SUMMARY
A case-control study involving 109 in-patients with chronic liver disease and 190 in-patients with no apparent liver disease was conducted to evaluate the seroprevalence of anti-HEV antibodies and the possible association with chronic liver disease. Among cases, the anti-HEV prevalence was 36·6% which increased significantly by age; among controls, the prevalence was 12·1% (P<0·05) and was similar among age groups <60 years. Among cases, aged >50 years (OR 4·0, 95% CI 1·4–11) and the presence of end stage liver disease (ESLD) (OR 4·3, 95% CI 1·4–12·8) were associated independently with anti-HEV positivity. The mean optical density, determined by anti-HEV immunoenzymatic test, was significantly higher among patients with ESLD, compared to the other patients. These results indicate that there is a high seroprevalence of anti-HEV in patients with chronic liver disease and a possible association between HEV infection and/or anti-HEV production and advanced stage chronic liver disease.
INTRODUCTION
The prevalence of antibodies to hepatitis E virus (HEV) has been considered as an indicator of exposure to HEV in various general populations worldwide, and it has been found that anti-HEV antibodies are present in all geographical areas. Whereas both endemic and epidemic forms of HEV infection have been reported in Southeast and Central Asia, Africa, and Mexico, the prevalence among the general populations of the economically developed countries of Europe is low, ranging from 1 to 3%. However, this range is relatively high when compared with the low rate of clinically evident HEV disease in these areas [1, 2].
In non-endemic countries, travellers to endemic areas are at risk for HEV infection; however, several studies have reported cases not associated with travel to endemic areas and unexplained transmission routes [3–7]. Some studies have reported a high prevalence of HEV antibodies in patients with chronic liver disease and others have suggested that superinfection with HEV in patients with underlying chronic liver disease can cause severe hepatic decompensation, leading to increased morbidity and mortality [8, 9]. We conducted a study to evaluate the association between anti-HEV infection and chronic liver disease, using serum samples collected from patients with chronic liver disease in a hospital in Albania [10].
STUDY POPULATION AND METHODS
The sera from all 299 patients included in this study had been tested for hepatitis B virus (HBV), hepatitis C virus (HCV) and hepatitis D virus (HDV) in a previous case-control study aimed at investigating the relationships of these infections and alcohol intake with chronic hepatitis and cirrhosis [10]. The study population consisted of the 109 in-patients with chronic liver disease who were consecutively admitted in 1995 to the Liver Unit of the University Hospital Centre of Tirana, the most important centre for the diagnosis and treatment of liver disease in Albania, and for whom a frozen serum sample was available. Primary or secondary biliary cirrhosis and autoimmune or metabolic liver diseases were excluded. The presence of chronic hepatitis or liver cirrhosis, according to the Child–Pugh classification, was as follows: 47 (43%) patients had chronic hepatitis or Child A liver cirrhosis, as shown by liver biopsy, and 62 (57%) patients, based on either liver biopsy or clinical, laboratory, and imaging findings, had Child B or C liver cirrhosis [10, 11]. These last patients were classified as having end stage liver disease (ESLD). The control group consisted of 190 in-patients with no apparent liver disease consecutively admitted for the first time to the same hospital during the same period for diseases different from and not related to liver disease. All participating patients had been interviewed by the same physician using a standard questionnaire containing information on age, gender, level of education and occupation. In patients with chronic liver disease, information was also collected on the number of hospitalizations and any endoscopic and laparoscopic procedures as possible risk factors for transmission of HEV infection. The age distribution was similar for cases and controls, whereas cases seemed to have less formal education than controls [10].
Laboratory analyses
Serum samples, which had been frozen at −40°C, were tested for specific IgG antibodies to HEV by a commercial enzyme immunoassay (Abbott HEV EIA, Abbott Laboratories, Abbott Park, IL, USA), which uses recombinant antigens from open reading frames (ORF) 2 and 3 of the HEV genome. Samples were considered as positive when repeatedly reactive, with a sample cut-off ratio of ⩾1·2. Serum samples were also tested for anti-HAV by enzyme-linked immunosorbent assay (HAVAB EIA) (Abbott Laboratories).
