provides a baseline for future monitoring.
Surveillance of illnesses caused by certain pathogens provides information about selected known bacteria or viruses but it cannot provide data on the majority of pathogens causing illness, some of which are as yet unknown [6, 7]. Identification of population groups, seasons and localities where the occurrence of gastroenteritis is higher than average can be usefully combined with data from surveillance of known serious pathogens to guide strategic direction of resources to high-risk areas.
Most estimates of the frequency of gastroenteritis in different populations around the world depend on a definition based on three or more loose stools in 24 h, sometimes with other criteria. The estimated incidence of gastroenteritis in developed countries ranges from 0·2 and 0·3 cases/person per year in the United Kingdom and The Netherlands respectively [3, 7], to 0·8 cases/person per year in Melbourne, Australia and 0·8 in the United States [4, 8]. It is clear that at least some of these differences can be attributed to differences in methodology. For example, the same US data that were used to estimate 0·8 cases/person per year [8], were also used to estimate 1·4 cases/person per year [6] when a different definition of gastroenteritis was applied. This highlights the need for standardization of terms and methods when comparing outcomes.
Enteric pathogens and bacterial enterotoxins can be transmitted to humans via food or water, by person-to-person contact, exposure to animals, or acquired from the environment. Reports from a number of developed countries indicate that rates of gastroenteritis in the community vary by person, place and time, both seasonally and by longer term trends [9–12]. In Australia there are distinct regional differences in climate, environmental conditions and population subgroups and variation in infectious diseases is to be expected across the country. The Northern Territory (NT), a tropical, low-latitude region, has double the incidence of notified cases of salmonellosis compared to the temperate southern states [13]. Surveillance notifications also indicate that campylobacteriosis increases in spring and salmonellosis and shigellosis peak in autumn [13].
However, rates based on public health surveillance are highly dependent on reporting practices which vary across states in Australia and reflect only a small proportion of gastroenteritis from a limited number of pathogens. A more reliable assessment of the change of gastroenteritis seasonally and geographically is only possible if standardized methods are used to collect data and data are collected on all types of episodes regardless of the pathogen. We conducted a national community survey between September 2001 and August 2002 to estimate the number of cases of gastroenteritis nationally and across the states of Australia. A second objective was to identify any regional, seasonal, demographic and socioeconomic risk factors for gastroenteritis.
METHODS
A retrospective cross-sectional design was implemented by applying stratified random sampling across six Australian states and the NT. The Australian Capital Territory was included with New South Wales because of its small population and location entirely within the state (see Fig. 1). All data were collected over a 1-year period by Computer Assisted Telephone Interviews (CATI).
Fig. 1.
States and Territories of Australia.
Study population and sample
The study population comprised all people in Australia living in residential households connected to a land telephone line. Interpreters were provided for six languages; Italian, Greek, Cantonese, Mandarin, Vietnamese and Arabic. The sample frame did not include people in institutions and overseas visitors, those who were unable to answer because of an incapacity such as deafness or intellectual disability, people who did not speak sufficient English to answer the questionnaire and spoke a language other than those where an interpreter was available, and people living in households without a land telephone line.
Random digit dialling was used to select a sample of households within each state or territory. Interviewers asked to speak to the person who had the most recent birthday in each house. Carers answered for children <15 years of age. If the selected respondent was not at home, nine further attempts were made to contact the person at different times of the day before moving on to the next randomly selected household. The sample of 860 in each jurisdiction was spread out over the year to allow assessment of seasonal changes. The target sample size was 6000 in total.
Interviews
Interviewers asked all respondents about their demographic details, occurrence of vomiting and/or diarrhoea in the last 4 weeks, chronic illness, food safety perceptions and socioeconomic status. If the respondent reported diarrhoea or vomiting, they were asked for more details regarding symptoms and timing, use of health-care services, diagnostic methods, treatment practices, and the effect of their illness on work and daily activities. Further details of these data are reported elsewhere [14].
Case definition for analysis
To reduce misclassification of ‘infectious gastroenteritis’, respondents with loose stools and/or vomiting who identified a non-infectious cause for their symptoms (pregnancy, alcohol, chronic illness, medications) were excluded from analysis. There is evidence that some people with a respiratory infection may have symptoms of vomiting/diarrhoea [15, 16]. An adjustment was therefore made to take account of a proportion of people with respiratory infections with secondary gastrointestinal symptoms. Individuals reporting respiratory symptoms in addition to loose stools/vomiting were only included if the gastrointestinal symptoms were at a higher threshold. We defined infectious gastroenteritis as at least three loose stools or two vomits in 24 h, or at least four loose stools or three vomits in 24 h if respiratory symptoms were present.
