Abstract
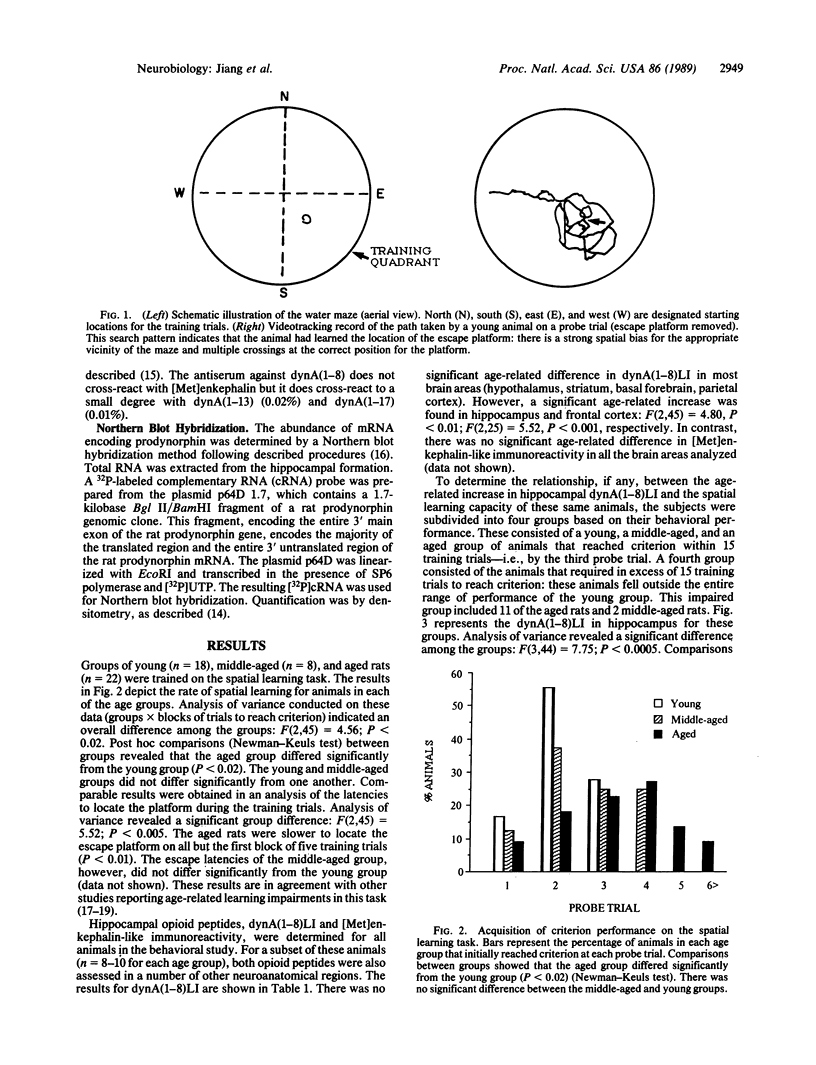
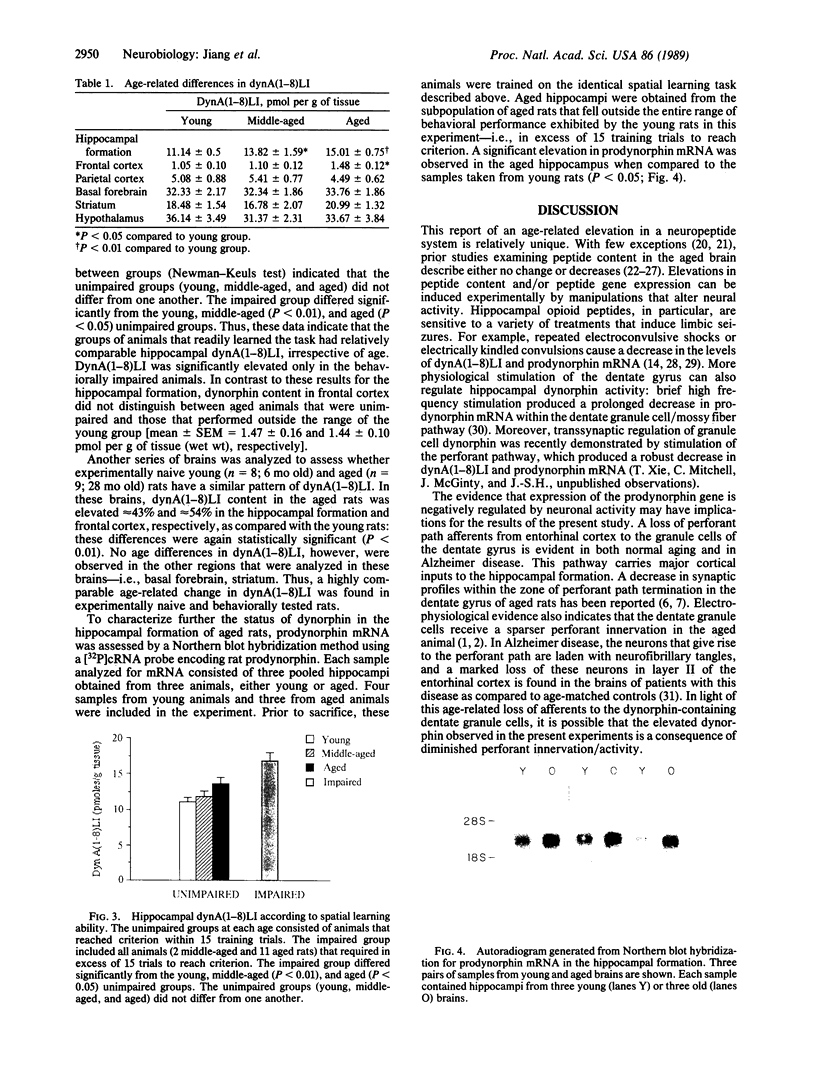
Radioimmunoassay revealed increased dynorphin A(1-8)-like immunoreactivity [dynA(1-8)LI] in the aged rat brain. Among a number of brain regions examined, an age-related dynA(1-8)LI elevation was found only in the hippocampal formation and frontal cortex. Moreover, the increase in dynA(1-8)LI in the aged hippocampus was associated with a decline in spatial learning ability: dynA(1-8)LI distinguished aged rats that were behaviorally impaired from aged cohorts that learned the spatial task as rapidly as younger animals. Northern blot hybridization using a 32P-labeled complementary RNA probe encoding rat prodynorphin indicated that the abundance of prodynorphin mRNA was also significantly increased in the hippocampal formation of aged rats with identified spatial learning impairments.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball M. J. Neuronal loss, neurofibrillary tangles and granulovacuolar degeneration in the hippocampus with ageing and dementia. A quantitative study. Acta Neuropathol. 1977 Feb 28;37(2):111–118. doi: 10.1007/BF00692056. [DOI] [PubMed] [Google Scholar]
- Barden N., Dupont A., Labrie F., Mérand Y., Rouleau D., Vaudry H., Boissier J. R. Age-dependent changes in the beta-endorphin content of discrete rat brain nuclei. Brain Res. 1981 Mar 9;208(1):209–212. doi: 10.1016/0006-8993(81)90634-x. [DOI] [PubMed] [Google Scholar]
- Barnes C. A., McNaughton B. L. Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J Physiol. 1980 Dec;309:473–485. doi: 10.1113/jphysiol.1980.sp013521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes C. A. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979 Feb;93(1):74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Brizzee K. R., Ordy J. M. Age pigments, cell loss and hippocampal function. Mech Ageing Dev. 1979 Jan;9(1-2):143–162. doi: 10.1016/0047-6374(79)90126-x. [DOI] [PubMed] [Google Scholar]
- Buck S. H., Deshmukh P. P., Burks T. F., Yamamura H. I. A survey of substance P, somatostatin, and neurotensin levels in aging in the rat and human central nervous system. Neurobiol Aging. 1981 Winter;2(4):257–264. doi: 10.1016/0197-4580(81)90033-6. [DOI] [PubMed] [Google Scholar]
