SUMMARY
The concomitant occurrence of a case of haemolytic–uraemic syndrome (HUS) and 62 cases of mild gastroenteritis in schools of a small rural community in southern Italy induced the health authorities to suspect a foodborne outbreak of shiga-toxin-producing Escherichia coli (STEC) infection. The schools were closed and the catering service involved was investigated. However, STEC were not isolated from the HUS case or from the 56 cases of gastroenteritis examined, and the HUS case and the outbreak of gastroenteritis were probably just coincidental. A retrospective cohort study failed to show any correlation with consumption of school meals and suggested that the outbreak probably started outside the school setting and then spread within the schools by person-to-person transmission. All the cases examined were negative for common enteric pathogens and the responsible agent for the cases of gastroenteritis was not identified. The concern raised in the small community by the occurrence of a severe case of HUS and the lack of a rapid epidemiological assessment excluding the occurrence of a STEC outbreak, turned an epidemic episode of mild gastroenteritis into a public health emergency with relevant socioeconomic consequences. Prompt intervention in outbreaks following timely and effective risk communication are crucial for taking the most appropriate control measures and avoiding the spread of fear and panic in the community.
INTRODUCTION
The haemolytic–uraemic syndrome (HUS) is the most common cause of acute renal failure in children and is also characterized by thrombocytopaenia, and microangiopathic haemolytic anaemia [1]. More than 80% of HUS cases occur as a complication of intestinal infections with shiga-toxin (ST)-producing Escherichia coli (STEC) [2–4].
STEC are zoonotic pathogens and represent an important cause of diarrhoea and bloody diarrhoea worldwide [3, 5]. The most important reservoir of these pathogens is the gastrointestinal tract of ruminants, mainly cattle, and the agent can be transmitted to humans via contaminated meat products, unpasteurized milk and other foods or beverages, direct contact with animals, or contamination of the environment [3, 6, 7]. Person-to-person transmission is frequent, because of the high potential of transmission and the low infectious dose [3].
In Italy, a surveillance system for paediatric HUS coordinated by the Istituto Superiore di Sanità (ISS) and the ‘Bambino Gesù’ Paediatric Hospital in Rome has been active since 1988. The system involves most Italian paediatric nephrology units, which notify HUS cases and collect samples for laboratory diagnosis of STEC infection, carried out at the ISS. Between 1988 and 2000, the surveillance system has observed 342 cases of HUS, 73% of which were shown to be associated with STEC infection [8].
In this paper we report the investigations carried out on a case of HUS associated with a concomitant outbreak of mild gastroenteritis involving school children and their relatives in a small rural community in southern Italy. The possible links between these events and their public health and socioeconomic impact are evaluated and discussed.
METHODS
Background
On 30October 2001, a case of HUS in a 3-year-old child residing in the municipality of Drapia, Calabria region, southern Italy, was notified to the ISS. The child developed diarrhoea with acute abdominal pain on 26 October and was administered oral neomycin and bacitracin. Diarrhoea became bloody on 27 October and on 28 October she was admitted to the local hospital. On 29 October she developed renal failure and anuria, and was transferred to the ‘Ospedale Bambino Gesù’ Paediatric Hospital in Rome, where she underwent dialysis and surgery for intestinal perforation. The parents’ interview carried out on 4 November and preliminary contact with local health authorities on 5 November revealed that, in the same period of time, many cases of gastroenteritis had occurred among children attending the nursery and primary schools of the area, which had been closed on 31 October together with the catering service which provided the meals to the schools. Since the HUS case had prodromal haemorrhagic colitis and had eaten hamburgers in the school cafeteria on 25 October, an outbreak of STEC infection was suspected. On 6 November, the Regional Health Authority requested the collaboration of the ISS. Information concerning the situation in the affected municipality and the seriousness of the condition of other sick people were not available when the investigation started.
Settings
The municipality of Drapia is constituted of three small neighbouring rural villages, identified as A, B and C, with a total population of ∼2200 people. Each village has a school district including a nursery for children between 3 and 5 years of age and a primary school with children aged from 6 to 11 years. Nurseries and primary schools were served by the same catering service, which also served other cafeterias in the area. The caterer delivered meals in refrigerated boxes to the school kitchen, located in one of the nurseries. Meals were warmed up in the kitchen and distributed to all the schools by car within 30 min. The daily menu was the same in all the school districts.
Epidemiological investigation
Preliminary information was collected by interviewing the local family paediatrician and the school personnel on 7 November. Then a retrospective cohort study on children and staff members attending the six schools was carried out from 7 to 9 November. We tested several case definitions. However, since most children complained of mild symptoms and a more strict case definition would have left only a few cases, a case of gastroenteritis was defined as the occurrence of vomiting and/or diarrhoea, and/or abdominal pain. Diarrhoea was defined as the passing of two or more liquid or semi-liquid stools per day. A standard questionnaire was administered to collect information on clinical features, frequency of meal consumption at the school cafeteria and food items consumed, attendance at children’s parties or other recreational activities, exposure to known risk factors for STEC infection such as living close to cattle or sheep dairy operations, raw milk consumption, using well water, having a garden where vegetables for home consumption were grown [7].