Quality control
Interviewers were trained and monitored for response rates and maintenance of random selection in each state. Small quality-control studies were run in parallel with the main survey and are discussed elsewhere [14].
Approval for the conduct of this work was obtained from the ethics committees of the Australian Government Department of Health and Ageing, Australian National University and various state government health departments.
Analysis
The first aim of the analysis was to estimate the annual number of cases of infectious gastroenteritis in Australia in a single year. The period prevalence in the last 4 weeks was also estimated by region, season and socio-demographic status. These estimates were adjusted for known differences between the survey sample and the target population by weighting to the 2001 resident population for age, sex and household size [17]. A program from the Australian Bureau of Statistics was used, which utilized generalized regression estimation and iterative proportional fitting to population benchmarks. Standard errors were produced by a jackknife technique [18].
The second aim was to identify risk factors for gastroenteritis. Multivariate logistic regression was used to produce odds ratios (ORs) for each of the geographic, demographic and socioeconomic variables while controlling for the possible confounding influence of other factors in the dataset. To do this each factor was treated as a categorical variable, and because of the potential for all factors to exert a confounding influence, all variables were forced into the model. The effect of regional stratified sampling was accounted for by including state in the model. There was no clustering in the design. Weighting was not included in the logistic regression since the purpose of the analysis was to identify relative ORs among risk factors. Analysis was performed using the statistical packages SPSS version 11 [19], and stata version 8.2 [20].
RESULTS
Overall, 67% (6087/9085) of contacted households participated in the survey.
Period prevalence
Any diarrhoea or vomiting in the 4 weeks prior to the survey was reported by 11·2% (unweighted: 683/6087) of respondents, and 7·4% (unweighted: 450/6087) met the criteria for the definition of infectious gastroenteritis.
Males reported less gastroenteritis at 6·3% [95% confidence interval (CI) 4·7–7·8] compared to females at 7·7% (95% CI 6·1–9·4). The prevalence across states varied from New South Wales with 6·6% (95% CI 4·7–8·6) to the NT at 9·6% (95% CI 6·3–12·9). The prevalence of gastroenteritis was highest in young children at 11% (95% CI 5·2–16·8). Among adults >70 years only 3% (95% CI 0·8–5·7) reported gastroenteritis. The prevalence among Indigenous respondents was 14·9% (95% CI 2·5–27·2).
Extrapolation of the data to the Australian population indicated that there were 17·2 million cases (95% CI 14·5–19·9) of infectious gastroenteritis in Australia in one year, equating to an incidence of 0·92 (95% CI 0·77–1·06) cases of gastroenteritis per person per year.
Risk factors for infectious gastroenteritis
The OR of having gastroenteritis when all socio-demographic, regional and seasonal factors were in a multivariate logistic regression model are shown in the Table.
Table.
Logistic regression model* showing association between gastroenteritis and region, season and demographic/socioeconomic factors

OR, Odds ratio; CI, confidence interval.
Estimates were generated by forcing all risk factors into the model.
Region
The NT was identified as a statistically significant risk factor in the multivariate logistic regression with an OR of 1·8 when compared with Queensland. All other states had a non-significant OR of <1 compared with Queensland.
Living in urban areas compared with rural was not identified as a statistically significant risk factor. However, there was variation by state. Among urban respondents, the prevalence of gastroenteritis was high in the NT (12%) compared to 6–7% for all other jurisdictions. Among rural respondents, the prevalence was high in New South Wales (9·5%) and the NT (9%) compared to 5–6% in all other jurisdictions. Unlike other jurisdictions, the rural prevalence in New South Wales was considerably higher than the urban level (data not shown).
Season
The multivariate model indicates a statistically significant variation in gastroenteritis across seasons (P=0·02) with a greater odds of gastroenteritis in summer compared with spring (OR 1·2) and a lower odds in autumn (OR 0·7). The odds of gastroenteritis in winter was similar to spring.
Age and sex
A statistically significant difference in risk across age groups was identified in the model (P<0·001). When comparing the odds of gastroenteritis with children 0–4 years of age, most adult age groups had an OR of ∼0·5 or less, with a slightly higher OR of ∼0·6–0·7 for adults aged 15–45 years.
