SUMMARY
The objectives of these studies were to analyse the effect of mass influenza immunization in children on the morbidity of unvaccinated non-institutionalized elderly during an influenza epidemic. A mass vaccination campaign with vaccine was conducted in children aged 3–6 years attending kindergartens (57·4% of 6374) and aged 7–17 years attending schools (72% of 34 237) in two communities of the Moscow region. The clinical effectiveness of vaccination was 60·9% for kindergartens and 68·8% for schools. There were 3·4 times fewer episodes of influenza-like illnesses and 1·7–2·6 fewer episodes in all seven diseases which are possible complications of influenza out of the 10 evaluated diseases in 158 451 unvaccinated non-institutionalized elderly people during the influenza epidemic compared with the control communities. The differences were found to be statistically significant. Mass vaccination of children attending child institutions brought about a significant reduction of both influenza-like illnesses in children and influenza-associated illnesses in unvaccinated non-institutionalized elderly persons living in the home setting.
INTRODUCTION
It has been shown in several studies that children of both pre-school and school age are more frequently infected by influenza than adults and play a major role in the spread of the infection [1–10]. A study conducted in Tecumseh (USA) showed that immunization of 85% of all schoolchildren against influenza gave a three-fold reduction in the infection rate in other age groups, compared with a neighbouring community where schoolchildren were not targeted for immunization [10, 11]. Recently, it was shown in Japan that immunization of 50–85% of schoolchildren was associated with a significant decrease in mortality among non-immunized elderly persons during influenza epidemics [12]. In addition to reducing morbidity in non-immunized populations, mass immunization of children is found to be cost-effective for society [13, 14].
While persons aged >65 years of age are infected with influenza less frequently than other age groups, the influenza-related death rate is the highest in this age group [15, 16]. For example, in the United States during the 1999–2000 epidemic season, 40% of 110 000 patients admitted to hospital for influenza-related causes were elderly, and the elderly also accounted for 90% of 20 000 influenza fatalities [17].
The objectives of this study were to evaluate both the clinical effectiveness of mass immunization in children's institutions and the impact of mass immunization of children on the occurrence of influenza-like illnesses (ILI) and other influenza-associated diseases in unvaccinated non-institutionalized elderly people during an epidemic of influenza.
METHODS
The vaccine
A trivalent inactivated subunit influenza vaccine Influvac¯ in disposable prefilled syringes (manufactured by Solvay Pharma, Weesp, The Netherlands) with antigens to A/New Caledonia/20/99 (H1N1), A/Moscow/10/99 (H3N2) and B/Sichuan/379/99 influenza virus strains (recommended by the WHO for use in influenza vaccines in the 2001–2002 season) was used in the study [18].
The population
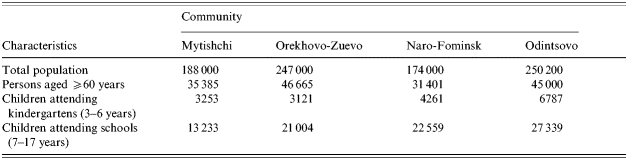
The study was conducted in four areas of the Moscow region, Russia. These were the Mytishchi area (M), the Orekhovo-Zuyevo area (OZ), the Odintsovo area (O), and the Naro-Fominsk area (N) (see Table 1). Socio-economic and smoking conditions were similar, but there was some difference in demography – M+OZ had 9·3% children but N+O had 14·3%.
Table 1.
Demographic characteristics of the Moscow region communities studied
Immunization of children
Mass immunization of children against influenza was conducted in kindergartens (children aged 3–6 years) and schools (children aged 7–17 years) in the M and OZ areas. The target population included healthy children without contraindications whose parents had given their consent for immunization. The commercial vaccine Influvac¯ was used for immunization. Children were vaccinated in November of 2001 with 0·5 ml of the vaccine administered as a single intramuscular injection into the deltoid muscle. The N and O areas where children were largely not immunized, the coverage being below 1%, were taken as control communities.
