SUMMARY
This review focuses on current and future prevention of invasive cervical cancer (ICC), the second most common cancer among women worldwide. Implementation of population-based cytological screening programmes, using the ‘Pap’ smear to detect pre-cancerous lesions in the cervix, has resulted in substantial declines in mortality and morbidity from ICC in North America and some European countries. However, cases of, and deaths from, ICC continue to occur. Primary prevention of infection with high-risk human papillomavirus (HPV) types, the central causal factor of ICC, could further reduce incidence of and mortality from ICC. This is particularly the case in developing countries, which bear 80% of the burden of ICC, and where effective Pap screening programmes are extremely difficult to implement. Very promising results from several trials of synthetic HPV type-specific monovalent (HPV 16) and bivalent (HPV 16 and 18) vaccines have recently been published, showing high efficacy against type-specific persistent HPV infection and development of type-specific pre-cancerous lesions. Large-scale phase III trials of a number of such vaccine candidates are currently underway, and there is real hope that an effective vaccine capable of protecting against infection with HPV types 16 and 18 (which together account for ∼70% of cervical cancer cases worldwide), and thereby of preventing development of a very significant proportion of cases of ICC, could be available within the next 2 years.
INTRODUCTION
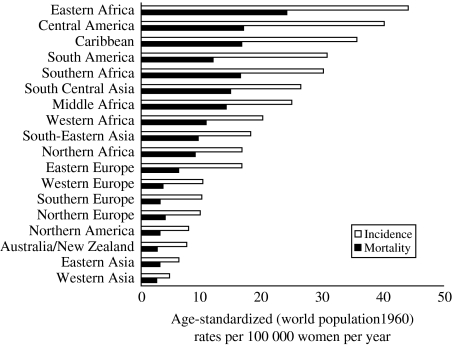
More than 450 000 cases of invasive carcinoma of the uterine cervix are diagnosed worldwide each year, resulting in nearly a quarter of a million deaths [1]. Despite being a theoretically preventable disease, cervical cancer is still the second most common cancer in women (after breast cancer) worldwide [2], and the fifth most frequent cancer overall, with an estimated prevalence of 1·4 million cases. Incidence rates are highest in developing countries, which bear 80% of the burden of cervical cancer (Fig. 1). It is the most common cause of cancer-related mortality among women in many countries of Africa, South and Central America and the Caribbean.
Fig. 1.
Annual invasive cervical cancer incidence and mortality rates in different regions of the world (IARC/GloboCan 2000). Rates are standardized according to age distribution of world population in 1960. (Figure provided by Eduardo Franco.)
In Western Europe, ∼33 500 new cases of cervical cancer are diagnosed each year and 15 000 women die from the disease [3]. In the United States, an estimated 13 000 new cases of cervical cancer, and 4000 deaths, occurred in 2003 [4]. Incidence of cervical cancer increases with age, rising sharply to 15 cases/100 000 between the ages of 20 and 35 years, then fluctuating around 15–20 cases/100 000 in older women. Median age at diagnosis is 48 years. Costs of treatment are high and rising: in the United States in 1994, it was estimated that the combined costs of treating cervical cancer exceeded $4.5 billion, more than any other single sexually transmitted infection (STI) with the exception of HIV [5].
Most cervical cancers (at least 75%) are of the squamous cell type. Adenocarcinomas account for ∼15%, and have been increasing in incidence during the last few decades, particularly in younger women [6, 7]. The term invasive refers to tumours in which the malignant cells have penetrated the underlying basement membrane and have infiltrated the stroma, with vascular and/or lymphatic invasion [5]. Invasive squamous cell cancers are graded as well-, moderately- or poorly-differentiated. Non-invasive squamous cell lesions are classified as pre-cancerous (atypia, dysplasia or cervical intra-epithelial neoplasia – CIN 1/2/3), or as carcinoma in situ (CIS), based on the thickness of epithelium occupied by undifferentiated basaloid cell types (cells resembling the basal cell layer of the epithelium). CIN lesions share some morphological features with CIS cells, and are thought to represent the earliest morphological changes associated with invasive cervical cancer (ICC). It is widely accepted that CIN and CIS are stages in the development of ICC, with CIS lesions thought to represent incipient ICC [5].
WHAT CAUSES CERVICAL CANCER?
Human papillomavirus (HPV)
The clinical and epidemiological profile of cervical cancer has long been recognized as suggestive of a sexually transmitted process, and numerous studies have confirmed the association between sexual exposure and development of CIS and ICC, stimulating a search for specific sexually transmitted agents that might act as carcinogens in genital cancers [5]. There is now consistent and convincing evidence that cervical cancer is in fact a rare consequence of infection of the genital tract by some mucosatropic types of HPV [2].
HPV, a small (∼8000 bp), double-stranded DNA virus which infects epithelial cells, was first isolated and linked to cervical cancer pathogenesis in the early 1980s. Strong clinical, epidemiological and molecular biological evidence indicates that specific types of sexually transmitted HPVs are the central causal factor in at least 95% of ICC cases [2, 8]. Mounting evidence also implicates HPV infection in a considerable proportion of other cancers of the ano-genital tract, including cancers of the vulva, vagina, anal canal, penis and perianal skin; as well as in some oropharyngeal and oesophageal carcinomas [9, 10].
There are over 100 types of HPV defined on the basis of DNA homology, of which over 40 strains can infect the epithelial lining of the ano-genital tract. Clinical and subclinical HPV infection is the most common STI today, with asymptomatic cervical HPV infection detectable in 5–40% of women of reproductive age [8, 11, 12], and an estimated lifetime risk of infection with any genital HPV strain of 50–80%. Prevalence of HPV DNA (a measure of HPV exposure at a given time point) and HPV seroprevalence (a measure of cumulative HPV exposure) are strongly associated with number of lifetime and recent sexual partners [13–16]. Women tend to become HPV positive soon after initiation of sexual activity [11, 17]. Around 20–30% of HPV-infected women harbour multiple HPV types [18–20]. HPV infection generally persists for 6–12 months in the genital tract (with HPV 16 tending to persist longer than other types) and then becomes undetectable [11, 21], although it is unclear what fraction of infections are completely cleared rather than maintained in a latent or persistent state [22]. In general, prevalence peaks in women under 25 years of age, followed by a sharp decline to very low levels in older women. This may be due to acquired immunity to HPV infection over time and with multiple exposures [12].