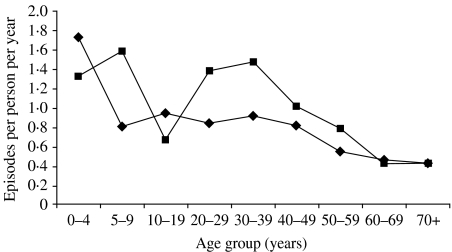
Being female had an OR of 1·3 (P=0·01). This was largely due to a higher rate of gastroenteritis among women aged 20–40 years (Fig. 2).
Fig. 2.
Incidence of gastroenteritis per person per year, by age and sex (weighted to the Australian population). –✦–, Males; –▪–, females.
Eighteen percent of households where a woman aged 25–45 years had gastroenteritis in the last 4 weeks also had at least one child <5 years who had had gastroenteritis. This compares with only 5% of households where women of the same age did not have gastroenteritis.
Indigenous status
When controlling for state in the multivariate logistic model, Indigenous status was not significant due to small numbers of Indigenous respondents.
Household size
The variation in gastroenteritis across households was not significant when controlling for age in the multivariate model (Table).
Socio-economic status
The association between gastroenteritis and the socioeconomic explanatory variables of income, education and health insurance was complex. The multivariate analysis showed that the variation across income groups was significant (P=0·02) although increasing income was not associated with a consistent trend of increasing risk of gastroenteritis. Compared with the other income groups, households with incomes over $AUD100 000 had increased odds of reporting gastroenteritis.
The prevalence of gastroenteritis was higher in respondents living in households with a higher education level over year 10 at high school, although there was not a consistent trend to increasing risk of gastroenteritis from lowest to highest education level. Figure 3 shows the relationship between education and income: for those with higher education, income had little effect. For those with lower education, reported gastroenteritis showed a U-shaped relationship with income with higher levels of gastroenteritis among the lowest and highest income households.
Fig. 3.
Period prevalence of gastroenteritis in the past 4 weeks, by income and education (education level: –✦–, < year 10; –▪–, ⩾ year 11), Australia, 2001–2002 (weighted to the Australian population).
Those without health insurance were more likely to report gastroenteritis (Fig. 4). Gastroenteritis was highest in those without health insurance but with high incomes.
Fig. 4.
Period prevalence of gastroenteritis in the past 4 weeks, by health insurance (–▪–, yes; –✦–, no insurance) and income, Australia, 2001–2002 (weighted to the Australian population).
DISCUSSION
The survey clearly demonstrates a massive burden of gastroenteritis in Australia, with ∼17·2 million episodes in a single year (95% CI 14·5–19·9 million). Australia has an annual incidence rate of 0·92 (95% CI 1·24–1·53) similar to results from studies in the United States and Canada [8, 21], and higher than the United Kingdom, Ireland and The Netherlands [3, 7, 22]. However, the different case definitions and methodologies used in these studies make comparison problematic. The UK and The Netherlands gastroenteritis studies were a prospective longitudinal design while cross-sectional telephone interviews were used in Australia, Ireland and the United States. The UK study included a check component to compare the incidence based on a retrospective recall method similar to that used in the US and Australian studies, and found that the incidence was about 0·6 cases/person per year by this methodology, compared with 0·2 cases/person per year using the prospective diary method. Whether prospective studies might tend to cause an underestimate, possibly due to participants having to supply a stool sample and, therefore, being reluctant to report symptoms, or whether a recall method causes an overestimate due to ‘telescoping’ events into a shorter time frame is unknown. Clearly, differences in data collection methods have an impact on the final estimate. The definition of gastroenteritis should be the same when comparing across countries and times, as even an apparently small change in the definition can cause a large impact on the incidence.
The NT had the highest level of infectious gastroenteritis in Australia. This is Australia's warmest state and has a population comprising ∼30% Aboriginal people, while most other states are about 2% Indigenous [17]. The small numbers of Indigenous respondents in most states makes interpretation of the influence of Indigenous status difficult and since the survey was by telephone, remote Indigenous communities would not have been included. The NT rates were higher than elsewhere for both the Indigenous and non-Indigenous respondents which suggests that overall environmental factors in the NT, such as a widespread environmental exposure to pathogens or warmer climate, are probable influences on increased gastroenteritis in this state.