In the previous study, the serum samples had been tested for HBsAg and anti-HBc by Monoclonal Auszyme and Ausab EIA (Abbott Diagnostics) respectively. Anti-HCV reactivity had been tested using a third-generation ELISA (EIA-3, Ortho HCV 3rd generation; Ortho Diagnostic Systems, Rariton, NJ, USA) and confirmed by third-generation RIBA-3 (Chiron Corporation, Emeryville, CA, USA, and Ortho Diagnostic Systems).
Statistical analysis
Differences in proportions were evaluated by χ2 test, χ2 for linear trend and Fisher's exact test, when appropriate. For continuous variables the results were expressed as means and standard deviation (s.d.). Differences in means were evaluated by Student's t test. A P value of <0·05 was considered as statistically significant. The crude odds ratios (OR) for associations between HEV infection and sociodemographic characteristics were evaluated by univariate analysis. The independent effect of the sociodemographic characteristics on anti-HEV positivity was evaluated by logistic regression analysis using as reference the category with the most favourable level of exposure.
RESULTS
The anti-HEV prevalence among the 299 cases and controls was 21·1% (63/299). None of the patients had travelled to areas considered as endemic for HEV infection. The anti-HEV prevalence by sociodemographic characteristics and positivity for HBsAg and anti-HCV is shown in Table 1. At the univariate analysis, anti-HEV prevalence was found to be associated with age >50 years [OR 3·6, 95% confidence interval (CI) 2·0–6·4], ⩽8 years of formal education (OR 1·8, 95% CI 1·0–3·2), and positivity for HBsAg (OR 2·4, 95% CI 1·3–4·5). No association was observed between people who lived in the village and/or those who had occupations with close contact with animals and anti-HEV positivity (data not shown). At multivariate analysis, significant associations remained for age >50 years (OR 3·4, 95% CI 1·8–6·6) and HBsAg positivity (OR 2·4, 95% CI 1·2–4·6).
Table 1.
Prevalence of anti-HEV by sociodemographic characteristics and positivity for HBsAg and anti-HCV in 109 patients with chronic liver disease and 190 controls; Albania, 1995
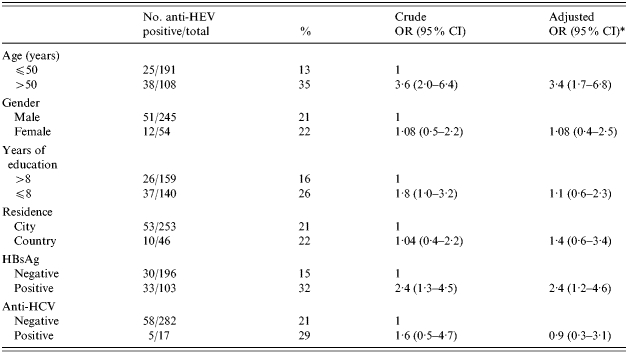
OR, Odds ratio; CI, confidence interval.
Adjusted for all other variables in the table.
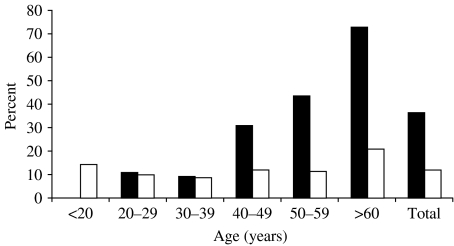
The anti-HEV prevalence was 36·6% among cases (40/109) and 12·1% among controls (23/190) (P<0·05). As shown in the Figure, among cases the prevalence of anti-HEV increased significantly by age (χ2 for linear trend: P<0·001), from 11% in patients <30 years of age to 73·0% among those >60 years of age. In the control group, the highest anti-HEV prevalence was 21% and it was found for patients >60 years of age; among the other age groups, the prevalence was fairly similar. The overall anti-HAV prevalence was 100% in both cases and controls.
Fig.
Age-specific prevalence of anti-HEV in 109 patients with chronic liver disease (▪, cases) and 190 patients with no apparent liver disease (□, controls).