Infection with HPV types classified as of low or no oncogenic risk (predominantly types 6 and 11) may cause subclinical infection and benign genital lesions (including low-grade CINs) and ano-genital warts (condylomata acuminata). Infection with high-risk, oncogenic HPV types (predominantly types 16 and 18) can lead to development of cervical cancer (Fig. 2). However over 90% of such infections, and the lesions (cytological abnormalities) caused by them, are transient or intermittent and resolve spontaneously [11, 17, 21]. Evidence suggests that, in general, cervical cancer develops only in the small proportion of women (<10%) with persistent (or latent) HPV infection [15, 23].
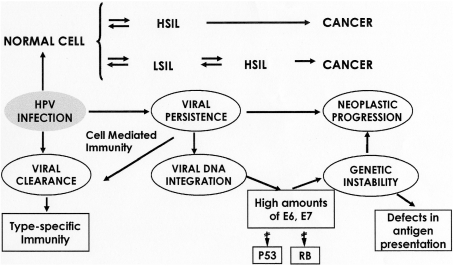
Fig. 2.
Mechanisms of human papillomavirus carcinogenesis. HSIL, High-grade squamous intra-epithelial lesion; LSIL, low-grade squamous intra-epithelial lesion; RB, retinoblastoma gene. [Reproduced with permission from Bosch et al. [2] (courtesy of John Schiller).]
HPV type 16 is the most commonly occurring oncogenic HPV type, and is present in ∼50% of cervical cancers and high-grade cervical intra-epithelial neoplasias (CIN 2/3), and in ∼25% of low-grade cervical intra-epithelial neoplasias (CIN 1). It is estimated that ∼20% of adults become infected with HPV 16 at some stage of their lifetime. HPV types 16 and 18 together account for ∼70% of ICC cases worldwide [24]. The remaining ∼30% of cancers contain a ‘local cocktail’ of other oncogenic HPV types, most commonly 31, 33 and 45, and less commonly, types 35, 51, 52, 58, 59 [2, 25]. The presence of other HPV types more rarely encountered in cervical cancer specimens, including HPV 39, 56, 68, 73 and 82, may be due to their oncogenic potential or to chance [25, 26]. Unclassified HPV types are also detected in a small proportion of cervical cancers. HPV type distribution in the population and in patients with cervical cancer shows some geographical variation, which has yet to be fully characterized [2].
In the IARC (International Agency for Research on Cancer) multi-centre case-control study, the pooled, age- and centre-adjusted odds ratio for presence of the 10 most common HPV types and cervical cancer was estimated at 83·3 [2]. The risk of development of cervical cancer linked to infection with multiple HPV types (the proportion of which varies across studies and particularly according to the sensitivity of the HPV detection method used), does not appear to vary significantly from that linked to single HPV types [2].
Co-factors
Given that only a very small subset of the many women infected with oncogenic types of HPV ever develop cervical cancer, interest has focused on the identification of other risk factors that might act in conjunction with HPV to increase the risk of persistent/latent HPV infection and/or of rates of progression of pre-cancerous lesions to high-grade cervical neoplasia and cancer. In addition to markers of risky sexual behaviour, including age at first sexual intercourse and number of sexual partners, other relevant co-factors include infection with other STIs, particularly Chlamydia trachomatis and herpes simplex virus type 2 (HSV-2), smoking, socio-economic status, diet and hormonal factors, including parity and oral contraceptive use. In the case of chlamydial infection, case-control and longitudinal nested case- control studies indicate that C. trachomatis seropositivity increases the risk of development of cervical squamous cell carcinoma, possibly through induction of chronic inflammation and/or production of mutagenic metabolites [27, 28]. Evidence is conflicting with respect to the role of HSV-2 infection [29–31]. Host factors may also be important in susceptibility to development of ICC following HPV infection, including major histocompatibility complex (HLA) types and p53 (tumour suppressor gene) polymorphisms [14, 32].
Lack of male circumcision has also been identified as a potential risk factor for ICC. Male circumcision is associated with a reduced risk of penile HPV infection, and, in the case of men with a history of multiple female sex partners, a reduced risk of cervical cancer in their current female partners [33].
Thus, both environmental and host factors may indeed modulate the effect of HPV infection on cervical cancer development, and may to some extent account for the geographical variation in cervical cancer incidence and the variability in risk estimates reported in different populations. For example, in addition to high HPV infection rates, risk or co-factors for cervical cancer, including other STIs, young age at marriage, parity, low socio-economic status and poor health-seeking behaviour are more prevalent in developing countries. While more research is necessary to clarify the exact roles of some of these modulating factors, the elucidation of the central and consistent role of HPV infection in the development of cervical cancer has nevertheless enabled a clear focus to emerge in terms of its primary and secondary prevention.
Prevention of cervical cancer
Secondary prevention: cytological screening
The cornerstone of current cervical cancer prevention programmes is cytology-based screening employing Papanicolaou staining of cervical swab or cytobrush specimens containing exfoliated cervical cells (the Pap smear). This cytological staining process enables microscopic detection of cellular changes characteristic of HPV infection (koilocytosis, dyskariosis) and associated with various stages of the development of ICC (dysplasia, CIN 1/2/3, CIS†). Women with pre-cancerous or cancerous lesions identified through Pap screening are referred for repeat Pap screening, colposcopy, biopsy and, where appropriate, treatment. The development and implementation of population-based Pap smear screening programmes for the early detection of pre-cancerous cervical lesions, together with aggressive treatment of women with abnormal biopsies, are thought to be largely responsible for the significantly reduced incidence of and mortality from ICC seen in many developed countries since the 1950s [8, 35–39].
The sensitivity of the Pap smear for detection of precursors of ICC is however sub-optimal and variable, ranging from around 30–90% in different studies, and is highly dependent on adequacy of sample collection, slide preparation and slide interpretation [8, 37, 40]. The specificity of the test varies between 85% and 100%, and thus, its predictive value for accurately predicting the risk of development of CIS and ICC is imperfect. Approximately 7% of women who undergo Pap testing in the United States are diagnosed with a cytological abnormality requiring additional follow-up or evaluation, although the vast majority of these abnormalities would regress without intervention [41]. Identification of the small proportion of women with low-grade cytological abnormalities who are at risk for development of significant cervical disease is a major current challenge.