- Civelli O., Douglass J., Goldstein A., Herbert E. Sequence and expression of the rat prodynorphin gene. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4291–4295. doi: 10.1073/pnas.82.12.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier T. J., Routtenberg A. Selective impairment of declarative memory following stimulation of dentate gyrus granule cells: a naloxone-sensitive effect. Brain Res. 1984 Sep 24;310(2):384–387. doi: 10.1016/0006-8993(84)90166-5. [DOI] [PubMed] [Google Scholar]
- De Ceballos M. L., Boyce S., Taylor M., Jenner P., Marsden C. D. Age-related decreases in the concentration of Met- and Leu-enkephalin and neurotensin in the basal ganglia of rats. Neurosci Lett. 1987 Mar 20;75(1):113–117. doi: 10.1016/0304-3940(87)90085-1. [DOI] [PubMed] [Google Scholar]
- Decker M. W. The effects of aging on hippocampal and cortical projections of the forebrain cholinergic system. Brain Res. 1987 Nov;434(4):423–438. doi: 10.1016/0165-0173(87)90007-5. [DOI] [PubMed] [Google Scholar]
- Dupont A., Savard P., Mérand Y., Labrie F., Boissier J. R. Age-related changes in central nervous system enkephalins and substance P. Life Sci. 1981 Nov 30;29(22):2317–2322. doi: 10.1016/0024-3205(81)90565-8. [DOI] [PubMed] [Google Scholar]
- Gage F. H., Dunnett S. B., Björklund A. Spatial learning and motor deficits in aged rats. Neurobiol Aging. 1984 Spring;5(1):43–48. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Gallagher M., Bostock E., King R. Effects of opiate antagonists on spatial memory in young and aged rats. Behav Neural Biol. 1985 Nov;44(3):374–385. doi: 10.1016/s0163-1047(85)90688-0. [DOI] [PubMed] [Google Scholar]
- Gallagher M., King R. A., Young N. B. Opiate antagonists improve spatial memory. Science. 1983 Sep 2;221(4614):975–976. doi: 10.1126/science.6879198. [DOI] [PubMed] [Google Scholar]
- Gambert S. R., Garthwaite T. L., Pontzer C. H., Hagen T. C. Age-related changes in central nervous system beta-endorphin and ACTH. Neuroendocrinology. 1980 Oct;31(4):252–255. doi: 10.1159/000123083. [DOI] [PubMed] [Google Scholar]
