SUMMARY
The risk for a pathogen to cross the species barrier depends on the rate of efficient contacts between the species. However, contact rates between species have rarely been estimated from observations. Here we estimate contact rates and exposure of chamois Rupicapra rupicapra and Alpine ibex Capra ibex exposed to domestic pasteurellosis and brucellosis carried by sheep or cattle herds summering in mountain pastures. We use field observation data on animal positions treated in a geographic information system (GIS). Comparing 10 pastures, we show that the management of domestic herds influences the risk of inter-species transmission. Exposure to direct transmission of pasteurellosis is high when herds are not guarded nor enclosed, whereas exposure to indirect transmission of brucellosis is increased on epidemiological dangerous points such as salt deposits. Our preliminary results need further investigation, but they underline the importance of both herd management and pathogen transmission mode when the aim is to reduce the risk of contamination of wild populations by a pathogen associated with domestic pathogens.
INTRODUCTION
Horizontal inter-species transmission is a central mechanism in the emergence of diseases in wild-living populations [1–4]. The probability for a pathogen to cross the species barrier from a ‘source’ to a ‘receptor’ species is likely to depend on the susceptibility of the receptor and the rate of efficient contacts between the species [5–7], which in turn depend on the relationships between the two species. Competition is favourable to the transmission of parasites that may be able to infect both competitors. However, it is supposed to lead to specific avoidance behaviours [8]. If individuals modify their spatial behaviour when they are in contact with the other species, then contact rates cannot be inferred only from the presence of both species in a given area and field studies are necessary to estimate them. However, contacts rates between species have rarely been estimated from observations [9–11].
In mountainous areas, the abundance of domestic herds and the increase of wild-living populations, partly due to human manipulation such as introduction or reinforcing, lead to novel cohabitation situations. The spillover of disease from domestic to wild-living ungulates has been largely reported during the last 20 years [6, 12–15]. Domestic and wild-living ungulates are competitors for food, which results in pasture sharing and, thus, to the transmission of parasites, especially indirectly transmitted ones. However, the spatial behaviour of individuals may be variable according to the species considered, environmental conditions (natural barriers) and human management of wild and domestic herds. In mountain pastures, these conditions are variable and can be compared. Mountain ungulates thus appear as a good biological model to study inter-species transmission and develop methods that could be adapted to other situations.
As a case study, we considered two pathogenic bacteria differing in their transmission mode (direct vs. indirect) and for which domestic herds may serve as reservoirs of infection for the chamois (Rupicapra rupicapra) and the Alpine ibex (Capra ibex), two ungulate species phylogenetically related to domestic ruminants and largely present in the French Alps. The first model is the agent of pasteurellosis in domestic ruminants, Mannheimia haemolytica (M.h.; formerly known as Pasteurella haemolytica), thereafter called domestic pasteurellosis. The bacteria are transmitted by ‘nose to nose’ contact or inhalation of aerosols [16]. In domestic ruminants, M.h. is usually present in the upper respiratory tract but it may cause disease after stress or primary infection. The clinical expression is acute with general and respiratory symptoms in adult animals and bacteraemia in lambs [17]. In northern America, the transmission of M.h. from healthy carrier sheep to highly susceptible bighorn sheep Ovis canadensis caused several deadly outbreaks of bronchopneumonia [18–20]. Management plans now include buffer zones between domestic sheep and bighorn sheep to avoid pasteurellosis transmission [6]. The transmission of M.h. from sheep to wild chamois is suspected in alpine mountains where outbreaks of acute bronchopneumonia occurred in adult populations of chamois in 1976 [21], 1998 [22] and 2001 (Gauthier, personal communication).
The second pathogen we considered was Brucella spp., which causes brucellosis in domestic ruminants (B. melitensis biovar. 3 in sheep and B. abortus biovar. 1 in cattle). Brucellosis is transmitted by direct or indirect contacts with infected genital secretions or abortion products, Brucella being able to survive for several months on the ground [23]. Because brucellosis may entail economic losses to stock farming and cause severe disease in humans, prophylactic measures have been undertaken in France to eradicate brucellosis and as a result, the prevalence of B. melitensis and B. abortus in domestic livestock is now very low, except in ovine herds in south-eastern France where 5% of herds were still infected in 2000 [24]. However, outbreaks of brucellosis in chamois and Alpine ibex have been reported since 1990 and in all cases the source of infection is thought to be domestic animals [25, 26].