Table 2 reports the anti-HEV prevalence by selected characteristics for the patients with chronic liver disease. At the univariate analysis, age >50 years (OR 6·4, 95% CI 2·7–15) and the presence of ESLD (OR 6·5, 95% CI 2·5–16·7) were associated with anti-HEV positivity. These associations continued to be significant when each variable was adjusted for the confounding effect of other variables at the logistic regression analysis [age >50 years (OR 4·0, 95% CI 1·4–11); ESLD (OR 4·3, 95% CI 1·4–12·8)].
Table 2.
Prevalence of anti-HEV by selected characteristics of 109 patients with chronic liver disease; Albania, 1995
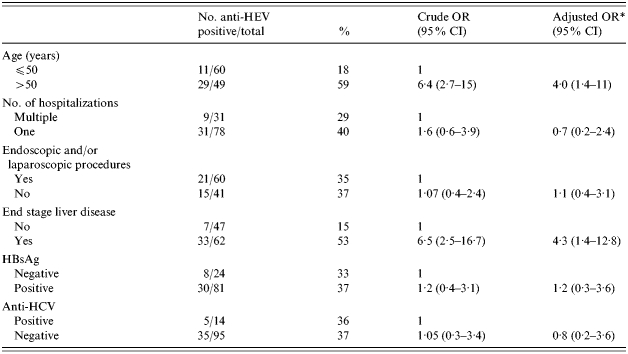
OR, Odds ratio; CI, confidence interval.
Adjusted for all other variables in the table.
The mean optical density (OD) given by anti-HEV immunoenzymatic test in patients with ESLD, in the remaining patients with chronic liver disease (chronic hepatitis or Child A liver cirrhosis) and in controls was respectively 0·370 (s.d.=0·250), 0·253 (s.d.=0·120), 0·173 (s.d.=0·09) with a cut-off of 0·310. The OD in patients with ESLD was significantly higher (P<0·05) in comparison with the other two groups. No differences in the OD values given by the anti-HAV immunoenzymatic test were observed in patients with ESLD, chronic hepatitis and controls (data not shown).
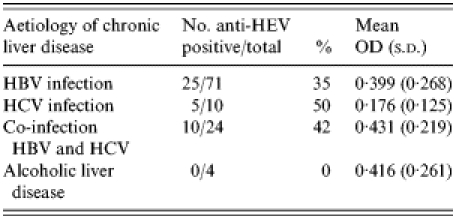
The underlying cause of chronic liver disease with the anti-HEV positivity rate and mean test OD is shown in Table 3. No significant association was observed.
Table 3.
Prevalence of anti-HEV and mean optical density (OD) values for anti-HEV by the aetiology of chronic liver disease
DISCUSSION
Albania is a Mediterranean country where HAV and HBV infections are hyperendemic and there is an apparently low prevalence of HCV and HDV infection in patients with chronic liver disease and in a variety of groups in the population (e.g. Albanian refugees in Italy and Greece) [10, 12, 13].
In studies conducted among Albanian refugees, the anti-HEV prevalence has been found to be 2–4·8% [12, 14]. In our study, the prevalence of HEV infection for the overall study population (i.e. cases and controls combined) was quite high (21·1%) and the prevalence among controls was significantly higher (12·1%) than that reported in HEV non-endemic countries [5–7]. However, the previous studies as well as the present one were conducted among selected groups of individuals and the reported anti-HEV prevalence does not necessarily represent the real anti-HEV prevalence in Albania's general population.
Travel to geographical areas endemic for anti-HEV is the most common risk factor among clinical cases from non-endemic countries [3, 4, 7]. Occupation with direct contact with animals is also reported to be one of the most common possible risk factors for acquiring HEV [15, 16]. In most sporadic cases of HEV infection, the mode of transmission remains unclear. None of our patients had travelled to areas where HEV infection is considered as endemic and no association was found with occupations involving direct contact with animals. Although there is no strong evidence that HEV is transmitted through the transfusion of blood or blood products, an apparent association between HEV and HCV infection has been reported [17–20]. In accordance with the data of the previous study conducted in the Albanian refuges, the prevalence of HEV infection in our study was independently associated with HBsAg positivity, suggesting that these infections could have overlapping routes of transmission [14]. However, these data might just reflect the significantly higher anti-HEV prevalence observed in our patients with chronic liver disease secondary to HBV infection in most cases. However, when we analysed only the cases, HBsAg was not a factor independently associated with anti-HEV positivity.