Immunized children were followed up to evaluate the safety of the vaccine and monitor serious adverse events. All children were under direct observation by a doctor or nurse for 30–60 min immediately after the injection. Their parents were instructed either to call for a doctor or bring the child to the polyclinic if they noticed any unease, a serious local reaction to the vaccination or a more serious adverse event such as a body temperature rise above 38°C or any other unusual reaction. On days 3–5 after the immunization, a doctor or nurse checked the children's attendance at school/kindergarten by telephone and made home visits where necessary. Observed adverse events were immediately reported to the local Centre for Sanitation and Epidemiology, which forwarded the information via the regional Centre for Sanitation, and Epidemiology to the State Control Institute for Standardization and Control of Medical Biological Preparations where the causes of the observed adverse events were analysed.
Children attending kindergartens and schools were followed up throughout the epidemic season from December 2001 to April 2002, both in the target and control communities. Children were considered affected by an ILI only when a physician had diagnosed the disease. The clinical diagnosis of ILI was made in accordance with WHO clinical case definition: sudden onset of fever of >38°C and cough or sore throat. All cases clinically close to this definition were classified as ILI. In all four communities, data on the occurrence of ILI in children and schools throughout the epidemic season (December 2001–March 2002) were collected and analysed separately for schools and kindergartens. Similar data were available on the occurrence of ILI in the same areas in 1999, 2000 and 2001 when children were generally not immunized against influenza.
Evaluation of morbidity in the elderly
Out of a total of 158 451 unvaccinated non-institutionalized elderly persons (>60 years) followed up in all four study areas, 82 050 were residents of the two communities (M and OZ) where mass immunization of children had been conducted and the other 76 401 were from the control communities (N and O). Approximately 60–70% of children were vaccinated in the M+OZ communities and not more than 1% in the N+O communities. The overwhelming majority of the elderly lived in the same household as their children/grandchildren. These groups of elderly in the four communities were comparable in terms of demographics, health status and habits. The immunization coverage rates among the elderly in the study communities did not exceed 1% due to a shortage of the influenza vaccine in the Moscow region. Morbidity was determined based on information in the special registration forms kept at local polyclinics and containing clinical diagnoses made by a district physician after his/her home visit to the patient or the presentation of the patient at the doctor's surgery. It should be noted that district physicians and polyclinic staff in all areas were not informed if children in the area were vaccinated or not. Data on the following diseases were included in the final analysis: ILI, pneumonia, bronchial asthma (exacerbation), chronic bronchitis (exacerbation), cardiovascular diseases, diabetes mellitus, gastrointestinal diseases, chronic pyelonephritis (exacerbation) were selected as possible complication of symptomatic influenza and the next three – pancreatitis, cholecystitis, and rheumatoid arthritis – were selected as ‘control’ diseases that were not associated with influenza incidence. In all four communities, data on the occurrence of each of the above diseases were collected and analysed throughout the epidemic season (December 2001–March 2002). Similar data on the occurrence of the above diseases in the elderly were also collected in August–September 2002 when no ILI was reported.
Immunization of health workers
Over 80% of all medical staff working in kindergartens, schools and polyclinics (for outpatient care) were immunized with inactivated influenza vaccine in November–December 2001 in all study communities.
Statistical analysis
The clinical effectiveness (E) of the vaccine was calculated based on the following formula:
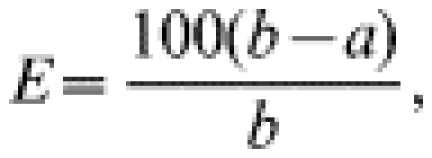 |
where a is the attack rate of ILI in immunized children and b is the attack rate of ILI in the non-immunized study population. Statistical analysis was calculated based on χ2 and Fisher tables. Differences at P<0·05 were considered statistically significant.