Because of the involvement of children, the questionnaires were administered to their parents, who also provided information on other household members with clinical signs suggestive of gastroenteritis.
Sample collection
Samples of minced meat were collected on 2 November at the butchery that supplied the meat to the catering service. Stool and serum samples were collected from the child with HUS at 5, 15, and 25 days after onset of intestinal symptoms. Faecal and serum specimens were also collected between 6 and 8 November from 56 gastroenteritis cases (41 children and 15 adults). The median interval between onset of symptoms and sample collection was 11 days (range 4–19 days). Each stool specimen was split: some was stored at −20°C and sent to ISS for examination for the presence of STEC and Norovirus, and the rest was examined for common enteric pathogens at the laboratory of the local hospital within 24 h from collection.
Laboratory investigations
Stools were examined for the presence of free faecal VT by the Vero cell cytotoxicity assay [9] and streaked onto MacConkey agar for STEC isolation. STEC strains were identified by testing colony sweeps for VT production by Vero cells [9] and for the presence of VT genes by PCR amplification [10]. Salmonella, shigella, and campylobacter were sought by standard methods. Search for noroviruses was performed on 26 stool samples by RT–PCR using generic primers JV12I and JV13Y [11], and primers SR80 and JV33 were used in a Sapovirus-specific RT–PCR [12]. Serum samples were tested for antibodies to the lipopolysaccharide (LPS) of five major STEC serogroups (O157, O26, O103, O111, and O145) by ELISA, as previously described [9, 13, 14]. Minced meat samples were examined for E. coli O157 by using an O157-specific immunomagnetic separation enrichment technique [10]. Supernatants of pre-enrichment broth cultures were also examined for the presence of VT by the Vero cell assay [9].
Data analysis
The questionnaires were entered in an Epi-Info version 6.04 b database (Centers for Disease Control and Prevention, Atlanta, GA, USA), univariate analysis with odds ratio (OR) and 95% confidence interval (CI) calculation was carried out with the same software. Outcomes that provided statistically significant association in the univariate analysis were included in a multivariate model, run by a backward deletion procedure with SPSS version 9.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Descriptive epidemiology
Questionnaires were collected from all the 42 school teachers and assistants and from 139 out of the 188 children attending the three school districts. Sixty-two cases were identified; eight of them were among the school personnel (median age 42 years) and 54 were children (median age 5 years). The rate of illness among the school personnel was significantly lower than among children (19·0% vs. 38·8%; RR=0·5, P=0·02). The overall attack rate in the schools was 34·2%, but it was significantly higher in district A (57·1%), where most of the cases (34 children and 6 adults) occurred (Table 1). Also a decreasing trend from the schools of village A to those of village C was observed. The attack rate in the nursery schools was significantly higher than in the primary schools (43·4% vs. 26·8%, P<0·01). No pattern of illness among the school personnel by school was observed.
Table 1.
Attack rates of gastroenteritis observed in the nursery and primary schools of the three villages in the municipality of Drapia, Calabria, southern Italy, October–November, 2001
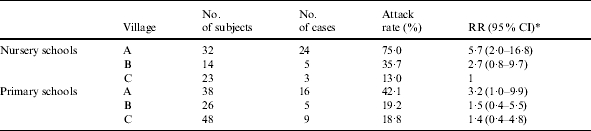
RR (95% CI) indicates the point value of the relative risk (RR), and the lower and the upper limits of the 95% confidence interval (95% CI).
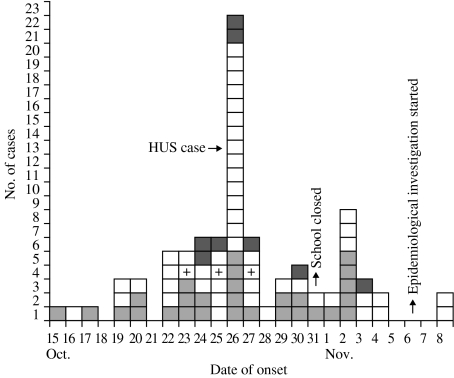
An additional 32 cases, 10 children and 22 adults, were identified among the household contacts of 19 children with gastroenteritis (35·2%) and three children without symptoms (3·5%). Twenty-five out of 32 were contacts of children attending district A schools. In 14 of the 26 household cases for whom information was available the onset of symptoms occurred before or on the same day as in the school child. Cases occurred between 15 October and 8 November, with a peak (22 cases) on 26 October (Fig.). The clinical features included abdominal pain (75·5%), diarrhoea (57·4%), vomiting (34·0%), and fever (27·7%). The symptoms were generally mild and in ∼50% of cases lasted 1 day only. Two children needed hospitalization: one was the HUS case and the other one was a child admitted to the emergency room of the local hospital on 25 October with acute abdominal pain, but discharged after a few hours.