In this paper, we aim to estimate rates of inter-specific contacts between wild and domestic ungulates living in the alpine mountains of France and, taking into account the potential emission of pathogens by domestic animals and to estimate risk of exposure of chamois and Alpine ibex to pathogens harboured by the domestic animals. We also study whether population characteristics (species and population size), domestic herd management (pastoral practices) and external factors (hours) influence exposure to disease.
MATERIALS AND METHOD
Study area and data collection
This study is part of a research programme dedicated to the risk of pathogen spillover between wild and domestic ruminants. For this programme, data have been collected in different areas of the National Park at La Vanoise, the wild game and wildlife reserve at Les Bauges [27] and massifs of Belledonne and Beaufort [28]. All areas are situated in the northern French Alps, which are the natural range for the chamois and Alpine ibex. In the present study, we analysed data collected between 1996 and 2001 in the central area of the Vanoise National Park, where pastoral activities are permitted but hunting prohibited. Field observations of 10 pastoral situations where inter-species contacts and exposure were possible (observation units, Table 1) were carried out during the grazing season between June and October. The sites were chosen because they were representative of the different pastoral practices in the area [29, 30] and they were easily accessible.
Table 1.
Description of the observation units and available data (for ibex locations ‘absence’ mean that no ibex live on the mountain pasture). The mean number of chamois or ibex is calculated per year; minimum and maximum values are given when observations have been repeated many years on one site
The observations lasted 1–5 consecutive days and were repeated 1–5 times over the course of a summer. The observer was stationed at a viewpoint from which he/she could observe the whole mountain-side including the pasture. The observer scanned the mountain-side using a field glass and reported the positions of wild and domestic ungulates on a map every 3 h from 06:00 to 21:00 hours. Points represented groups of wild ungulates and polygons represented the area occupied by domestic individuals. The observer noted the species and group size for each point or polygon.
Paper maps were manually digitized and imported into a GIS to generate spatial objects into a vector model: points for wild (‘wild points’) and polygons for sheep or cattle (‘domestic polygons’). Each geographical feature was linked to specific attribute information: hour, date, species and number of animals. The spatial objects were manipulated using the GIS software Arcview 3.1¯ and its script Nearest Feature extension [31], allowing us to calculate all distances separating wild points from domestic polygons. Situations corresponding to direct or indirect contacts were extracted from the GIS and processed in a tabular form.
Direct contact rates and exposure to pasteurellosis
We first estimated the contact rate between wild and domestic ungulates and then the degree of exposure (thereafter called exposure). Dixon et al. [32] showed that strains of M.h. nebulized into a wind tunnel can remain viable over a distance of ∼20 m, suggesting that an aerosol is potentially infectious up to 20 m away from an infected source. This estimate is in accordance with previous data on the low survival of M.h. in aerosols [33]. Therefore, we assumed that a direct contact occurs when the simultaneous locations of a wild individual and a domestic polygon were within a 20-m horizontal distance. This hypothesis assumes that (1) bacterial survival in mountain areas can be estimated from the in vitro experiments and (2) the vertical distance can be neglected.
To estimate contact rates, we followed the principle of Courtenay et al. [9] but did not use longitudinal data. Instead we pooled all observations of wild individuals made at one observation unit to estimate the contact rate between the wild ‘exposed population’ of observation unit i, i.e. wild animals using the mountain-side, and the domestic herd. We thus assumed that the observed wild individuals constitute a homogeneous group regarding the rate of contact. For each observation scan j, we obtained the number of with individuals in direct contact with livestock nij and the total number of wild individuals observed Nj. We estimated the direct contact rate of the wild exposed population i as the total number of contacts observed with population i, Σjnij divided by the total number of locations of wild individuals recorded in the area N=ΣjNj. The direct contact rate for the wild population of observation unit i thus reads [9]:
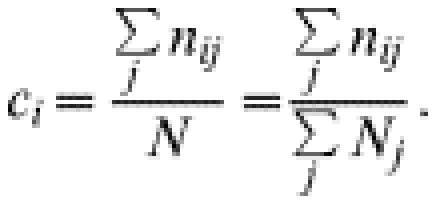 |
(1) |
We did not estimate contact rates for each scan nor for each observation day because they would not constitute independent measures as they involve the same domestic herd.