RESULTS
Characterization of the influenza epidemic in the study areas of the Moscow region
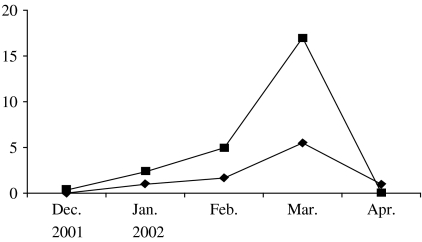
According to the WHO's report [19], in many European countries the influenza epidemic of the 2001–2002 season lasted from late December, 2001 to late March, 2002 and was moderately severe. The influenza epidemic in the study areas of the Moscow region was also moderate in the 2001–2002 season. According to clinical and laboratory ILI diagnostic data, the epidemic started in mid-December and was over by the end of April, its peak activity being observed in March, 2002 (Fig.). In January, only A/H3N2 influenza virus was found to be circulating in the communities while in February–March both A/H3N2 and B influenza viruses were isolated at approximately the same rate as in other countries in the 2001–2002 season [20]. The analysis of the antigenic specificity of the influenza virus isolates showed a good antigenic match between the A/H3N2 and B influenza viruses which were circulating in the Moscow region and the A/Moscow/10/99(H3N2) and B/Sichuan/379/99 virus strains used in the vaccine. It should be noted that peak activity of ILI was observed in March in children as well as in elderly persons.
Fig.
✦—✦, Influenza-like illnesses (ILI) in children in vaccinated (Mytishchi+Orekhovo-Zuevo) communities; ▪—▪, ILI in children in control (Naro-Fominsk+Odintsovo) communities.
Clinical effectiveness of immunization of children against influenza
Prior to the mass immunization campaign in children, data on the ILI rates in the four study areas were also collected and analysed in 1999, 2000 and 2001. During these years no mass immunization against influenza was conducted in these communities. A comparison of the morbidity data showed that the M+OZ communities taken together had had a somewhat higher ILI rate both in the whole population and in children <14 years, compared to the N+O communities (Table 2). Therefore, the M and OZ communities were selected as targets for mass immunization of children while the N and O communities were taken as the control.
Table 2.
Incidence of ILI in the study areas of the Moscow region by year (overall data per year)
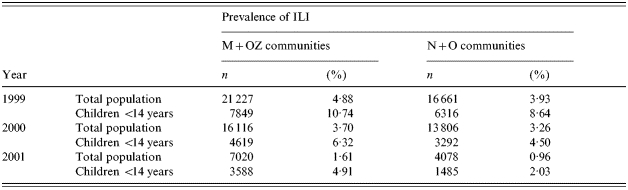
ILI, Influenza-like illnesses; M, Mytishchi; OZ, Orekhovo-Zuevo; N, Naro-Fominsk; O, Odintsovo.
In the M+OZ communities, 28 309 children were vaccinated (3658 in kindergartens and 24 651 in schools) out of a total of 40 611. The coverage rates were 57·4 and 72·0% respectively. In the control communities (N+O) 11 048 and 49 898 children from kindergartens and schools respectively were followed up. The number of children vaccinated against influenza in the control communities did not exceed 1%. Not one single serious adverse reaction to the vaccine used was observed immediately after the injection or during the follow-up check on days 3–5 after injection.
Table 3 shows the ILI incidence rates in children attending kindergartens and schools in the target and control communities (morbidity was calculated as the percentage of children with ILI out of all children in the groups).
Table 3.
ILI in children in mass vaccination vs. control communities (overall data for December 2001–March 2002)
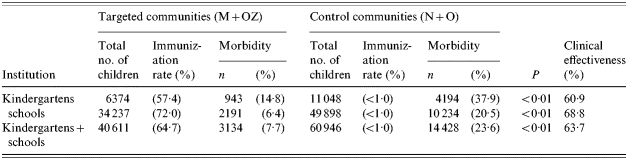
ILI, Influenza-like illnesses; M, Mytishchi; OZ, Orekhovo-Zuevo; N, Naro-Fominsk; O, Odintsovo.
Analysis of morbidity throughout the epidemic season (December 2001–March 2002) showed that M+OZ kindergarten children had 2·56 times (60·9%) fewer ILI cases than kindergarten children in the control communities N+O. M+OZ schoolchildren had 3·2 times (68·8%) fewer ILI cases compared with the control communities. The differences were significant (P<0·01). In analyses of totals of kindergartens and schools in vaccinated and non-vaccinated communities the clinical effectiveness was 63·7%. All children in this study were aged >3 years and received only one injection of the vaccine. But clinical effectiveness of the immunization was 60·9–68·8%. Perhaps with two injections of the vaccine the effectiveness would be better but in our study the number of doses of the vaccine was limited.