HAV and HEV have common characteristics: they are transmitted by the faecal–oral route and generally cause an acute self-limited illness followed by complete recovery. The two infections differ in terms of epidemiological patterns, however, we could not evaluate a possible association between anti-HAV and anti-HEV seroprevalences as the whole study population were HAV antibody positive.
It is still not clear how long HEV antibodies persist after exposure, and some studies have reported that they have a short life [21]. However, our finding that HEV prevalence was independently associated with age >50 years, as also previously described, could potentially be explained by a long-term persistence of HEV antibody and a consequently higher seroprevalence among older individuals [6, 22]. A previous study detected HEV IgG in 47% of persons 14 years after acute HEV infection, and another follow-up study conducted among the general population reported that antibody persisted in only 37% of seropositive subjects after 5 years [23, 24]. As a consequence, the past spread of HEV infection cannot completely explain the significant trend in HEV prevalence by age. In addition, this trend was only observed for patients with chronic liver disease and not for controls. This is consistent with findings from a previous study and further suggests that there is another explanation [25].
Regarding the potential association between HEV infection and chronic liver disease, the prevalence of anti-HEV among persons with this condition was high (i.e. 36·6%), and it was significantly higher than that among persons with no apparent liver disease. These findings are consistent with the high anti-HEV prevalence among patients with chronic liver disease reported by other recent studies [8, 9, 14]. As previously mentioned, we also found that the anti-HEV prevalence significantly increased with age among cases yet not among controls. These results apparently cannot be explained by exposure to risk factors such as endoscopic procedures and frequent hospitalization in patients with chronic liver disease. In a recent study, the severity of chronic liver disease did not differ when comparing anti-HEV-positive patients to those who were anti-HEV negative, although the course of chronic liver disease in patients with HEV superinfection was severe [9]. In our patients with chronic liver disease, the presence of ESLD and age >50 years were both independently associated with anti-HEV positivity. Moreover the mean OD obtained by an immunoenzymatic test was significantly higher in patients with ESLD compared to the remaining patients with chronic liver disease and with controls. These associations could be explained by possible HEV superinfection of patients with chronic liver disease, particularly in cases with high OD values. Otherwise, in patients with ESLD, an antigen-independent pathway of T-cell and consequently B-cell activation or enhanced immune response against HEV that could have remained in the liver after HEV infection, could also explain our findings. In addition, in patients with ESLD, increased IgG levels could be due to increased interleukin production, particularly of IL-6 [26–29]. However, the lack of an available commercial test to confirm the specificity of the anti-HEV seroreactivity does not allow us to exclude that the higher prevalence of anti-HEV in cases compared to controls is an unspecific result due to increased IgG.
With regard to the specific means of detecting anti-HEV, studies have shown that tests based on ORF 3 of the HEV genome are of limited value for seroepidemiological studies, whereas tests based on ORF 2 have broad utility and yield reproducible data [30, 31]. The anti-HEV reactivity in the sera of our patients was based on an immunoenzymatic assay that used recombinant antigens from both ORF 2 and ORF 3.
In conclusion, our data indicate that the seroprevalence of anti-HEV is significantly higher and that it increases significantly by age among Albanian patients with chronic liver disease compared to individuals from the same geographical area with no apparent liver disease. Our data also suggest that the presence of anti-HEV is associated with advanced stages of chronic liver disease, although the specific role, if any, of HEV infection on the severity of chronic liver disease and/or the meaning of enhanced HEV antibody production in patients with ESLD remains to be determined.
ACKNOWLEDGEMENTS
The authors thank Mark Kanieff for editorial assistance and linguistic revision of the text and Romina Tomasetto for secretarial and editorial assistance. This work was supported by Istituto Superiore di Sanità ‘Viral Hepatitis National Projects’ (D. leg.vo no. 502) and by the Istituto Superiore di Sanità ‘Ethiological, Diagnostic, and Structural Characterization of Hepatitis Viruses Project’ (no. C3NE).