Variable standards of screening, the inherent performance characteristics of the Pap smear, along with inappropriate screening regimens and insufficient population coverage rates (particularly for women of low socio-economic status who are most at risk for HPV infection and the development of ICC) are likely to continue to result in unacceptable rates of morbidity and mortality from cervical cancer. In the United States, around 50% of cervical cancers (∼7000/year) are diagnosed in patients who are being screened [41]. In the developed world overall, it is estimated that 90 000 cases of and 40 000 deaths from ICC still occur. In the European Union, despite extensive population-based Pap screening efforts in many countries, ∼22 000 new cases of cervical cancer are diagnosed each year and 13 000 women die from it [42]. Indeed, evidence from several European countries suggests that incidence of cervical cancer has increased in recent years, probably as a result of increasing HPV infection rates due to changes in sexual behaviour, especially decreases in age at sexual debut [26, 43].
In developing countries, where cervical cancer rates are highest, effective, high-quality population-based cytology screening programmes have proved very difficult to implement [44]. Where they exist, screening programmes lack coverage, accessibility, effectiveness and acceptability. Due to their inadequacy, cervical cancer is often detected at a late stage, with incidence often equal to mortality.
This situation, together with the rising cost of traditional cytology-based cervical cancer control in developed countries, has raised interest in a number of new approaches to cervical cancer control. These include: redesigning cytology-based screening strategies in terms of age at which screening commences and frequency of screening; introduction of thin-layer liquid-based cytology (LBC) to improve the performance (through improving smear quality and visibility) and sensitivity of cytology for detection of pre-cancerous lesions; and instituting a more conservative approach to the clinical management of low-grade cytological abnormalities [8]. Screening for high-risk HPV types in cervical samples, using DNA hybrid capture and nucleic acid amplification technology, is being explored for its utility both as a primary screening tool (particularly in women over 30 where HPV prevalence is lower), and as an adjunctive test in the management of women referred for abnormal cervical cytology [34, 45–48]. Such approaches may decrease the numbers of women who undergo unnecessary aggressive treatment for low-grade cytological lesions identified through traditional Pap screening, and may improve detection rates of CIN 2+ without increasing the colposcopy referral rate.
Given the difficulties involved in implementing effective screening programmes, primary prevention of infection with high-risk HPV types may be the most efficient and logistically feasible preventive intervention for cervical cancer, particularly in developing countries.
Primary prevention: prophylactic vaccines
HPV is in many ways an ideal target for vaccine development. It is a simple virus, with a small, stable genome which is not prone to mutation. DNA sequences of genital HPV types, particularly HPV 16, are highly conserved globally. It is possible to synthesize DNA-free, non-infectious virus-like particles (VLPs) in the laboratory through expression and self-assembly of the major capsid protein antigen L1 in eukaryotic cells. VLPs mimic the natural structure of the virion, and are capable of generating a potent humoral immune response with neutralizing antibodies in both animals and humans, with evidence of T-cell responses also reported in some studies [22].
In 2002, Koutsky and colleagues [49] reported on a randomized double-blind proof-of-concept trial of a monovalent synthetic vaccine consisting of DNA-free, HPV 16 L1 capsid protein-containing VLPs synthesized in a yeast expression system. In the trial, 2392 women aged 16–23 years received intramuscular injections of either vaccine or placebo at day 0, month 2 and month 6. After a median follow-up period of 17·4 months, no cases of persistent HPV 16 infection had occurred in the vaccine group (n=768 after exclusion and loss to follow-up), while all nine cases of HPV 16-related CIN occurred in the placebo group (incidence 3·8/100 person-years). In according-to-protocol (ATP) analyses, the efficacy of the vaccine in preventing transient HPV infection, persistent HPV infection and pre-invasive disease was 91·7%, 100% and 100% respectively, at 18 months. A seroconversion rate of 99·7% was reported, and mean antibody titres were ∼60-fold higher in vaccinated women than in women naturally infected with HPV 16 at enrolment.
The high levels of protection seen even against transient HPV infection suggest that the vaccine may induce protective immunity in at least some cases, while in others it may reduce the viral load and limit rounds of re-inoculation [22]. The primary mediators of protection are thought to be virus-neutralizing antibodies, transudated from serum into the cervical mucus. Cell-mediated immunity may also be involved.
More recently, in a Phase II trial of a bivalent HPV 16/18 L1 VLP vaccine in 1113 women, with 2·5 years follow-up, both ATP and intention-to-treat (ITT) analyses showed high efficacy of the bivalent vaccine against both incident and persistent HPV 16 and 18 infections, even with use of vaginal self-sampling, the most sensitive method for HPV detection [50]. In the ITT analysis, vaccine efficacy was 95·1% against persistent HPV 16/18 infection, and 92·9% against cytological abnormalities associated with HPV 16/18 infection (CIN 1/2); ATP analyses also demonstrated high efficacy against incident HPV 16/18 infections. The efficacy of the bivalent vaccine against HPV 18 infection is particularly important, since HPV 18 is more closely associated with cervical adenocarcinoma, which is more difficult to detect by Pap screening than squamous cell carcinoma [51].
While these results are encouraging, it will be necessary to wait for the results of further ITT analyses, as well as longer-term efficacy data, to evaluate fully the effectiveness of HPV VLP vaccines in protecting against development of ICC.
Large-scale, multi-centre, multi-country Phase III efficacy trials of bivalent (16/18) VLP vaccines are now being carried out in Europe, North, Central and South America, and Asia [52]. The end-points of these trials are incident and persistent HPV infection (2–3 years follow-up) and associated cytological and histological lesions (CIN; 2–3 and 4–5 years follow-up). Phase II and III trials of a quadrivalent HPV vaccine (HPV 6, 11, 16 and 18), which should, in principle, simultaneously protect against infection with the predominant strains causing both ano-genital warts and ICC, are also currently underway [52], with promising preliminary results [53]; as are plans for development of second-generation HPV vaccines containing additional high-risk types. Therapeutic HPV vaccines – which eradicate or reduce numbers of HPV-infected cells – are also promising, although in the early stages of development [22, 54].
Issues related to implementation and effectiveness of vaccination programmes against cervical cancer
If the large-scale efficacy trials currently underway are successful, one or more prophylactic HPV vaccines could reach registration as early as 2006/2007. Several important unresolved issues remain with respect to implementation of a vaccination programme against HPV infection, and with respect to the effect of vaccination programmes on incidence of and mortality from ICC.