A comparison of morbidity of the elderly in the target and control communities
A total of 158 451 non-institutionalized elderly persons aged >60 years were included in the follow-up. Out of these, 82 051 resided in the target communities where the mass vaccination of children was conducted and 76 401 in the control communities. Immunization rates of the elderly in the study communities did not exceed 1%.
Data presented in Table 4 show that from December 2001 to March 2002, the ILI rate in the elderly of the immunized communities was 3·4 times lower than in the control communities. The difference was found to be significant.
Table 4.
Morbidity among elderly persons aged ⩾60 years in mass children vaccination (M+OZ) and control (N+O) communities (overall data for December 2000–March 2002 and for August–September 2002)
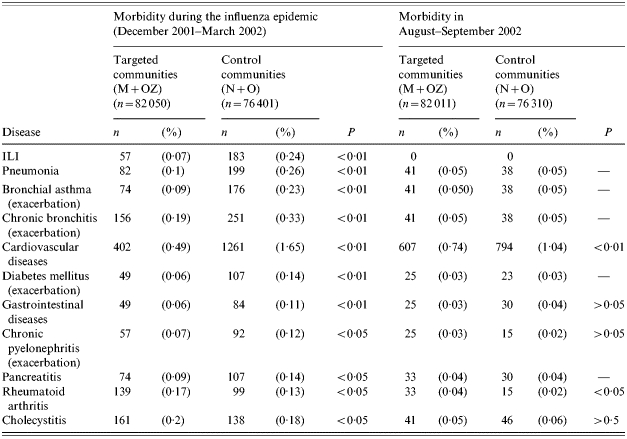
ILI, Influenza-like illnesses; M, Mytishchi; OZ, Orekhovo-Zuevo; N, Naro-Fominsk; O, Odintsovo.
Data on the morbidity of the elderly in December–March are indicative of a statistically significant reduction in the occurrence of most medical conditions evaluated in the study in the target communities vs. the control communities. It was found that compared with the control communities (N and O), in the M and OZ communities the reduction was for the seven diseases which were selected as possible complications of symptomatic influenza: 2·6-fold for pneumonia; 2·5-fold for bronchial asthma; 1·7-fold for chronic bronchitis; 3·4-fold or by 2·0-fold following adjustment for cardiovascular diseases (see below); 2·3-fold for diabetes mellitus; 1·8-fold for gastrointestinal diseases; and 1·7-fold for chronic pyelonephritis. All these differences were statistically significant (Table 4). As to the three diseases selected as ‘control’ the numbers of episodes of rheumatoid arthritis and cholecystitis in control communities were fewer in comparison with vaccinated communities. Surprisingly there were fewer episodes of pancreatitis in comparison with control communities. The target communities had a somewhat higher incidence of rheumatoid arthritis compared with the control communities. Differences for cholecystitis were found to be insignificant.
To check the validity of differences in the morbidity of the elderly in the immunized and control communities during the influenza epidemic, data on the occurrence of the same diseases in the same communities were collected in August–September 2002. At that time the number of cases of upper respiratory tract infections in elderly persons was very low, and only one case of ILI was diagnosed at that period. It was found (Table 4) that differences in the morbidity in the summertime were either nil or insignificantly small for all of the evaluated medical conditions, except for cardiovascular diseases. Indeed, elderly persons in the target communities had 1·4 times fewer episodes of cardiovascular diseases during this period compared to the control communities. During the influenza epidemic, the rate of cardiovascular diseases had been 3·4 times lower in the target communities, but it became 2·0 times lower following data adjustment. The incidence of rheumatoid arthritis was found to be higher in the areas with mass vaccination of children compared to the control communities, just as it had been during the influenza epidemic.
Therefore, there was a statistically significant 3·4-fold reduction of the ILI rate and a 1·7- to 2·6-fold reduction in elderly morbidity in the target vs. control communities during the influenza epidemic for 6 out of 10 evaluated medical conditions.