REFERENCES
- 1.Paul DA, Knigge MF, Ritter A et al. Determination of hepatitis E virus seroprevalence by using recombinant fusion proteins and synthetic peptides. J Infect Dis. 1994;169:801–806. doi: 10.1093/infdis/169.4.801. [DOI] [PubMed] [Google Scholar]
- 2.Balayan MS. Epidemiology of hepatitis E virus infection. J Viral Hepat. 1997;4:155–165. doi: 10.1046/j.1365-2893.1997.00145.x. [DOI] [PubMed] [Google Scholar]
- 3.Center for Disease Control and Prevention. Hepatitis E among U.S. travelers, 1989–1992. Morb Mortal Wkly Rep. 1993;42:1–4. [PubMed] [Google Scholar]
- 4.De Cock KM, Bradley DW, Sandford NL et al. Epidemic non-A, non-B hepatitis in patients from Pakistan. Ann Intern Med. 1987;106:227–230. doi: 10.7326/0003-4819-106-2-227. [DOI] [PubMed] [Google Scholar]
- 5.Zanetti AR, Dawson GJ. Hepatitis type E in Italy: a seroepidemiological survey. Study Group of hepatitis E. J Med Virol. 1994;42:318–320. doi: 10.1002/jmv.1890420321. [DOI] [PubMed] [Google Scholar]
- 6.Stroffolini T, Menchinelli M, Dambruoso V et al. Prevalence of hepatitis E in a central Italian town at high endemicity for hepatitis C virus. Ital J Gastroenterol. 1996;28:523–525. [PubMed] [Google Scholar]
- 7.Mast EE, Kuramoto IK, Favorov MO et al. Prevalence of and risk factors for antibody to hepatitis E virus seroreactivity among blood donors in Northern California. J Infect Dis. 1997;176:34–40. doi: 10.1086/514037. [DOI] [PubMed] [Google Scholar]
- 8.Ramachandran J, Eapen C, Kang G et al. Hepatitis E superinfection produces severe decompensation in patients with chronic liver disease. J Gastroenterol Hepatol. 2004;19:134–138. doi: 10.1111/j.1440-1746.2004.03188.x. [DOI] [PubMed] [Google Scholar]
- 9.Hamid SS, Atiq M, Shehzad F et al. Hepatitis E virus superinfection in patients with chronic liver disease. Hepatology. 2002;36:474–478. doi: 10.1053/jhep.2002.34856. [DOI] [PubMed] [Google Scholar]
- 10.Kondili LA, Tosti ME, Szklo M et al. The relationships of chronic hepatitis and cirrhosis to alcohol intake, hepatitis B and C, and delta virus infection: a case-control study in Albania. Epidemiol Infect. 1998;121:391–395. doi: 10.1017/s0950268898001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pugh RNH, Murray-Lyon IM, Dawson JL et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–654. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 12.Malamitsi-Puchner A, Papacharitonos S, Sotos D et al. Prevalence study of different hepatitis markers among pregnant Albanian refugees in Greece. Eur J Epidemiol. 1996;12:297–301. doi: 10.1007/BF00145420. [DOI] [PubMed] [Google Scholar]
- 13.Chironna M, Germinario C, Lopalco PL, Quarto M, Barbuti S. HBV, HCV and HDV infections in Albanian refugees in Southern Italy (Apulia region) Epidemiol Infect. 2000;125:163–167. doi: 10.1017/s0950268899004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalekos GN, Zervou E, Elisaf M et al. Antibodies to hepatitis E virus among several populations in Greece: increased prevalence in a haemodialysis unit. Transfusion. 1998;38:589–595. doi: 10.1046/j.1537-2995.1998.38698326339.x. [DOI] [PubMed] [Google Scholar]
- 15.Meng XJ. Novel strains of hepatitis E virus identified from humans and other animal species: is hepatitis E a zoonosis? J Hepatol. 2000;33:842–845. doi: 10.1016/s0168-8278(00)80319-0. [DOI] [PubMed] [Google Scholar]