We estimated exposure to M.h. of each wild exposed population i during scan j by taking into account the number of domestic animals infected with M.h. involved in each direct contact k, dk [9]. In France, 95% of sheep have been found to be healthy carriers of M.h. [16]. Other studies concerning cattle [34, 35] or different countries [36] or periods [34] also reported high prevalences. We thus assumed that all domestic sheep are potential sources of infection. We estimated the total exposure of a wild exposed population i as
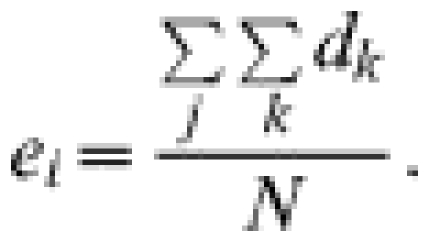 |
(2) |
We compared pastoral situations through their exposure to inter-species transmission and not through contact rates because exposure is most closely related to the risk of spillover.
Indirect contact rates and exposure to brucellosis
The transmission of Brucella occurs either through direct contact or through contact with a soil contaminated with abortion excreta from ruminants. These bacteria can survive on a pasture with a maximal persistence depending on UV radiations and vegetation for up to 15 days in pastures with good sunlight and for up to 25 days in those with low levels of sunlight [23]. Thus, we assumed that an indirect contact occurred when a wild ungulate is grazing on an area used by livestock up to 15 days (on sunned observation units: A, B and C) or 25 days (on partially sunned observation units D, E, F and G) before. In mountain conditions, cold temperatures (−0·5°C each 200 m of altitude [37]) are expected to increase the survival time of bacteria [23]. Therefore, it is possible that our calculation underestimates exposure to brucellosis.
As for direct transmission, we obtained for each scan j the number of contacts of wild ungulates with the potentially infectious area during scan jnij, and the total number of locations collected for this wild exposed population Nj. We estimated the indirect contact rate of a wild exposed population i, ci, as described in equation (1). In France, the eradication of bovine brucellosis is nearing completion: 25 outbreaks were detected in 1999 vs. 109 in 1995 [24]. In contrast, ovine brucellosis remains at a high level in south-eastern France, with more than 5% of herds infected in 2000 [24]. Most herds summering in the study area come from this area. Thus, we assumed that the risk of brucellosis is proportional to the number of domestic animals using a given pasture. We calculated exposure to Brucella of each wild exposed population by using the number of ovine or bovine individuals dk using the area involved in contact k [9] and we estimated the total exposure of a wild exposed population using equation (2).
Factors influencing exposure
After calculating rates of contact and exposure, we investigated the factors that may influence exposure. The domestic herds used in the present study have different agro-pastoral characteristics according to the livestock species (cattle or sheep), herd size and pastoral driving practices (guarded herds, i.e. enclosed or accompanied by dogs and shepherd, vs. free herds) (Table 1). We tested the effect of the domestic species on exposure because the exploratory behaviour of domestic individuals and the reaction of wild individuals may differ between cattle and sheep. Moreover, we expected that large herds would constitute a higher risk because they would be more at risk to include at least one infected individual. Concerning driving practices, we expected that free herds would be more at risk than guarded herds because individuals would be allowed to enter in contacts with wild individuals. In parallel, the type of species and size of the wild populations in the 10 observational units were variable. We tested the effect of the wild species considered because chamois and Alpine ibex may differ in their shyness towards domestic herds. We used the mean number of wild individuals observed during 3-h periods as an index indicating the size of the wild local population. We expected the wild population size to be positively correlated with exposure because crowding may lead to more contacts and more infection. Finally, we investigated whether exposure was higher before 09:00 hours or after 18:00 hours, which are the periods when chamois and Alpine ibex are most active [30].
Because of the expected non-normality in the contact rates (many zero values), we used non-parametric tests: Spearman's rank correlation tests for the effect of herd size and wild population size, Mann–Whitney U tests for the effects of the domestic species and driving practices. We used paired tests (Wilcoxon signed-ranks test) to compare chamois and Alpine ibex where both species were observed and Friedman test to assess the effect of the activity rhythm (to compare before 09:00, 09:00–18:00 and after 18:00 hours) [38]. We used bilateral tests unless a clear alternative hypothesis was available and we considered P values <0·05 as significant. Due to the low number of replicates, we did not search for relationships among explicative variables and confounding was only investigated when a specific hypothesis was tested.
RESULTS
After 924 h of observations, corresponding to 79 days in the field, 751 domestic polygons, 9145 chamois points and 3126 ibex points were digitized into the GIS.