DISCUSSION
Children are more frequently infected with influenza than adults and play a major role in its spread. Mass vaccination of children against influenza was found to result in a reduced morbidity among unvaccinated persons [10, 11] and mortality in the elderly [12]. Vaccination of 80% of troops in barracks led to a substantial decrease in morbidity among unvaccinated servicemen [21]. No case of influenza was reported during the influenza epidemic in a small community in Australia following vaccination of 84% of the population [22].
The main objective of this study was to investigate the effect of mass vaccination of children on the occurrence of ILI and some other influenza-associated diseases in unvaccinated elderly persons living mainly in families with their children/grandchildren. The immunization procedure using inactivated influenza vaccine available in disposable pre-filled syringes and administered intramuscularly took no longer than intranasal administration of live attenuated vaccine. It can, therefore, be concluded that currently available inactivated vaccines supplied in disposable pre-filled syringes can be effectively used for mass vaccination.
A comparison of incidence rates of ILI in vaccinated and unvaccinated children populations showed that there was a statistically significant reduction in the occurrence of ILI in vaccinated children compared to unvaccinated children in the control communities throughout the epidemic season (December 2001–March 2002). The clinical effectiveness of children's vaccination in kindergartens was 60·9% and in schools it was 68·8%. In general, the efficacy of the vaccine in preventing ILI in this study was comparable to that observed in other studies on immunization of healthy subjects [23–25].
It was found that in our studies during the epidemic (December 2001–March 2002), unvaccinated non-institutionalized elderly persons aged ⩾60 years living in the communities with mass immunization of children had 3·4 times fewer ILI and 1·7–2·6 times fewer episodes of all seven diseases which were selected as possible complications of symptomatic influenza compared to those elderly people who lived in the control communities. This was true for pneumonia, bronchial asthma, chronic bronchitis, cardiovascular disease, diabetes mellitus, gastrointestinal disease, and pyelonephritis. As to three ‘control’ diseases we surprisingly found data about fewer episodes of pancreatitis in unvaccinated communities in comparison with control areas. It should be noted that validity of differences in the morbidity of the elderly in the vaccinated and control communities were confirmed by studies of elderly morbidity with the same diseases in the same communities during August–September 2002 when the number of cases of upper respiratory tract infections was very low. This study shows that differences in the morbidity in summertime were either nil or insignificant. As noted above, the target communities M+OZ had 9·3% children and the control communities had 14·3% children but we supposed that the 5% difference cannot substantially affect the difference of the morbidity in the elderly in the target and control communities found in our study.
It is possible that the incidence of ILI in elderly persons was somewhat under-reported. Most of the elderly in the Moscow region are non-working retired people living in households together with their family, and in mild cases of ILI without complications, not all elderly people called or visited a doctor. In cases of more serious diseases (such as those analysed in our studies) elderly people did seek medical advice.
It should be noted that the analysis of an influenza simulation model suggested that immunization of only 50% of schoolchildren in a community may result in a 49–65% reduction in attack rates of influenza in other unvaccinated population groups [26].
Influenza infection is known to produce malfunction in many organs such as lungs, heart, vessels, liver, kidneys, muscles, and other body systems [27]. In many published studies, vaccination of elderly persons against influenza was shown to result in fewer cardiopulmonary and other influenza-associated complications [27–30]. Moreover, adults living in families together with their children were found to acquire influenza more often than those in childless families [31]. Immunization of children can reduce the risk of secondary transmission of influenza within families [32, 33].