Effectiveness of vaccination in prevention of cervical cancer at the population level
Both natural and induced immunity to HPV infection appear to be largely type-specific. A number of studies have found that different HPV types are serologically distinct and do not produce strong cross-neutralizing antibody responses [55]. This is borne out by the results from one trial of a monovalent HPV 16 VLP vaccine, where an equal number of cases (22 in each group) of CIN that were not associated with HPV 16 occurred in the placebo and vaccine recipient groups [49].
If this is the case, the proportion of cases of cervical cancer prevented by vaccination will depend on the proportion attributable to the specific genotypes in the vaccine. A recent pooled analysis of data from international surveys on HPV type distribution in cervical cancer suggests that a vaccine including types 16 and 18 could potentially prevent 71% of cervical cancers worldwide [56]. The cost of including additional HPV types in a multivalent vaccine will have to be balanced against the additional fraction of cases of cervical cancer prevented, and this issue is further complicated by the fact that prevalence of certain oncogenic HPV types varies in different regions of the world. Thus, the percentage of cases potentially prevented by a 16/18 vaccine would be higher in Asia and Europe/North America – where there is a higher prevalence of HPV 16/18 in cervical cancers – than in other regions of the world, including sub-Saharan Africa and Central/South America, where higher proportions of types 45 and 31 respectively, are seen [56]. A vaccine containing the seven most common HPV types (16, 18, 45, 31, 33, 52 and 58) would theoretically prevent ∼87% of cervical cancers worldwide, with little regional variation. That a number of different HPV types are implicated in cervical cancer is a challenge for the development of effective vaccines.
However, although while the balance of evidence suggest that different HPV types behave as independent STIs [57], some studies suggest that, in natural infections, low levels of interaction may occur between certain types which are closely phylogenetically related (including types 16, 31 and 33; and types 18 and 45) [58, 59]. These interactions may occur either directly at the level of the virus itself (through competition for ecological niches) or indirectly, for example at the level of the immune response (through cross-reactivity of antibodies and/or T-cell-mediated responses). Some evidence also exists for antagonistic interactions between HPV 16 and HPV 6/11 [20, 60]. Low-level immunological interaction may be due at least in part to T-cell responses to HPV gene products that would not be contained in the vaccine.
If competing risks for infection between HPV types do exist, the equilibrium of other oncogenic types might be affected if a type-specific vaccine successfully prevented HPV 16/18 infection, due to filling in of ecological niches created by reduction or removal of the most prevalent HPV types [61]. Reduced cross-protection from disease could also result from a decrease in prevalence of cross-protective (antagonistic) HPV types [60]. It is also possible that, as a result of protection against infection with HPV 16/18 and subsequent development of 16/18-related cervical cancer, the pool of women susceptible to development of ‘replacement’ cervical cancer, after a longer period of infection with other, less virulent, non-vaccine, oncogenic HPV types, could increase [62, 63].
On the other hand, artificial VLPs are somewhat different to natural HPV virions, and it has been suggested that the immune response stimulated by HPV 16/18 vaccination might also protect against genetically related HPV types, such as 33 or 45. Low-level cell-mediated cross-reactivity between genetically related HPV types may occur because of the different (not so conformation dependent) nature of the epitopes [57]. Cross-reactive cell-mediated immunity could potentially keep the oncogenic non-vaccine types under control due to naturally occurring boosting by the benign non-vaccine included types [26]. Under this scenario, it is possible that the prevalence of other (non-vaccine) genetically related HPV types could decrease rather than increase following vaccination.
With current knowledge, it is thus difficult to predict whether the existence of low-level interactions between HPV types would decrease or increase the predicted fraction of cervical cancer cases prevented by a 16/18 vaccine, in relation to the current prevalence of these types in ICC. Monitoring systems for surveillance of breakthrough infections, and of the epidemiological distribution of vaccine and non-vaccine HPV types, will be necessary following introduction of vaccination programmes.
Cost-effectiveness of vaccination in the context of continuing screening programmes
Given, in any event, that the vaccines currently under evaluation will not protect against all cervical oncogenic viruses, continuation of Pap screening programmes will be necessary, even with widespread vaccination programmes. One could expect, however, that with an effective vaccine there would be a reduced frequency of abnormal Pap smears and pre-invasive disease – and, thus, of the costs of follow-up – as well as of ICC [64]. Vaccination could also complement screening programmes, and further decrease incidence of cervical cancer if women who do not regularly attend for screening (where ∼50% of cervical cancers are diagnosed [65]), can be reached by vaccination programmes. Preliminary studies suggest that in the United States, vaccinating adolescent girls for high-risk HPV infections in combination with screening is a cost-effective approach, particularly if it were possible to delay the age at which screening commences, as well as to reduce the frequency of screening [4, 66]. These projections are, however, sensitive to the cost of the vaccine and the length of protection – two presently unknown variables. Furthermore, cervical screening programmes are expensive in the United States compared with European countries, costing an estimated US $6 billion annually [52].
Moreover, given the lower prevalence of HPV 16 and 18 in low-grade CIN than in ICC, there is uncertainty about the exact proportion of cases of CIN that would be prevented by vaccination, and some mathematical modelling studies have suggested that the effect of vaccination on overall HPV prevalence, and on prevalence of low-grade cervical abnormalities, may not be greatly reduced [64]. On the other hand, if vaccination significantly reduces the population prevalence of HPV 16/18 infection, as well as of its sequelae [63], HPV testing could eventually replace cervical cytology as a primary screening tool, as the specificity and, therefore, positive predictive value of primary HPV screening could be significantly increased. This could decrease screening costs and improve the performance of screening programmes [45].
Finally, the duration of the antibody response induced by the HPV vaccine remains to be determined. To be truly efficacious, the vaccine would need to confer protection lasting from adolescence for several decades. Studies on the duration of protection conferred against sexual transmission of hepatitis B (HBV) after childhood immunization with a HBV vaccine – which is technically similar to the HPV VLP vaccine – indicate that boosters are likely to be needed at least at 10-year intervals to maintain high efficacy against infection [67]. In the monovalent HPV 16 VLP vaccine trial, results presented at the American Society for Microbiology Conference in Washington in November 2004 indicated that vaccine efficacy remained high four years after vaccination, with protection waning for only a small proportion of women (7/755 vaccinated women having developed HPV 16 infections as opposed to 111/750 women who received placebo injections). Further studies are needed to determine whether booster vaccination is necessary and indeed efficacious, and at what time intervals it should be carried out. The need for and cost of booster vaccines to extend duration of protection will be critical in terms of both the design and cost-effectiveness of vaccination programmes.