Seasonally, when controlling for state, socioeconomic and demographic factors, the likelihood of gastroenteritis was highest in summer. Together with the higher levels in the warmest region, this suggests that climatic variation may influence gastroenteritis in Australia. A number of studies suggest that longer term warming due to climate change may increase the rates of gastroenteritis [23–26]. However, it is interesting to note that the timing of seasonal peaks of illness due to certain pathogens do not occur in summer; for example, campylobacteriosis increases in the spring [27] and rotavirus infection increases in winter [28, 29]. Mechanisms of seasonality are thought to vary for the different pathogens; some multiply in food under warmer conditions [30] while others have enhanced spread in drier conditions [31]. Others have increased carriage in animals in certain seasons [32], while some have enhanced replication in warmer sea water [33]. There could also be seasonal changes in the contamination of raw-food products, water supplies or general environment; for example, due to increased contaminated water run-off at certain times of the year when rainfall is high. Our understanding of the causal pathway underlying the association between gastroenteritis and the weather is still speculative and it is also likely that there are many as yet unknown pathogens with differing mechanisms of seasonality. Investigation of the causes of the summer-dominant rate of gastroenteritis should be a priority for future research and surveillance.
The age groups most at risk of acquiring gastroenteritis in Australia were children <5 years of age and women aged between 20 and 40 years of age, which was similar to findings in the United States [8, 34]. It is likely that the behaviour of young children may increase their exposure to pathogens via person-to-person and environmental transmission. Women with gastroenteritis who were aged between 20 and 40 years, had a higher probability of having a young child with gastroenteritis in the house, suggesting that transmission between a child and their carer may be an issue. In contrast, teenagers reported a ‘lower’ prevalence of gastroenteritis than children and young adults which may reflect a reluctance to self-report gastroenteritis. In younger teenagers up to 15 years, parents who answered the questionnaire may be unaware of symptoms in their adolescents. The elderly have a lower risk of contracting gastroenteritis, possibly due to conservative and safe food habits.
Identification of groups of people vulnerable to gastroenteritis is useful to guide resource allocation. Young children are clearly at risk, and potentially their parents also, so promotion of high-level hygiene in childcare centres and in the home is important. Older people living in the community do not appear at risk of increased episodes of gastroenteritis and are, therefore, not a high priority for public health prevention. Resources might be better targeted to other known high-risk areas, such as nursing homes.
There was a tendency for people with higher household incomes over $AUD100 000 per year and higher education levels to be more likely to report gastroenteritis, a finding similar to that in the United States [8]. This is possibly due to a reporting bias, with more wealthy and better-educated households more likely to perceive that they have symptoms, or may be due to more risky behaviours in this group, such as eating out more frequently, and eating higher risk foods like soft cheeses and rare meat. Within each income category, those with health insurance were less likely to report gastroenteritis. Possibly those with health insurance are more conscious about health and take more care with food practices. Further study of the interactions between education, income and health insurance may help explain the relationship between these variables.
In summary, this study identified that gastroenteritis imposes a significant burden across Australia. Certain factors are associated with an increased likelihood of gastroenteritis and these findings can assist in targeting further investigation and, ultimately, strategic interventions to reduce the incidence in certain groups. Examination of the kind of transmission that is most important for the spread of gastroenteritis in warmer ambient temperatures is warranted. Likewise, investigation of child–adult transmission pathways and how to interrupt these would be beneficial. Comparison of food preparation and consumption patterns in high- and low-risk age groups may identify ‘safe’ and ‘unsafe’ practices. Further study of the high level of gastroenteritis in those of higher socioeconomic status may help elucidate whether this is an effect of greater reporting or riskier habits. These findings can assist in targeting interventions to reduce the incidence of gastroenteritis in high-risk groups.
ACKNOWLEDGEMENTS
We thank Edmond Hsu and Niels Becker for assistance with the weighting and statistical advice; Anne Taylor from the Department of Human Services of South Australia for assisting with organizing data collection; Harrison's Health Research Pty Ltd for conducting the interviews; Scott Cameron and David Jordan for helpful comments on the manuscript. The Australian Government Department of Health & Ageing provided funding for this study. The questionnaire was modified from population survey instruments developed by the FoodNet active surveillance programme of the Centers for Disease Control and Prevention in the United States.