- Geinisman Y., Bondareff W., Dodge J. T. Partial deafferentation of neurons in the dentate gyrus of the senescent rat. Brain Res. 1977 Oct 14;134(3):541–545. doi: 10.1016/0006-8993(77)90828-9. [DOI] [PubMed] [Google Scholar]
- Geinisman Y., de Toledo-Morrell L., Morrell F. Loss of perforated synapses in the dentate gyrus: morphological substrate of memory deficit in aged rats. Proc Natl Acad Sci U S A. 1986 May;83(9):3027–3031. doi: 10.1073/pnas.83.9.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genisman Y., Bondareff W. Decrease in the number of synapses in the senescent brain: a quantitative electron microscopic analysis of the dentate gyrus molecular layer in the rat. Mech Ageing Dev. 1976 Jan-Feb;5(1):11–23. doi: 10.1016/0047-6374(76)90003-8. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Yang H. Y., Fratta W., Costa E. Rat striatal methionine-enkephalin content after chronic treatment with cataleptogenic and noncataleptogenic antischizophrenic drugs. J Pharmacol Exp Ther. 1978 Apr;205(1):141–147. [PubMed] [Google Scholar]
- Hyman B. T., Van Hoesen G. W., Damasio A. R., Barnes C. L. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984 Sep 14;225(4667):1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Kanamatsu T., McGinty J. F., Mitchell C. L., Hong J. S. Dynorphin- and enkephalin-like immunoreactivity is altered in limbic-basal ganglia regions of rat brain after repeated electroconvulsive shock. J Neurosci. 1986 Mar;6(3):644–649. doi: 10.1523/JNEUROSCI.06-03-00644.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfield P. W., Baskin R. K., Pitler T. A. Brain aging correlates: retardation by hormonal-pharmacological treatments. Science. 1981 Oct 30;214(4520):581–584. doi: 10.1126/science.6270791. [DOI] [PubMed] [Google Scholar]
- Mani R. B., Lohr J. B., Jeste D. V. Hippocampal pyramidal cells and aging in the human: a quantitative study of neuronal loss in sectors CA1 to CA4. Exp Neurol. 1986 Oct;94(1):29–40. doi: 10.1016/0014-4886(86)90269-4. [DOI] [PubMed] [Google Scholar]
- McGinty J. F., Henriksen S. J., Goldstein A., Terenius L., Bloom F. E. Opioid peptide identity and localization in hippocampus. Life Sci. 1982 Oct 18;31(16-17):1797–1800. doi: 10.1016/0024-3205(82)90213-2. [DOI] [PubMed] [Google Scholar]