Direct exposure to pasteurellosis
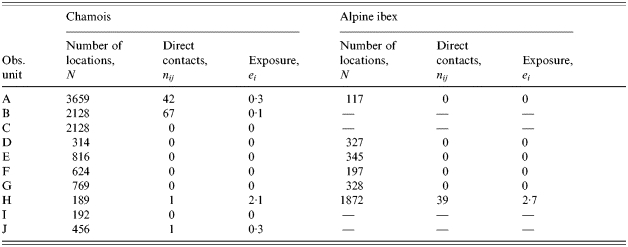
Among the 10 observed pastoral situations, four show non-zero exposure to pasteurellosis (Table 2). Neither the species or size of the wild populations (Wilcoxon test, P=0·655; Spearman test, P=0·094 respectively) nor the species or size of the domestic herds (Mann–Whitney U test, P=0·449; Spearman test, P=0·281 respectively) explain the variability of exposure. On the contrary, all four domestic herds responsible for direct contacts with chamois or Alpine ibex are free herds: the free ranging practice is a significant risk factor (Mann–Whitney U test, P=0·005). We suspected that there may be a confounding between the domestic species considered and the pastoral driving practice if sheep and cattle had different driving practices. However, in our sample, four of six sheep herds and two of five cattle herds were free, thus, the relationship is not clear. With a mean exposure of 1·64 (s.d.=1·59), hours of cohabitation before 09:00 hours are close to be a statistically significant risk factor compared to hours after 09:00 hours (0·48, s.d.=0·88; Friedman test, P=0·101) as expected from the pattern of activity of these species.
Table 2.
Estimates of contact rates and exposure for a model of direct transmitted disease, pasteurellosis, between domestic herd and wild ungulates on 10 alpine pastures of the Vanoise National Park
Indirect exposure to brucellosis
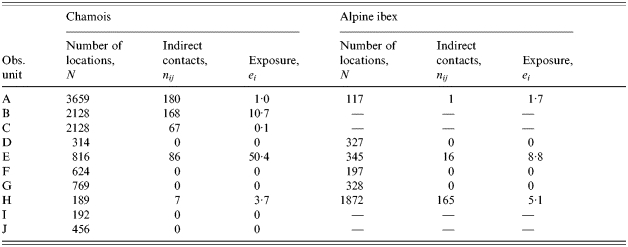
Five of the ten observed pastoral situations express a non-zero exposure to brucellosis for chamois and three for Alpine ibex (Table 3). Exposure was higher in pastures used by sheep (7·41, s.d.=14·06) compared to cattle (0·01, s.d.=0·02; Mann–Whitney U test, P=0·061). The difference was not statistically significant but it is plausible that sheep explore more remote areas of the pasture and, thus, are in contact with wild ruminants more often. In contrast, the pastoral driving practice, free or guarded, was not an explicative factor (Mann–Whitney U test, P=0·233). The size of the domestic herd (Spearman test, P=0·082) and the number (Spearman test, P=0·225) or the species (Wilcoxon test, P=1·000) of wild ungulates did not clearly influence exposure to brucellosis, although exposure tended to be high when the domestic herd was large, as expected. Again, when testing the influence of the activity rhythm of wild ungulates on the exposure, hours before 09:00 hours and after 18:00 hours (mean exposure: 11·87, s.d.=19·69) appear to be an important risk factor (mean exposure between 09:00 and 18:00 hours=0·83, s.d.=1·38; Friedman test, P=0·025).
Table 3.