APPENDIX. The OzFoodNet Working Group
At the time this study concluded the OzFoodNet Working Group comprised: G. V. Hall, M. D. Kirk, R. Ashbolt, R. Stafford, K. Lalor, Robert Bell, Barry Combs, Scott Crerar, Craig B. Dalton, Karen Dempsey, Rod Givney, Joy Gregory, Brigid Hardy, Geoff Hogg, Janet Li, Tony Merritt, Ian McKay, Geoff Millard, Lillian Mwanri, Jennie Musto, Leonie Neville, Jane Raupach, Paul Roche, Mohinder Sarna, Craig Shadbolt, Nola Tomaska, Leanne Unicomb, Kefle Yohannes, Craig Williams, and Jenny Williams.
REFERENCES
- 1.Black RE, Brown KH, Becker S, Yunus M. Longitudinal studies of infectious diseases and physical growth of children in rural Bangladesh: I patterns of morbidity. Am J Epidemiol. 1982;115:305–314. doi: 10.1093/oxfordjournals.aje.a113307. [DOI] [PubMed] [Google Scholar]
- 2.Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;8:197–204. [PMC free article] [PubMed] [Google Scholar]
- 3.de Wit MA, Hoogenboom-Verdegaal AM, Goosen ES, Sprenger MJ, Borgdorff MW. A population-based longitudinal study on the incidence and disease burden of gastroenteritis and Campylobacter and Salmonella infection in four regions of The Netherlands. Eur J Epidemiol. 2000;16:713–718. doi: 10.1023/a:1026754218713. [DOI] [PubMed] [Google Scholar]
- 4.Hellard ME, Sinclair MI, Harris AH, Kirk M, Fairley CK. Cost of community gastroenteritis. J Gastroenterol Hepatol. 2003;18:322–328. doi: 10.1046/j.1440-1746.2003.02959.x. [DOI] [PubMed] [Google Scholar]
- 5.Withington SG, Chambers ST. The cost of campylobacteriosis in New Zealand in 1995. N Z Med J. 1997;110:222–224. [PubMed] [Google Scholar]
- 6.Mead PS, Slutsker L, Dietz V et al. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wheeler JG, Sethi D, Cowden JM et al. Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. Br Med J. 1999;318:1046–1050. doi: 10.1136/bmj.318.7190.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herikstad H, Yang S, Van Gilder TJ et al. A population-based estimate of the burden of diarrhoeal illness in the United States: FoodNet, 1996–7. Epidemiol Infect. 2002;129:9–17. doi: 10.1017/s0950268801006628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FoodNet. Incidence of foodborne illnesses: preliminary data from the Foodborne Diseases Active Surveillance Network (FoodNet) – United States, 1998. Morb Mortal Wkly Rep. 1999;48:189–194. [PubMed] [Google Scholar]
- 10.Neimann J, Engberg J, Molbak K, Wegener HC. A case-control study of risk factors for sporadic campylobacter infections in Denmark. Epidemiol Infect. 2003;130:353–366. doi: 10.1017/s0950268803008355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skirrow MB. A demographic survey of campylobacter, salmonella and shigella infections in England. A Public Health Laboratory Service Survey. Epidemiol Infect. 1987;99:647–657. doi: 10.1017/s0950268800066504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.OzFoodNet. Foodborne disease in Australia. Incidence, notifications and outbreaks. Annual report of the OzFoodNet network, 2002. Commun Dis Intell. 2003;27:209–243. doi: 10.33321/cdi.2003.27.45. [DOI] [PubMed] [Google Scholar]
- 13.NNDSS. 2003. http://www.cda.gov.au/surveil/index.htm. http://www.cda.gov.au/surveil/index.htm National Notifiable Diseases Surveillance System Database. Australian Department of Health and Ageing, ). Accessed June 2003.
- 14.Hall G. National Centre for Epidemiology and Population Health, Australian National University; Canberra: 2004. and the OzFoodNet Working Group. Results from the national gastroenteritis survey 2001–2002. NCEPH Working Paper Number 50. [Google Scholar]
- 15.Monto AS, Koopman JS. The Tecumseh study. XI. Occurrence of acute enteric illness in the community. Am J Epidemiol. 1980;112:323–333. doi: 10.1093/oxfordjournals.aje.a112998. [DOI] [PubMed] [Google Scholar]
- 16.Leder K, Sinclair MI, Mitakakis TZ, Hellard ME, Forbes A. A community-based study of respiratory episodes in Melbourne, Australia. Aust N Z J Public Health. 2003;27:399–404. doi: 10.1111/j.1467-842x.2003.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 17.ABS. http://www.abs.gov.au/ausstats/abs@census.nsf. 2003. http://www.abs.gov.au/ausstats/abs@census.nsf Australian Census 2001. Basic community profile series. Canberra. Australian Bureau of Statistics; 2002 ( ). Accessed June .