- McGinty J. F., Kanamatsu T., Obie J., Dyer R. S., Mitchell C. L., Hong J. S. Amygdaloid kindling increases enkephalin-like immunoreactivity but decreases dynorphin-A-like immunoreactivity in rat hippocampus. Neurosci Lett. 1986 Oct 30;71(1):31–36. doi: 10.1016/0304-3940(86)90252-1. [DOI] [PubMed] [Google Scholar]
- Meaney M. J., Aitken D. H., van Berkel C., Bhatnagar S., Sapolsky R. M. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988 Feb 12;239(4841 Pt 1):766–768. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- Mitchell C. L., Grimes L., Hudson P. M., Hong J. S. Stimulation of the perforant path alters hippocampal levels of opioid peptides, glutamine and GABA. Brain Res. 1987 Dec 1;435(1-2):343–347. doi: 10.1016/0006-8993(87)91621-0. [DOI] [PubMed] [Google Scholar]
- Morris B. J., Feasey K. J., ten Bruggencate G., Herz A., Höllt V. Electrical stimulation in vivo increases the expression of proenkephalin mRNA and decreases the expression of prodynorphin mRNA in rat hippocampal granule cells. Proc Natl Acad Sci U S A. 1988 May;85(9):3226–3230. doi: 10.1073/pnas.85.9.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. G., Garrud P., Rawlins J. N., O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982 Jun 24;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Rapp P. R., Rosenberg R. A., Gallagher M. An evaluation of spatial information processing in aged rats. Behav Neurosci. 1987 Feb;101(1):3–12. doi: 10.1037//0735-7044.101.1.3. [DOI] [PubMed] [Google Scholar]
- Rogers J., Shoemaker W. J., Morgan D. G., Finch C. E. Senescent change in tissue weight and immunoreactive beta-endorphin, enkephalin, and vasopressin in eight regions of C57BL/6J mouse brain and pituitary. Neurobiol Aging. 1985 Spring;6(1):1–9. doi: 10.1016/0197-4580(85)90064-8. [DOI] [PubMed] [Google Scholar]
- Steger R. W., Sonntag W. E., Van Vugt D. A., Forman L. J., Meites J. Reduced ability of naloxone to stimulate LH and testosterone release in aging male rats; possible relation to increase in hypothalamic met5-enkephalin. Life Sci. 1980 Sep 1;27(9):747–753. doi: 10.1016/0024-3205(80)90328-8. [DOI] [PubMed] [Google Scholar]