Estimates of contact rates and exposure to an indirect transmitted disease, brucellosis, between domestic herd and wild ungulates on 10 observed pastures of the Vanoise National Park
Influence of local characteristics
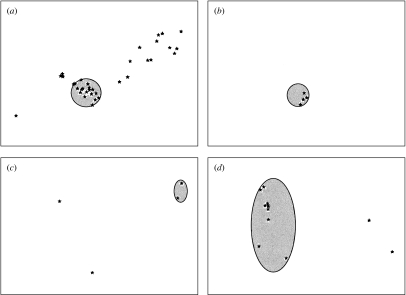
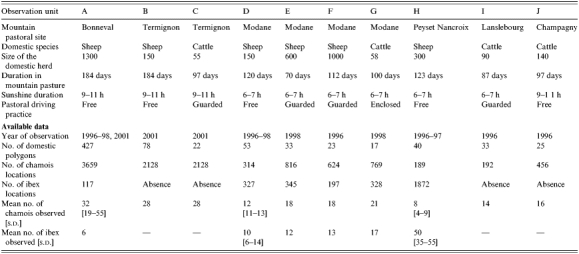
A visual inspection of the GIS maps showed that, in observation units B, C and E, most points representing indirect contacts were spatially aggregated (Fig.). When studying which factors may be at the origin of the clusters we found that most contacts occurred either in salt deposits, i.e. areas where salt is made available to domestic ruminants, or in a particular zone known by field agents of the National Park as a ‘refuge’ for chamois, i.e. an area where chamois are observed to go when they are disturbed in the pasture. The Figure shows the location of the indirect contacts in pastures B, C and E also with salt deposits or refuge zones. That salted areas were significant risk factors for indirect exposure was demonstrated by the fact that whereas salt deposits represented only 6% of the total grazing areas in units B and C, the salted deposits accounted for 37 and 100% respectively of the total number of chamois deemed to have been exposed to infection in units B and C. In unit E, the salt deposit (3% of the total grazing area) constitutes a high-risk area (82% of the total exposure) only for ibex: the refuge area representing 25% of the grazing area explains 78% of exposure to brucellosis for this species. Exposures calculated separately in high-risk and low-risk areas were significantly different (50·80, s.d.=25·25 vs. 11·20, s.d.=13·51; Wilcoxon test, P=0·030).
Fig.
Indirect contacts (black stars) and high-risk areas (grey areas). (a) Pasture B, indirect contacts between chamois and sheep and salt deposits. (b) Pasture C, indirect contacts between chamois and cattle and salt deposits. (c) Pasture E, indirect contacts between ibex and sheep and salt deposits. (d) Pasture E, indirect contacts between chamois and sheep and wild refuge zone.
DISCUSSION
Methods
Our results constitute a first attempt to estimate contact rate and exposure to inter-species disease transmission, however, both field observations and assumptions of the model have several limitations. First, observations were realized by day so none of our results takes into account the nocturnal activity of individuals. Concerning directly transmitted diseases, night and days rates may be different. For indirectly transmitted diseases, the longer the domestic individual stays in one place, the higher the risk of contamination of this place, which probably occurs during the night. The number of wild animals can also be underestimated in midday because animals are inactive [30]. We used the mean number of chamois or ibex being observed on a mountain-side as an index of the size of wild exposed populations. Other individuals may stand on non-visible sides of the mountain but may frequent the observed side, thus, we probably underestimated the exposed population and overestimated contact rates. As a whole, the observed numbers of individuals must be taken as first estimates and be further elaborated. Last, indirect contacts were based on discontinuous observations of both domestic sheep and wild animals. Therefore, some indirect contacts may not have been observed and exposure to brucellosis could have been underestimated. A solution to avoid biases due to activity pattern during the day, lack of observations during the night and under-observation of some individuals or sheep would have been to use VHF or GPS collars. However, this would require observing numerous individuals with collars and, thus, must be balanced against the need to repeat the observations on contrasted sites.
When formulating the contact rates and exposure, we hypothesized homogeneity of contacts, without discrimination of age or sex of wild individuals. However, some individuals may have specific risk because of their behaviour. In an outbreak of brucellosis in the Italian Alps, more males were positive than females and this was attributed to males using low pastures more often than females [12]. On the other hand, pastures and salt may specifically attract females who have to fill specific physiological needs during pregnancy and lactation. Preliminary observations in chamois in other sites show that females stay closer to domestic herds than males (Fromont et al., unpublished observations).
We considered transmission from domestic to wild species because the maintenance of diseases in wild animals is not relevant in the cases we studied, either because domestic species are virtually all infected (pasteurellosis) [16] or because spill-back transmission between wild to domestic species is unrealistic (brucellosis) [25]. When estimating contact rates and exposure, we also made specific assumptions concerning the properties of both M.h. and Brucella spp. For direct contacts, we assumed that any wild individual standing within 20 m of a domestic herd has the same risk for disease, whereas this risk is probably modulated by the precise distance to the domestic herd and by the specific behaviour during contact. This distance of 20 m may be revised if new data on the survival of M.h. are proposed, but we hypothesize that changing this distance would not affect the comparison among sites. Concerning brucellosis, the survival time of the bacteria influences the contact rate. However, in both cases, the same assumptions are made for all sites, thus, we hypothesize no bias in the collected data. Finally, the number of replicates (observation units) is low and so is the power of the tests, thus, we considered P values between 0·05 and 0·1 as interesting trends to be investigated further.