- 18.ABS. Canberra: 2001. Generalized regression estimator method and jackknife approach to estimation of standard errors. : Household Surveys Facilities, Australian Bureau of Statistics; . Program written by P Bell. [Google Scholar]
- 19.SPSS Inc. Chicago, IL, USA: 2002. SPSS for Windows release 11.5.0. [Google Scholar]
- 20.Stata Corp. College Station, TX, USA: 2003. Intercooled STATA 8.2 for Windows. [Google Scholar]
- 21.Majowicz SE, Dore K, Flint JA et al. Magnitude and distribution of acute, self-reported gastrointestinal illness in a Canadian community. Epidemiol Infect. 2004;132:607–617. doi: 10.1017/s0950268804002353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scallan E, Fitzgerald M, Collins C et al. Acute gastroenteritis in northern Ireland and the Republic of Ireland: a telephone survey. Commun Dis Public Health. 2004;7:61–67. [PubMed] [Google Scholar]
- 23.D'Souza RM, Becker NG, Hall G, Moodie KB. Does ambient temperature affect foodborne disease. Epidemiology. 2004;15:86–92. doi: 10.1097/01.ede.0000101021.03453.3e. [DOI] [PubMed] [Google Scholar]
- 24.Bentham G, Langford IH. Climate change and the incidence of food poisoning in England and Wales. Int J Biometeorol. 1995;39:81–86. doi: 10.1007/BF01212585. [DOI] [PubMed] [Google Scholar]
- 25.Checkley W, Epstein LD, Gilman RH et al. Effects of El Nino and ambient temperature on hospital admissions for diarrhoeal diseases in Peruvian children. Lancet. 2000;355:442–450. doi: 10.1016/s0140-6736(00)82010-3. [DOI] [PubMed] [Google Scholar]
- 26.McMichael AJ, Campbell-Lendrum DH, Kovats RS 2002. , et al. Comparative risk assessment for the effects of climate change on human health.
- 27.Hall GV, D'Souza RM, Kirk MD. Foodborne disease in the new millennium: out of the frying pan and into the fire. Med J Aust. 2002;177:614–618. doi: 10.5694/j.1326-5377.2002.tb04984.x. [DOI] [PubMed] [Google Scholar]
- 28.Barnes GL, Uren E, Stevens KB, Bishop RF. Etiology of acute gastroenteritis in hospitalized children in Melbourne, Australia, from April 1980 to March 1993. J Clin Microbiol. 1998;36:133–138. doi: 10.1128/jcm.36.1.133-138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Velasco AC, Mateos ML, Mas G, Pedraza A, Diez M, Gutierrez A. Three-year prospective study of intestinal pathogens in Madrid, Spain. J Clin Microbiol. 1984;20:290–292. doi: 10.1128/jcm.20.2.290-292.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hocking A, Arnalod G, Jenson I Foodborne microorganisms of public health significance. Sydney: Australian Institute of Food Science and Technology. Food Microbiology Group: NSW Branch; 1997. , et al. ( [Google Scholar]
- 31.Guerrant RL, Kirchhoff LV, Shields DS et al. Prospective study of diarrheal illnesses in northeastern Brazil: patterns of disease, nutritional impact, etiologies, and risk factors. J Infect Dis. 1983;148:986–997. doi: 10.1093/infdis/148.6.986. [DOI] [PubMed] [Google Scholar]
- 32.Bang D, Nielsen D, Knudsen EM, Madsen K. A one-year study of campylobacter carriage by individual Danish broiler chickens as the basis for selection of Campylobacter spp. strains for a chicken infection model. Epidemiol Infect. 2003;130:323–333. doi: 10.1017/s095026880200821x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rose JB, Epstein PR, Lipp EK et al. Climate variability and change in the United States: potential impacts on water- and foodborne diseases caused by microbiologic agents. Environ Health Perspect. 2001;109(Suppl 2) yes:211–221. doi: 10.1289/ehp.01109s2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imhoff B, Morse D, Shiferaw B et al. The burden of self-reported acute diarrheal illness in FoodNet surveillance areas, 1998–1999. Clin Infect Dis. 2004;38(Suppl 3) yes:S219–S226. doi: 10.1086/381590. [DOI] [PubMed] [Google Scholar]