Our findings lead us to conclude that mass vaccination of children attending school or kindergarten with inactivated vaccine not only prevents ILI in most children, but additionally results in a substantial reduction in the occurrence of ILI in unvaccinated non-institutionalized elderly persons and prevents several serious illnesses in this population. Although these findings may not be applicable immediately in many developed countries, where a high proportion of elderly people are vaccinated on an annual basis, the finding may be far more relevant in a pandemic situation where there is insufficient vaccine available to cover both children and the elderly and some from of prioritization has to be introduced. This paper suggests that targeting school- and kindergarten-age children for vaccination against influenza may protect the unvaccinated elderly and may contribute towards preparing pandemic vaccination strategies.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Glezen N, Paredes A, Taber L. Influenza in children: relationship to other respiratory agents. Journal of the American Medical Association. 1980;243:1345–1349. doi: 10.1001/jama.243.13.1345. [DOI] [PubMed] [Google Scholar]
- 2.Neuzil K. Burden of interpandemic influenza in children younger than 5 years: a 25-year prospective study. Journal of Infectious Diseases. 2002;185:147–152. doi: 10.1086/338363. et al. [DOI] [PubMed] [Google Scholar]
- 3.Jordan W. The mechanism of spread of Asian influenza. American Review of Respiratory Disease. 1961;2:29–35. doi: 10.1164/arrd.1961.83.2P2.29. [DOI] [PubMed] [Google Scholar]
- 4.Fox J. Influenza virus infections in Seattle families, 1975–1979. Study design, methods and the occurrence of infections by time and age. American Journal of Epidemiology. 1982;116:212–227. doi: 10.1093/oxfordjournals.aje.a113407. et al. [DOI] [PubMed] [Google Scholar]
- 5.Glezen W, Couch R. Interpandemic influenza in the Houston area, 1974–76. New England Journal of Medicine. 1978;298:587–592. doi: 10.1056/NEJM197803162981103. [DOI] [PubMed] [Google Scholar]
- 6.Longini I. Estimating household and community transmission parameters for influenza. American Journal of Epidemiology. 1982;115:736–751. doi: 10.1093/oxfordjournals.aje.a113356. et al. [DOI] [PubMed] [Google Scholar]
- 7.Foy H, Cooney M, Allan L. Longitudinal studies of types A and B influenza among Seattle schoolchildren and families, 1968–1974. Journal of Infectious Diseases. 1976;134:362–369. doi: 10.1093/infdis/134.4.362. [DOI] [PubMed] [Google Scholar]
- 8.Gruber W., Nicholson K, Webster R, Hay A. Textbook for Influenza. Blackwell Science; 1998. Children as a target for immunization; pp. 433–444. : pp. [Google Scholar]
- 9.Wright P. Influenza in the family. New England Journal of Medicine. 2000;343:1331–1332. doi: 10.1056/NEJM200011023431808. [DOI] [PubMed] [Google Scholar]
- 10.Monto A. Effect of vaccination of a school-age population on the course of an A2/Hong Kong influenza epidemic. Bulletin of the World Health Organization. 1969;41:537–542. et al. [PMC free article] [PubMed] [Google Scholar]
- 11.Monto A. Modification of an outbreak of influenza in Tecumseh, Michigan by vaccination of schoolchildren. Journal of Infectious Diseases. 1970;122:16–25. doi: 10.1093/infdis/122.1-2.16. et al. [DOI] [PubMed] [Google Scholar]
- 12.Reichert T. The Japanese experience with vaccinating schoolchildren against influenza. New England Journal of Medicine. 2001;344:889–896. doi: 10.1056/NEJM200103223441204. et al. [DOI] [PubMed] [Google Scholar]
- 13.White T, Lavoie S, Nettelman M. Potential cost saving attributed to influenza vaccination of school-age children. Pediatrics. 1999;103:1273. doi: 10.1542/peds.103.6.e73. : electronic abstract; p. [DOI] [PubMed] [Google Scholar]
- 14.Cohen G, Nettleman M. Economic impact of influenza vaccination in preschool children. Pediatrics. 2000;106:973–976. doi: 10.1542/peds.106.5.973. [DOI] [PubMed] [Google Scholar]
- 15.Glezen W. Serious morbidity and mortality associated with influenza epidemics. Epidemiologic Reviews. 1982;4:25–44. doi: 10.1093/oxfordjournals.epirev.a036250. [DOI] [PubMed] [Google Scholar]
- 16.Couch R, Kasel G, Glezen W. Influenza; its control in person and population. Journal of Infectious Diseases. 1986;153:431–440. doi: 10.1093/infdis/153.3.431. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Prevention and control of influenza; recommendations of the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report. 2000;49:1–37. [PubMed] [Google Scholar]
- 18.Recommendation composition of influenza virus vaccines for use in the 2001–2002 season. Weekly Epidemiological Record. 2001;76:40–44. [Google Scholar]
- 19.Communicable Disease Surveillance and Response. http://www.who.int/emc/diseases/flu/country. 2002. http://www.who.int/emc/diseases/flu/country ). Accessed .