RESULTS
Despite the above caveats, repeated observations allowed us to estimate exposure to M.h. and Brucella spp. and to test for the effect of herd management. As we do not follow marked individuals, we cannot discriminate whether the number of wild individuals, Nj includes the same individuals observed in different scans or if different individuals are present in each scan. We provide a mean exposure valid for an hypothetical individual with a median behaviour. If Nj are not independent, mean values remain valid, but a minority of individuals support the majority of risk. This hypothesis is biologically plausible but can only be studied with observations on marked individuals. Contrary to our hypothesis, we showed no correlation between exposure and size of domestic or wild herds. This is in agreement with the results of Gauthier & Durand [39] who observed no relationship between pastoral pressure and seasonal succession or long-distance cohabitation. A possible interpretation is that contacts mainly depend on environmental conditions and human management, thus, the presence few individuals may be sufficient to entail high contact rates. Finally, direct exposure appears to be linked to pastoral practices. Gauthier & Durand [39] showed that the less a herd is guarded, the closer the cohabitation with chamois. Here we directly link pasture management with exposure to a directly transmitted pathogen and demonstrate that free-ranging herds may be a significant risk factor for the exposure of free-living animals to M.h. Risk factors of exposure appear to be different between the two diseases studied. Exposure to brucellosis tends to increase when the pasture is occupied by sheep or large herds. This observation is important since the prevalence of Brucella spp. is higher in sheep than in cattle [24]. Maps generated by the GIS also enable us to stress ‘at risk’ areas: salted areas represent attraction points not only for domestic but also for wild ungulates and can be qualified as ‘Epidemiological Dangerous Points’ [40].
Risk of spillover
The estimation of exposure is a first step towards the evaluation of the risk of spillover [41]. The second necessary point is to investigate the susceptibility of the receptor species to the bacteria. Presently, the molecular typing of M.h. and Brucella spp. isolated from dead wild animals in pastures shared by domestic animals is under investigation. Further studies on the cytotoxic effects on macrophages obtained from chamois by strains of M.h. would help to clarify the pathogenesis of this organism in wild ungulates.
CONCLUSION
Although they remain to be confirmed and further investigated, these results could probably be applied to pathogens with similar life-history traits, for example parainfluenza virus or Mycoplasma [42] for direct transmission or echtyma poxvirus or tuberculosis for indirect transmission [12]. Practically, our results suggest that there is not a single way to limit the sanitary risk related to summering herds: management options would differ with the type of pathogen. In our case, limiting salt deposits is expected to be an efficient way to control the risk of brucellosis transmission. As suggested by Nishi et al. [43], such applications would lead to the better integration of conservation biology with agricultural livestock policy to develop management options.
ACKNOWLEDGEMENTS
The authors thank all who participated in the data collection. This study is part of the research programme ‘Espaces Protégés: Cohabitation et transmission de pathogènes’ from the French Ministère de l'Ecologie et du Développement Durable. This programme is coordinated by the French National Natural History Museum and involved participation from the Departmental Veterinary Laboratory of Savoie, the Vanoise National Park, the National Veterinary School of Lyon and the French National Institute for Agricultural Research.
REFERENCES
- 1.Thorne ET, Williams ES. Disease and endangered species: the black-footed ferret as a recent example. Conserv Biol. 1988;2:66–74. [Google Scholar]
- 2.Simonetti JA. Wildlife conservation outside parks is a disease-mediated task. Conserv Biol. 1995;9:454–456. [Google Scholar]
- 3.Ostherhaus A. Catastrophes after crossing species barriers. Proc R Soc Lond B. 2001;356:791–793. doi: 10.1098/rstb.2001.0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lafferty KD, Gerber LR. Good medicine for conservation biology: the intersection of epidemiology and conservation theory. Conserv Biol. 2002;16:593–604. [Google Scholar]
- 5.Combes C. Parasitism: ecology and evolution of intimate interactions. Chicago University Press; 2001. p. 552. pp. [Google Scholar]
- 6.Cleaveland S, Laurenson MK, Taylor LH. Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Proc R Soc Lond B. 2001;356:991–999. doi: 10.1098/rstb.2001.0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woolhouse MEJ, Taylor LH, Haydon DT. Population biology of multihost pathogens. Science. 2001;292:1109–1112. doi: 10.1126/science.1059026. [DOI] [PubMed] [Google Scholar]