Fig.
Cases of gastroenteritis among school children, school personnel, and household contacts by date of onset, Drapia, Calabria, Italy, October–November 2001.  , School personnel;
, School personnel;  , household contacts; □, school children; +, STEC-positive cases.
, household contacts; □, school children; +, STEC-positive cases.
Analytical epidemiology
Food exposures at school
Among the 181 school personnel and children, 22 usually did not consume food served at the school cafeteria, but brought their meals from home. Five of them (22·7%) were cases, against 57 out of the 159 who usually consumed the school meals (35·8%, P=0·22). Moreover, none of the food items served at the cafeteria from 22 to 26 October was statistically associated with cases.
Other exposures
ORs and 95% CIs were estimated for possible risk factors outside the school. Among the 181 subjects who attended the schools, significant association was observed for having household contacts with symptoms, attendance at children’s parties on 20 and/or 23 October in districts C and A respectively, living close to cattle or sheep dairy operations, habitual consumption of raw milk, having a garden where vegetables for home consumption were grown (Table 2). When the OR were adjusted for significant predictors, only residence in district A, and having household contacts with symptoms remained significant (Table 2). Attending children’s parties also maintained a high OR, but did not reach a level of statistical significance. In particular, known risk factors for STEC infection, e.g. the possible contact with ruminants and their manure or the habit of raw milk consumption were not independently associated with illness.
Table 2.
Crude and adjusted (logistic regression) odds ratios (OR) and 95% confidence interval (95% CI) for significant exposure variables and outcome variables
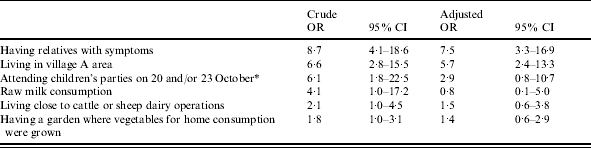
Parties were held in districts C and A respectively.
Microbiological results
Neither microbiological nor serological evidences of STEC infection were observed for the child with HUS. Stool and serum samples from her parents were also negative. Among the 56 cases with gastroenteritis examined, two had free VT2 in the stool but no STEC strains could be isolated. Examination of sera for LPS antibodies showed the presence of antibodies against E. coli O145 in two subjects, one of whom was also positive for free VT. One of the three children with evidence of STEC infection was the one admitted to hospital with acute abdominal pain; the other two had mild diarrhoea only. All the stool specimens were negative for salmonella, shigella, campylobacter and other common enteric pathogens. Search of Norovirus and Sapovirus RNA yielded negative results for all the 26 cases examined. Neither E. coli O157 nor other STEC were detected in the samples of minced meat.
DISCUSSION
The epidemiological investigation of a case of HUS revealed the concomitant occurrence of an outbreak of mild gastroenteritis in a small rural community, involving at least 94 people during a period of ∼3 weeks. Outbreaks of STEC-associated gastroenteritis in the community are often detected by the occurrence of cases of HUS [15, 16] but in this case several evidences suggest that the HUS case and the outbreak of gastroenteritis were not directly related. The presence of severe haemorrhagic colitis in the HUS case strongly suggests that this was due to a STEC infection, despite the negative laboratory results. Stool examination could have been affected by the previous antimicrobial therapy administered to the child. As for the serological analyses, the patient may have had an infection due to a STEC strain belonging to a serogroup that was not included in the LPS panel used in this study. Moreover, it is possible that patients with STEC infection do not have a serological response [17, 18]. Conversely, the possibility that the cases of uncomplicated gastroenteritis were due to STEC infection is unlikely, due to the very mild symptoms they complained of [19]. Laboratory evidence of STEC infection was obtained in three of the 46 cases examined with gastroenteritis, but no obvious epidemiological link among the three cases and between them and the HUS case was found. Two of these children, as well as the child with HUS, belonged to families of dairy farmers and contact with the farming environment is a well known risk factor for acquiring STEC infections [7, 20–22]. On the other hand, asymptomatic STEC infections have been frequently described among farm residents, who often develop immunity, probably due to recurrent exposure to less virulent strains [23, 24]. The multivariate analysis of exposure variables showed that risk factors for STEC infection, e.g. living close to cattle or sheep dairy operations, habit of raw milk consumption, and having a garden where vegetables for home consumption were grown, were not independently associated with illness, and this makes less likely a role of STEC as a causal agent of this outbreak.