- 16.Meng XJ, Wiseman B, Elvinger F et al. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J Clin Microbiol. 2002;40:117–122. doi: 10.1128/JCM.40.1.117-122.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pisanti F, Coppola A, Galli C. Association between hepatitis C and hepatitis E viruses in southern Italy. Lancet. 1994;344:746–747. doi: 10.1016/s0140-6736(94)92233-0. [DOI] [PubMed] [Google Scholar]
- 18.Barzilai A, Schulman S, Karetnyi YV et al. Hepatitis E virus infection in haemophiliacs. J Med Virol. 1995;46:153–156. doi: 10.1002/jmv.1890460213. [DOI] [PubMed] [Google Scholar]
- 19.Psichogiou MA, Tassopoulos NC, Papatheodoridis GV et al. Hepatitis E virus infection in a cohort of patients with acute non-A, non B hepatitis. J Hepatol. 1995;23:668–673. doi: 10.1016/0168-8278(95)80032-8. [DOI] [PubMed] [Google Scholar]
- 20.Psichogiou M, Tzala E, Boletis J et al. Hepatitis E virus infection in individuals at high risk of transmission of non-A, non-B hepatitis and sexually transmitted diseases. Scand J Infect Dis. 1996;28:443–445. doi: 10.3109/00365549609037936. [DOI] [PubMed] [Google Scholar]
- 21.Koshy A, Grover S, Hyams KC et al. Short-term IgM and IgG antibody responses to hepatitis E virus infection. Scand J Infect Dis. 1996;28:439–441. doi: 10.3109/00365549609037935. [DOI] [PubMed] [Google Scholar]
- 22.Arankalle VA, Tsarev SA, Chadha MS et al. Age-specific prevalence of antibodies to hepatitis A and E viruses in Pune, India, 1982 and 1992. J Infect Dis. 1995;171:447–450. doi: 10.1093/infdis/171.2.447. [DOI] [PubMed] [Google Scholar]
- 23.Khuroo MS, Kamili S, Dar MY, Moecklii R, Jameel S. Hepatitis E and long-term antibody status. Lancet. 1993;341:1355. [PubMed] [Google Scholar]
- 24.Rapicetta M, Kondili LA, Pretolani S et al. Seroprevalence and anti-HEV persistence in the general population of the Republic of San Marino. J Med Virol. 1999;58:49–53. doi: 10.1002/(sici)1096-9071(199905)58:1<49::aid-jmv7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 25.Ding X, Li TC, Hayashi S et al. Present state of hepatitis E virus epidemiology in Tokyo, Japan. Hepatol Res. 2003;27:169–173. doi: 10.1016/s1386-6346(03)00233-x. [DOI] [PubMed] [Google Scholar]
- 26.Tilg H, Wilmer A, Vogel W et al. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 1992;103:264–274. doi: 10.1016/0016-5085(92)91122-k. [DOI] [PubMed] [Google Scholar]
- 27.Menendez-Caro JL, Alvarez-Mon M, Giron JA et al. Increased IgM B cell differentiation lymphokine production by T lymphocytes from patients with primary biliary cirrhosis. J Hepatol. 1994;20:446–453. doi: 10.1016/s0168-8278(05)80488-x. [DOI] [PubMed] [Google Scholar]
- 28.Abrignani S. Antigen-independent activation of resting T-cells in the liver of patients with chronic hepatitis. Dev Biol Stand. 1998;92:191–194. [PubMed] [Google Scholar]
- 29.Streetz KL, Tacke F, Leifeld L et al. Interleukin 6/gp 130-dependent pathways are protective during chronic liver diseases. Hepatology. 2003;38:218–229. doi: 10.1053/jhep.2003.50268. [DOI] [PubMed] [Google Scholar]
- 30.Mast EE, Alter MJ, Holland PV, Purcell RH. Evaluation of assays for antibody to hepatitis E virus by a serum panel Hepatitis E Virus Antibody Serum Panel Evaluation Group. Hepatology. 1998;27:857–861. doi: 10.1002/hep.510270331. [DOI] [PubMed] [Google Scholar]
- 31.Ghabrah TM, Tsarev S, Yarbough PO et al. Comparison of tests for antibody to hepatitis E virus. J Med Virol. 1998;55:134–137. [PubMed] [Google Scholar]