- 8.Loehle C. Social barriers to pathogen transmission in wild animal populations. Ecology. 1995;76:326–335. [Google Scholar]
- 9.Courtenay O, Quinnell RJ, Chalmers WSK. Contact rates between wild and domestic canids: no evidence of parvovirus or canine distemper virus in crab-eating foxes. Vet Microbiol. 2001;81:9–19. doi: 10.1016/s0378-1135(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 10.Sauter CM, Morris RS. Behavioural studies on the potential for direct transmission of tuberculosis from feral ferrets (Mustela furo) and possums (Trichosurus vulpecula) to farmed livestock. N Z Vet J. 1999;43:294–300. doi: 10.1080/00480169./1995.35909. [DOI] [PubMed] [Google Scholar]
- 11.Hutchings MR, Harris S. Quantifying the risks of TB infection to cattle caused by badger excreta. Epidemiol Infect. 1999;122:167–174. doi: 10.1017/s0950268898001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudson PJ, Rizzoli A, Grenfell BT, Heesterbeek H, Dobson AP. The ecology of wildlife diseases. Oxford University Press; 2002. p. 187. pp. [Google Scholar]
- 13.Foreyt WJ, Jessup DA. Fatal pneumonia of bighorn sheep following association with domestic sheep. J Wildl Dis. 1982;18:163–168. doi: 10.7589/0090-3558-18.2.163. [DOI] [PubMed] [Google Scholar]
- 14.Callan RJ, Bunch TD, Workman GW, Mock RE. Development of pneumonia in desert bighorn sheep after exposure to a flock of exotic wild and domestic sheep. J Am Vet Med Assoc. 1991;198:1052–1056. [PubMed] [Google Scholar]
- 15.Frölich K, Thiede S, Kozikowski T, Jakob W. A review of mutual transmission of important infectious diseases between livestock and wildlife in Europe. Ann NY Acad Sci. 2002;969:4–13. doi: 10.1111/j.1749-6632.2002.tb04343.x. [DOI] [PubMed] [Google Scholar]
- 16.Casamitjana P. Respiratory disease in sheep. Bulletin des GTV. 1994;3:126–136. [Google Scholar]
- 17.Gilmour NJL. Pasteurella haemolytica infections in sheep. Vet Q. 1980;2:191–198. doi: 10.1080/01652176.1980.9693780. [DOI] [PubMed] [Google Scholar]
- 18.Foreyt WJ. Fatal Pasteurella haemolytica pneumonia in bighorn sheep after direct contact with clinically normal domestic sheep. Am J Vet Res. 1989;50:341–344. [PubMed] [Google Scholar]
- 19.Foreyt WJ, Snipes KP, Kasten RW. Fatal pneumonia following inoculation of healthy bighorn sheep with Pasteurella haemolytica from healthy domestic sheep. J Wildl Dis. 1994;30:137–145. doi: 10.7589/0090-3558-30.2.137. [DOI] [PubMed] [Google Scholar]
- 20.Sweeney SJ, Silflow RM, Foreyt WJ. Comparative leukotoxicities of Pasteurella haemolytica isolates from domestic sheep and free-ranging bighorn sheep (Ovis canadensis) J Wildl Dis. 1994;30:523–528. doi: 10.7589/0090-3558-30.4.523. [DOI] [PubMed] [Google Scholar]
- 21.Pairaudeau C, Moulin A, Prave M, Gastellu J, Hars J, Joubert L. About two outbreaks in the National Park of Vanoise: infectious keratoconjunctivitis in chamois and pneumonia in chamois and Alpine ibex. Travaux scientifique du Parc National de la Vanoise. 1977;VIII:157–172. [Google Scholar]
- 22.Grattarola C, Ferroglio E, Rossi L. Preliminary study on pneumonia in chamois. Bulletin d'Information sur la Pathologie des Animaux Sauvages. 1998;18:71–80. [Google Scholar]
- 23.Garin-Bastiji B. Brucellosis of cattle, sheep and goats: control and prevention. Point Vét. 1993;25:15–22. [Google Scholar]
- 24.Garin-Bastiji B, Delcueillerie F. Human and animal brucellosis in France in 2000. Epidemiological situation – control and eradication programmes. Méd Mal Infect. 2001;31:202–216. [Google Scholar]
- 25.Ferroglio E, Tolar F, Bollo E, Bassano B. Isolation of Brucella melitensis from Alpine ibex. J Wildl Dis. 1998;34:400–402. doi: 10.7589/0090-3558-34.2.400. [DOI] [PubMed] [Google Scholar]
- 26.Gauthier D, Hars J, Rossi L. Third Conference of the Wildlife Disease Association. Edinburgh, Scotland: 1998. Brucellosis in free ranging chamois (Rupicapra rupicapra) and its relationships with domestic breeding. [Google Scholar]
- 27.Pilar-Izquierdo M. Modalities of cohabitation between domestic livestock and wild ungulates in the National Reserve of wildlife, Les Bauge, 74pp. [post-doctoral report] Chambéry, France: Laboratoire Départemental Vétérinaire; 2000. [Google Scholar]
- 28.Ternois E. Pathogenic agent's transmission between domestic and wild ungulates, 227. pp. [thesisNantes, France: Veterinary Faculty; 2003 [Google Scholar]
- 29.Durand T. Mountain livestock breeding and wildlife, 144pp. [report] Chambéry, France: Parc National de la Vanoise; 1997. [Google Scholar]
- 30.Léna F.Wild ungulates and mountain livestock breedings: spatial and sanitary relationships 179. pp. [thesisLyon, France: Veterinary Faculty; 2002 [Google Scholar]
- 31.Jenness J. http;//arcscripts.esri.com/details.asp?dbid=10220. 2002. http;//arcscripts.esri.com/details.asp?dbid=10220 Nearest Features, with Distances and Bearings (v. 3.7a). Script for Arcview ( ). Accessed August .