Who and when to vaccinate?
HPV is a highly prevalent and widely distributed infection, and HPV prevalence and seroprevalence increase rapidly among young women once they become sexually active [11, 12, 58, 68, 69]. In order to achieve optimal efficacy, vaccination against HPV is, therefore, likely to be most effective if it targets adolescent or pre-adolescent girls before they become sexually active and are thus exposed to infection. This is particularly the case given the questionable efficacy of condoms in preventing HPV transmission, as well as evidence suggesting that transmission of HPV may occur through non-penetrative sex [22]. Schools-based vaccination programmes may be most effective at reaching significant proportions of the target population, as well as being cost-effective in terms of reducing costs of administering a three-dose regimen [4]. Initially, ‘catch-up’ vaccination of older cohorts of women, and/or of population subgroups at high risk of infection, would also be necessary to prevent further HPV infection in such groups [41], and also, possibly, to decrease the risk of developing cervical cancer in women already infected [52].
A further question is whether males should also be vaccinated, both to prevent occurrence of ano-genital warts (if the multivalent vaccines currently under evaluation do indeed protect against the latter), as well as to interrupt transmission of, and thus reduce the population prevalence of, oncogenic HPVs. Mathematical modelling studies suggest that vaccinating boys as well as girls would theoretically result in a greater decrease in HPV prevalence in girls than vaccinating only girls, due to herd immunity, although the additional proportion of cases estimated to be saved by vaccinating boys as well as girls varies across studies, and according to the model structures and parameter estimates used [63, 70]. On the other hand, there are as yet no data on the efficacy of HPV vaccination in preventing infection in boys, and some studies suggest that there may be a gender differential in the immune response to natural HPV infection [68], raising questions about the possible differential efficacy of a vaccine in boys (as has recently been demonstrated for the glycoprotein D vaccine against herpes simplex virus infection [71]). Cost-effectiveness considerations will also be paramount here. A recently published modelling study, using US data and an estimated cost of $US100 per vaccine dose, suggests that if high vaccine coverage of girls is achieved, vaccination of boys may not be the most cost-effective strategy, since it would result in only a small further reduction in rates of HPV infections and cancer cases, at a high cost [70]. In certain instances, however, such as those in which vaccine efficacy wanes rapidly without boosters, or overall vaccine coverage is low, vaccination of males could have a substantial effect and would be more cost-effective [70].
If the aim of vaccination is to reduce significantly HPV infection rates at the population level, in addition to preventing the development of cervical cancer at the individual level, high population coverage rates will be necessary, particularly if only girls are vaccinated [63].
Issues of parental and societal acceptability may well arise in relation to vaccination of young pre-sexually active girls against a STI [66], particularly since the general public have little or no knowledge of HPV or its involvement with cervical cancer [52, 72]. Appropriate and sensitive public information and education programmes will be necessary to communicate the public health benefits of vaccination to the general public. It will also be necessary to emphasize that the vaccine will not be fully effective in preventing cervical cancer, in order not to have an adverse effect on levels of risky sexual behaviour. Additionally, if women who are vaccinated perceive themselves to be at low risk for developing cervical cancer, and as a result do not participate in screening as recommended, gains from vaccination may be offset or even reversed [52, 66].
Vaccination in developing countries
Developing countries, where the burden of disease occurs, and where effective screening programmes are difficult to implement, stand to gain most from the introduction of an effective vaccine against cervical cancer. However, the cost of producing and administering a parenteral vaccine using the current VLP methodology is high and may well be prohibitive for developing countries, particularly given that cervical cancer is not necessarily a high priority in countries with many other competing health problems and needs [52]. Possibilities for cheaper and simpler vaccine production include the use of bacterial (E. coli) L1 expression systems to produce recombinant L1 major capsid proteins, which, after trypsin digestion, are capable of self-assembling into pentameric capsomeres which contain neutralization epitopes [22]. Animal models have shown such capsomeres to be protective against infection. HPV 11 L1 capsomeres have been successfully used to generate high-titre polyclonal antibodies in rabbits, which were capable of neutralizing HPV 11 virions in vitro [73].
Considerable efforts have been made by several groups to develop alternatives to parenteral vaccine delivery, that is so-called ‘needle-free’ methods such as oral/intranasal mucosal delivery and/or development of transgenic edible plant-based vaccines. Mucosally (orally) delivered vaccines are cheaper and easier to administer, as well as being more acceptable to recipients. Recent studies have shown that both HPV 16 and HPV 18 VLPs are immunogenic when administered orally, and that oral co-administration of mucosal adjuvants (E. coli heat-labile enterotoxin mutant R192G or CpG DNA) can significantly improve anti-VLP humoral responses in peripheral blood and in genital mucosal secretions [74]. Development of DNA vaccines, which are particularly suitable for developing countries because of their ease of production and delivery, is another possibility [22].
CONCLUSIONS
There is currently real hope that an effective vaccine capable of protecting against infection with HPV, and thereby of preventing the development of a significant proportion of cases of ICC, may be available within the next few years. Such a vaccine would be of great public health value in terms of reducing incidence of and mortality from ICC, and correspondingly the need for colposcopy, biopsy and treatment. A multivalent vaccine also protective against infection with HPV types 6 and 11 would additionally reduce incidence of ano-genital warts, which, although not fatal, are nevertheless a troublesome and difficult to treat consequence of infection.
There is, however, at present considerable uncertainty about the most effective strategy for vaccination, including: the age at which to vaccinate; the effect of vaccinating only women as opposed to both women and men; as well as the impact of vaccination on HPV and cervical cancer incidence at the population level. Questions remain concerning the exact pathways in the pathogenesis of cervical cancer; the factors determining the current epidemiological distribution of HPV types and the proportion of cervical cancer cases attributable to these types; the effect of type-specific immunity on the distribution of non-vaccine HPV types and the likely effect of this on overall ICC incidence; and the cost-effectiveness of a widespread vaccination programme given the necessity for continued cervical screening programmes. The answers to many of these questions will probably not be known until many years after the introduction of vaccination programmes.