- 20.Lin Y. Recent change among human influenza virus. Virus Research. 2004;103:47–52. doi: 10.1016/j.virusres.2004.02.011. et al. [DOI] [PubMed] [Google Scholar]
- 21.Sonoguchi T. Hemagglutination-inhibiting antibody response to and efficacy of inactivated Hong Kong influenza vaccine. Bulletin of the World Health Organization. 1969;41:517–519. [PMC free article] [PubMed] [Google Scholar]
- 22.Warburton M. Herd immunity following subunit influenza vaccine administration. Medical Journal of Australia. 1972;2:67–70. doi: 10.5694/j.1326-5377.1972.tb47154.x. et al. [DOI] [PubMed] [Google Scholar]
- 23.Nichol K., Nicholson K, Webster R, Hay A. Textbook of Influenza. Blackwell Science; 1998. Efficacy/clinical effectiveness of inactivated influenza virus vaccines in adults; pp. 358–372. ; pp. [Google Scholar]
- 24.Couch R, Brown L, Hampson A, Webster R. Option for the Control of Influenza III. New York: Elsevier; 1996. Prevention of influenza virus infection by current inactivated influenza virus vaccine; pp. 97–106. et al. ; pp. [Google Scholar]
- 25.Bridges C. Effectiveness and cost-benefit of influenza vaccination of health working adults. Journal of the American Medical Association. 2000;284:1655–1663. doi: 10.1001/jama.284.13.1655. et al. [DOI] [PubMed] [Google Scholar]
- 26.Evelback L. An influenza simulation model for immunization studies. American Journal of Epidemiology. 1976;103:152–165. doi: 10.1093/oxfordjournals.aje.a112213. et al. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson K., Nicholson K, Webster R, Hay A. Textbook of Influenza. Blackwell Science; 1998. Human influenza; pp. 219–266. ; pp. [Google Scholar]
- 28.Nichol K. Influenza vaccination and reduction hospitalization for cardiac disease and stroke among the elderly. New England Journal of Medicine. 2003;348:1322–1332. doi: 10.1056/NEJMoa025028. et al. [DOI] [PubMed] [Google Scholar]
- 29.Hak E. Influence of high risk medical conditions on the effectiveness of influenza vaccination among elderly members of three large managed-care organization. Clinical Infectious Diseases. 2002;35:370–377. doi: 10.1086/341403. et al. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen-Van-Tam J, Brown L, Hampson A, Webster R. Option for Influenza Control III. New York: Elsevier; 1966. Reduction in hospital admissions for pneumonia, influenza, bronchitis and emphysema associated with influenza vaccine during the 1989–90 epidemic in Leicestershire, UK; pp. 107–112. et al. ; pp. [Google Scholar]
- 31.Frank A, Taber L, Wells J. Comparision of infection rates and severity of illness for influenza A subtypes N1N1 and H3N2. Journal of Infectious Diseases. 1985;151:73–80. doi: 10.1093/infdis/151.1.73. [DOI] [PubMed] [Google Scholar]
- 32.Hurwitz E. Effectiveness of influenza vaccination of daycare children in reducing influenza-related morbidity among household contacts. Journal of the American Medical Association. 2000;284:1677–1682. doi: 10.1001/jama.284.13.1677. et al. [DOI] [PubMed] [Google Scholar]
- 33.Esposito S. Effectiveness of influenza vaccination of children with recurrent respiratory tract infection in reducing respiratory-related morbidity within the households. Vaccine. 2003;21:3162–3168. doi: 10.1016/s0264-410x(03)00253-6. et al. [DOI] [PubMed] [Google Scholar]