- 32.Dixon D, Rudolph K, Cowen L, Hunter DL, Ward ACS. Viability of air-borne Pasteurella spp. Northern Wild Sheep and Goat Council 13. Rapid City, South Dakota, USA: 2002. : 23–24 April. [Google Scholar]
- 33.Gilmour MI, Wathes CM, Taylor FG. The airborne survival of Pasteurella haemolytica and its deposition in and clearance from the mouse lung. Vet Microbiol. 1990;21:363–375. doi: 10.1016/0378-1135(90)90008-j. [DOI] [PubMed] [Google Scholar]
- 34.Adlam C, Rutter JM. Pasteurella and Pasteurellosis. New York: Academic Press; 1989. [Google Scholar]
- 35.Rowe HA, Poxton IR, Donachie W. Survival of Manheimia (Pasteurella) haemolytica in tracheobronchial washing of sheep and cattle. Vet Microbiol. 2001;81:305–314. doi: 10.1016/s0378-1135(01)00361-3. [DOI] [PubMed] [Google Scholar]
- 36.Sisay T, Zerihun A. Diversity of Mannheimia haemolytica and Pasteurella trehalosi serotypes from apparently healthy sheep and abattoir specimens in the highlands of Wollo, north east Ethiopia. Vet Res Com. 2003;27:3–14. doi: 10.1023/a:1022088005887. [DOI] [PubMed] [Google Scholar]
- 37.Lebreton P, Lebrun P, Martinot JP, Miquet A, Tournier H. Ecological approach to the Vanoise avifauna. Travaux Scientifiques du Parc National de la Vanoise. 1999;XXI:7–304. [Google Scholar]
- 38.Sokal RR, Rohlf FJ. Biometry. 3rd edn. New York: Freeman and Co; 1995. p. 887. pp. [Google Scholar]
- 39.Gauthier D, Durand T. Wroclaw Poland: 1996. pp. 16–21. Relationships between mountain livestock breeding and wild ungulates' health in National Park of Vanoise. In: Proceedings of the Second European Conference of the Wildlife Disease Association. [Google Scholar]
- 40.de la Rocque S, Michel JF, Cuisance D Satellite to microsatellite – the trypanosomosis risk: a global approach for a local decision. CIRAD; Montpellier, France: 2001. p. 151. pp. [Google Scholar]
- 41.Toma B, Dufour B, Saana M, Bénet JJ, Shaw A, Moutou F, Louza A. Applied veterinary epidemiology and the control of disease in populations. 2nd edn. Paris, France: A.E.E.M.A. Ed; 2001. p. 696. pp. [Google Scholar]
- 42.Chatelier N. Study of infectious keratoconjunctivitis in Alpine ibex (Capra ibex ibex) Chambéry, France: DEA Gestion des espaces montagnards, University; 1999. p. 61. pp. [report]. [Google Scholar]
- 43.Nishi JS, Stephen C, Elkin BT. Implications of agricultural and wildlife policy on management and eradication of bovine tuberculosis and brucellosis in free-ranging wood bison of northern Canada. Ann NY Acad Sci. 2002;969:236–244. doi: 10.1111/j.1749-6632.2002.tb04385.x. [DOI] [PubMed] [Google Scholar]