Further studies, including post-licensure, community-randomized phase IV trials, with long-term passive follow-up of cohorts of vaccines and non-vaccinees by population-based cancer registries, will be necessary to evaluate the effectiveness of vaccination on HPV prevalence and on associated disease at the population level, as well as to evaluate the efficacy of vaccinating both females and males compared to females only [26, 51]. In addition to the need for further empirical studies, mathematical modelling will be an important tool for predicting and assessing the effect of vaccination on HPV incidence and prevalence and on morbidity and mortality from ICC, including herd immunity effects; as well as for analysing the cost-effectiveness of vaccination programmes in different contexts. Intensive surveillance of both the prevalence of HPV types as well as of their proportional distribution in cervical cancer and its precursors (including to detect breakthrough infections) will be an essential part of vaccination programmes. Finally, in developing countries, which are most at need and stand to benefit most from vaccination, methods of vaccine production and delivery that are simpler and cheaper than those based on VLP technology will probably be necessary.
ACKNOWLEDGEMENTS
I thank Eduardo Franco and F. Xavier Bosch for providing me with figures, and my anonymous referees for their helpful comments on the manuscript.
DECLARATION OF INTEREST
None.
Footnotes
In the recently introduced US Bethesda terminology system, these stages correspond to atypical squamous cells (ASC-US or ASC-H: cells which are abnormal but not frankly reactive or dysplastic); low-grade squamous intra-epithelial lesions (LGSIL; mild dysplasia and the changes associated with HPV infection, known as koilocytosis); and high-grade squamous intra-epithelial lesions (HGSIL; moderate and severe dysplasia, carcimona in situ) [34].
REFERENCES
- Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000: the global picture. Eur J Cancer. 2001;37(Suppl 8) yes:S4–S66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Bray F, Pisani P, Parkin DM. 2004. http://www-depdb.iarc.fr/globocan/GLOBOframe.htm. http://www-depdb.iarc.fr/globocan/GLOBOframe.htm GLOBOCAN 2002: Cancer incidence, mortality and prevalence worldwide. IARC Cancer Base No. 5, version 2.0. IARC Press, Lyon, ). Accessed 2 July 2005.
- Sanders GD, Taira AV. Cost-effectiveness of a potential vaccine for human papillomavirus. Emerg Infect Dis. 2003;9:37–48. doi: 10.3201/eid0901.020168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviat N, Koutsky LA, Paavonen J., Holmes KK, Sparling PF, Mardh P-A, Lemon SM, Stamm WE, Piot P, Wasserheit JN. Sexually transmitted diseases, chapter 49. New York: McGraw-Hill; 1999. Cervical neoplasia and other STD-related genital tract neoplasias; pp. 669–684. [Google Scholar]
- Zheng T, Holford TR, Ma Z, Chen Y, Liu W, Ward BA, Boyle P. The continuing increase in adenocarcinoma of the uterine cervix: a birth cohort phenomenon. Int J Epidemiol. 1996;25:252–258. doi: 10.1093/ije/25.2.252. [DOI] [PubMed] [Google Scholar]
- Sasieni P, Adams J. Changing rates of adenocarcinoma and adenosquamous carcinoma of the cervix in England. Lancet. 2001;357:1490–1493. doi: 10.1016/S0140-6736(00)04646-8. [DOI] [PubMed] [Google Scholar]
- Franco EL, Duarte-Franco E, Ferenczy A. Cervical cancer: epidemiology, prevention and the role of human papillomavirus infection. Can Med Assoc J. 2001;164:1017–1025. [PMC free article] [PubMed] [Google Scholar]
- Lehtinen M, Paavonen J. Efficacy of preventive human papillomavirus vaccination. Int J STD AIDS. 2001;12:771–776. doi: 10.1258/0956462011924317. [DOI] [PubMed] [Google Scholar]
- Gillison ML, Shah KV. Role of mucosal human papillomavirus in nongenital cancers. J Natl Cancer Inst Monogr. 2003;31:57–65. doi: 10.1093/oxfordjournals.jncimonographs.a003484. [DOI] [PubMed] [Google Scholar]
- Woodman CB, Collins S, Winter H et al. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet. 2001;357:1831–1836. doi: 10.1016/S0140-6736(00)04956-4. [DOI] [PubMed] [Google Scholar]
- Burk RD, Kelly P, Feldman J et al. Declining prevalence of cervicovaginal human papillomavirus infection with age is independent of other risk factors. Sex Transm Dis. 1996;23:333–341. doi: 10.1097/00007435-199607000-00013. [DOI] [PubMed] [Google Scholar]
- Dillner J, Kallings I, Brihmer C et al. Seropositivities to human papillomavirus types 16, 18, or 33 capsids and to Chlamydia trachomatis are markers of sexual behavior. J Infect Dis. 1996;173:1394–1398. doi: 10.1093/infdis/173.6.1394. [DOI] [PubMed] [Google Scholar]
- Koutsky LA, Kiviat NB., Holmes KK, Sparling PF, Mardh P-A, Lemon SM, Stamm WE, Piot P, Wasserheit JN. Sexually transmitted diseases, chapter 49. New York: McGraw-Hill; 1999. Genital human papillomaviruses; pp. 669–684. [Google Scholar]
- Kjaer SK, van den Brule AJ, Paull G et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ. 2002;325:572. doi: 10.1136/bmj.325.7364.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson R, Jonsson M, Edlund K et al. Lifetime number of partners as the only independent risk factor for human papillomavirus infection: a population-based study. Sex Transm Dis. 1995;22:119–127. doi: 10.1097/00007435-199503000-00008. [DOI] [PubMed] [Google Scholar]
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- Franco EL, Villa LL, Sobrinho JP et al. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis. 1999;180:1415–1423. doi: 10.1086/315086. [DOI] [PubMed] [Google Scholar]
- Herrero R, Hildesheim A, Bratti C et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst. 2000;92:464–474. doi: 10.1093/jnci/92.6.464. [DOI] [PubMed] [Google Scholar]
- Rousseau MC, Villa LL, Costa MC, Abrahamowicz M, Rohan TE, Franco E. Occurrence of cervical infection with multiple human papillomavirus types is associated with age and cytologic abnormalities. Sex Transm Dis. 2003;30:581–587. doi: 10.1097/00007435-200307000-00010. [DOI] [PubMed] [Google Scholar]
- Elfgren K, Kalantari M, Moberger B, Hagmar B, Dillner J. A population-based five-year follow-up study of cervical human papillomavirus infection. Am J Obstet Gynecol. 2000;183:561–567. doi: 10.1067/mob.2000.106749. [DOI] [PubMed] [Google Scholar]
- Galloway DA. Papillomavirus vaccines in clinical trials. Lancet Infect Dis. 2003;3:469–475. doi: 10.1016/s1473-3099(03)00720-5. [DOI] [PubMed] [Google Scholar]
- Ho GY, Burk RD, Klien S et al. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. J Natl Cancer Inst. 1995;87:1365–1371. doi: 10.1093/jnci/87.18.1365. [DOI] [PubMed] [Google Scholar]
- Bosch FX, Manos MM, Munoz N et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- Munoz N, Bosch FX, de Sanjose S Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. , et al.; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. [DOI] [PubMed] [Google Scholar]
- Lehtinen M, Paavonen J. Effectiveness of preventive human papillomavirus vaccination. Int J STD & AIDS. 2003;14:787–792. doi: 10.1258/095646203322556084. [DOI] [PubMed] [Google Scholar]
- Anttila T, Saikku P, Koskela P et al. Serotypes of Chlamydia trachomatis and risk for development of cervical squamous cell carcinoma. J Am Med Assoc. 2001;285:47–51. doi: 10.1001/jama.285.1.47. [DOI] [PubMed] [Google Scholar]
- Smith JS, Munoz N, Herrero R et al. Evidence for Chlamydia trachomatis as a human papillomavirus cofactor in the etiology of invasive cervical cancer in Brazil and the Philippines. J Infect Dis. 2002;185:324–331. doi: 10.1086/338569. [DOI] [PubMed] [Google Scholar]
- Lehtinen M, Koskela P, Jellum E et al. Herpes simplex virus and risk of cervical cancer: a longitudinal, nested case-control study in the Nordic countries. Am J Epidemiol. 2002;156:687–692. doi: 10.1093/aje/kwf098. [DOI] [PubMed] [Google Scholar]
- Smith JS, Herrero R, Bosetti C et alHerpes simplex virus-2 as a human papillomavirus cofactor in the etiology of invasive cervical cancer. J Natl Cancer Inst. 2002;94:1604–1613. doi: 10.1093/jnci/94.21.1604. .; International Agency for Research on Cancer (IARC) Multicentric Cervical Cancer Study Group. [DOI] [PubMed] [Google Scholar]
- Zenilman JM. Chlamydia and cervical cancer: a real association? J Am Med Assoc. 2001;285:81–83. doi: 10.1001/jama.285.1.81. [DOI] [PubMed] [Google Scholar]
- Hemminki K, Dong C, Vaittinen P. Familial risks in cervical cancer: is there a hereditary component? Int J Cancer. 1999;82:775–781. doi: 10.1002/(sici)1097-0215(19990909)82:6<775::aid-ijc1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Castellsague X, Bosch FX, Munoz N et alMale circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med. 2002;346:1105–1112. doi: 10.1056/NEJMoa011688. .; International Agency for research on Cancer Multicenter Cervical Cancer Study Group. [DOI] [PubMed] [Google Scholar]
- Solomon D, Davey D, Kurman R et alThe 2001 Bethesda System: terminology for reporting results of cervical cytology. J Am Med Assoc. 2002;287:2114–2119. doi: 10.1001/jama.287.16.2114. .; Forum Group Members; Bethesda 2001 Workshop. [DOI] [PubMed] [Google Scholar]
- Gustafsson L, Ponten J, Zack M, Adami HO. International incidence rates of invasive cervical cancer after introduction of cytological screening. Cancer Causes Control. 1997;8:755–763. doi: 10.1023/a:1018435522475. [DOI] [PubMed] [Google Scholar]
- Levi F, Lucchini F, Negri E, Franceschi S, la Vecchia C. Cervical cancer mortality in young women in Europe: patterns and trends. Eur J Cancer. 2000;36:2266–2271. doi: 10.1016/s0959-8049(00)00346-4. [DOI] [PubMed] [Google Scholar]
- Martin-Hirsch PL, Koliopoulos G, Paraskevaidis E. Is it now time to evaluate the true accuracy of cervical cytology screening? A review of the literature. Eur J Gynaecol Oncol. 2002;23:363–365. [PubMed] [Google Scholar]
- Hakama M, Hristova L. Effect of screening for cancer in the Nordic countries on deaths, costs and quality of life. Acta Oncol. 1998;36:1–160. doi: 10.1080/0284186X.1997.11835453. [DOI] [PubMed] [Google Scholar]
- Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet. 2004;364:249–256. doi: 10.1016/S0140-6736(04)16674-9. [DOI] [PubMed] [Google Scholar]
- Nanda K, McCrory DC, Myers ER et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000;132:810–819. doi: 10.7326/0003-4819-132-10-200005160-00009. [DOI] [PubMed] [Google Scholar]
- Crum CP. The beginning of the end for cervical cancer? N Engl J Med. 2002;347:1703–1705. doi: 10.1056/NEJMe020121. [DOI] [PubMed] [Google Scholar]
- European Commission DG V F.2. http://crsg.ubc.kun.nl/quate/Guidelines/euindex.html. 2002. http://crsg.ubc.kun.nl/quate/Guidelines/euindex.html ‘Europe against cancer’ programme/Committee on quality assurance, training and education of the European Federation of Cytology Societies (QUATE). European guidelines for quality assurance in cervical cancer screening, CRSG, 1997 (Technical editor: Dr Chris de Woolf). ( ). Accessed 15 December .
- Laukkanen P, Koskela P, Pukkala E et al. Time trends in incidence and prevalence of human papillomavirus type 6, 11 and 16 infections in Finland. J Gen Virol. 2003;84:2105–2109. doi: 10.1099/vir.0.18995-0. [DOI] [PubMed] [Google Scholar]
- Goldie SJ, Kuhn L, Denny L, Pollack A, Wright TC. Policy analysis of cervical cancer screening strategies in low-resource settings: clinical benefits and cost-effectiveness. J Am Med Assoc. 2001;285:3107–3115. doi: 10.1001/jama.285.24.3107. [DOI] [PubMed] [Google Scholar]
- Cuzick J, Szarewski A, Cubie H et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet. 2003;362:1871–1876. doi: 10.1016/S0140-6736(03)14955-0. [DOI] [PubMed] [Google Scholar]
- Kulasingam SL, Hughes JP, Kiviat NB et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. J Am Med Assoc. 2002;288:1749–1757. doi: 10.1001/jama.288.14.1749. [DOI] [PubMed] [Google Scholar]
- Wright TC, Jr., Cox JT, Massad LS, Twiggs LB, Wilkinson EJ 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. J Am Med Assoc. 2002;287:2120–2129. doi: 10.1001/jama.287.16.2120. ; ASCCP-Sponsored Consensus Conference. [DOI] [PubMed] [Google Scholar]
- Peto J, Gilham C, Deacon J et al. Cervical HPV infection and neoplasia in a large population-based prospective study: the Manchester cohort. Br J Cancer. 2004;91:942–953. doi: 10.1038/sj.bjc.6602049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsky LA, Ault KA, Wheeler CM et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- Harper DM, Franco EL, Wheeler C et alEfficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–1765. doi: 10.1016/S0140-6736(04)17398-4. .; GlaxoSmithKline HPV Vaccine Study Group. [DOI] [PubMed] [Google Scholar]
- Lehtinen M, Paavonen J. Vaccination against human papillomaviruses shows great promise. Lancet. 2004;364:1731–1732. doi: 10.1016/S0140-6736(04)17410-2. [DOI] [PubMed] [Google Scholar]
- Schiller JT, Davies P. Delivering on the promise: HPV vaccines and cervical cancer. Nat Rev Microbiol. 2004;2:343–347. doi: 10.1038/nrmicro867. [DOI] [PubMed] [Google Scholar]
- Villa LL, Costa RL, Petta CA et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- Davidson EJ, Boswell CM, Sehr P et al. Immunological and clinical responses in women with vulval intraepithelial neoplasia vaccinated with a vaccinia virus encoding human papillomavirus 16/18 oncoproteins. Cancer Res. 2003;63:6032–6041. [PubMed] [Google Scholar]
- Kirnbauer R. Papillomavirus-like particles for serology and vaccine development. Intervirology. 1996;39:54–61. doi: 10.1159/000150475. [DOI] [PubMed] [Google Scholar]
- Munoz N, Bosch FX, Castellsague X et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111:278–285. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- Liaw KL, Hildesheim A, Burk RD et al. A prospective study of human papillomavirus (HPV) type 16 DNA detection by polymerase chain reaction and its association with acquisition and persistence of other HPV types. J Infect Dis. 2001;183:8–15. doi: 10.1086/317638. [DOI] [PubMed] [Google Scholar]
- Ho GY, Studentsov Y, Hall CB et al. Risk factors for subsequent cervicovaginal human papillomavirus (HPV) infection and the protective role of antibodies to HPV-16 virus-like particles. J Infect Dis. 2002;186:737–742. doi: 10.1086/342972. [DOI] [PubMed] [Google Scholar]
- Thomas KK, Hughes JP, Kuypers JM et al. Concurrent and sequential acquisition of different genital human papillomavirus types. J Infect Dis. 2000;182:1097–1102. doi: 10.1086/315805. [DOI] [PubMed] [Google Scholar]
- Luostarinen T. No excess risk of cervical carcinoma among women seropostiive for both HPV-16 and HPV6/11. Int J Cancer. 1999;80:818–822. doi: 10.1002/(sici)1097-0215(19990315)80:6<818::aid-ijc4>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Lipsitch M. Vaccination against colonizing bacteria with multiple serotypes. Proc Natl Acad Sci USA. 1997;94:6571–6576. doi: 10.1073/pnas.94.12.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett GP, Waddell HC. Public health paradoxes and the epidemiological impact of an HPV vaccine. J Clin Virol. 2000;19:101–111. doi: 10.1016/s1386-6532(00)00129-3. [DOI] [PubMed] [Google Scholar]
- Hughes JP, Garnett GP, Koutsky L. The theoretical population-level impact of a prophylactic human papilloma virus vaccine. Epidemiology. 2002;13:631–639. doi: 10.1097/00001648-200211000-00006. [DOI] [PubMed] [Google Scholar]
- Goldie SJ, Grima D, Kohli M, Wright TC, Weinstein M, Franco E. A comprehensive natural history model of HPV infection and cervical cancer to estimate the clinical impact of a prophylactic HPV-16/18 vaccine. Int J Cancer. 2003;106:896–904. doi: 10.1002/ijc.11334. [DOI] [PubMed] [Google Scholar]
- Sasieni PD, Cuzick J, Lynch-Farmery E. Estimating the efficacy of screening by auditing smear histories of women with and without cervical cancer. Br J Cancer. 1996;73:1001–1005. doi: 10.1038/bjc.1996.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulasingam SL, Myers ER. Potential health and economic impact of adding a human papillomavirus vaccine to screening programs. J Am Med Assoc. 2003;290:781–789. doi: 10.1001/jama.290.6.781. [DOI] [PubMed] [Google Scholar]
- Whittle H, Jaffar S, Wansbrough M et al. Observational study of vaccine efficacy 14 years after trial of hepatitis B vaccination in Gambian children. BMJ. 2002;325:569. doi: 10.1136/bmj.325.7364.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone KM, Karem KL, Sternberg MR et al. Seroprevalence of human papillomavirus type 16 infection in the United States. J Infect Dis. 2002;186:1396–1402. doi: 10.1086/344354. [DOI] [PubMed] [Google Scholar]
- Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218–226. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- Taira AV. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis. 2004;10:1915–1923. doi: 10.3201/eid1011.040222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanberry LR, Spruance SL, Cunningham AL et alGlycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347:1652–1661. doi: 10.1056/NEJMoa011915. .; GlaxoSmithKline Herpes Vaccine Efficacy Study Group. [DOI] [PubMed] [Google Scholar]
- Waller J, McCaffery K, Nazroo J, Wardle J. Making sense of information about HPV in cervical screening: a qualitative study. Br J Cancer. 2005;92:265–270. doi: 10.1038/sj.bjc.6602312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RC, White WI, Li M, Suzich JA, Lane C, Garcea RL. Human papillomavirus type 11 recombinant L1 capsomeres induce virus-neutralizing antibodies. J Virol. 1998;72:6151–6154. doi: 10.1128/jvi.72.7.6151-6154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber S, Lane C, Brown DM et al. Human papillomavirus virus-like particles are efficient oral immunogens when coadministered with Escherichia coli heat-labile enterotoxin mutant R192G or CpG DNA. J Virol. 2001;75:4752–4760. doi: 10.1128/JVI.75.10.4752-4760.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]