The involvement of other enteric pathogens could not be demonstrated. All the specimens were negative for common enteric pathogens like salmonella, shigella, campylobacter and Norovirus, even if the long interval between onset of symptoms and stool sample collection (median 11 days, range 4–19 days) could have affected the laboratory results. The involvement of food poisoning agents such as Clostridium perfringens, Bacillus cereus and Staphylococcus aureus seems unlikely because of the lack of association with food consumption; moreover, the high rate of cases with household contacts with symptoms suggests that person-to-person transmission had a role in the spread of the infection. Based on the frequency distribution of symptoms and the duration of illness, a comparison with other known causes of foodborne outbreaks [25] might suggest the involvement of Norovirus, even if the diagnostic assay attempted on a limited number of samples proved negative. On the other hand, the aetiology of a considerable proportion of sporadic and epidemic episodes of gastroenteritis remains unknown in many published studies [25–27].
As far as the source of the outbreak is concerned, the occurrence of a large number of cases in the schools between 25 and 27 October led the local health authorities to suspect a point source of infection, probably foodborne. This suspicion, together with the concern raised from the severe clinical conditions of the HUS case, prompted the authorities to close the schools and enquire into the catering service. However, the epidemiological investigation showed that: (i) the attack rate was significantly higher in the schools of district A, despite the same food being served in all the schools; (ii) no significant difference was observed between subjects who consumed food served at the school cafeteria and those who brought their meals from home; (iii) no food item served at the cafeteria was statistically associated with cases. Conversely, case finding showed that the outbreak probably started several days before the epidemic peak, with ∼50% of early cases outside the school setting. As a whole, these evidences suggest that the outbreak started in the community. The causative agent could have been carried into the schools by infected subjects and then spread with a person-to-person transmission mechanism, until the number of children with diarrhoea and/or vomiting attending school became high enough to cause the epidemic peak. The hypothesis of a major role of person-to-person transmission is supported by: (i) the high rate (35·8%) of cases with household contacts with gastroenteritis; (ii) the association with attending children’s parties outside the schools; (iii) the observation of a significantly higher attack rate among children in the nursery schools, among whom transmission of pathogens by the oral–faecal route is particularly frequent [28, 29].
In conclusion, the concern raised in a small community by a severe case of HUS and the potential occurrence of additional cases turned an epidemic episode of mild gastroenteritis, that otherwise would have been barely detected, into a public health emergency. This had relevant social and economic consequences, such as the closure of schools for 2 weeks and their thorough sanitization, and the charge of the catering service. These decisions were made before undertaking a correct epidemiological investigation. The local authorities could have performed a rapid epidemiological assessment to evaluate whether the interventions were indeed needed considering that: (i) the very mild symptoms complained of by almost all the cases were not compatible with the clinical manifestation usually associated with outbreaks of STEC infection; (ii) a foodborne point source of infection was unlikely, due to the very different attack rates between schools and the occurrence of disease among children bringing their meals from home and the many household contacts. Such a rapid epidemiological assessment together with a more effective risk communication effort might have been the best strategy for minimizing the public anxiety that accompanied this outbreak and for deciding more appropriate interventions. Unfortunately, this approach was not adopted, mainly due to an insufficient knowledge of the characteristics of STEC outbreaks, which are relatively uncommon in Italy [8, 26].
This experience strengthens the importance of a prompt intervention in outbreak settings for: (i) increasing the likelihood of detecting both the causative agent and the source of the outbreak; (ii) reassuring the population about the real risk they are exposed to; (iii) taking the most appropriate measures to stop the outbreak.
ACKNOWLEDGEMENTS
We acknowledge the support of the municipality of Drapia and of the staff of the schools involved in the outbreak for providing services and facilities to carry out the field investigation. We are also indebted to the families who kindly provided information during the interviews.
REFERENCES
- 1.Trompeter RS, Schwartz R, Chantler C et al. Haemolytic-uraemic syndrome: an analysis of prognostic features. Arch Dis Child. 1983;58:101–105. doi: 10.1136/adc.58.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karmali MA. Related infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffin PM, Tauxe AV. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 4.Siegler RL. The hemolytic uremic syndrome. Pediatr Clin North Am. 1995;42:1505–1529. doi: 10.1016/s0031-3955(16)40096-9. [DOI] [PubMed] [Google Scholar]
- 5.Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong GL, Hollingsworth J, Morris JG., Jr. Emerging foodborne pathogens: Escherichia coli O157:H7 as a model of entry of a new pathogen into the food supply of the developed country. Epidemiol Rev. 1996;18:29–51. doi: 10.1093/oxfordjournals.epirev.a017914. [DOI] [PubMed] [Google Scholar]
- 7.Coia JE, Sharp JC, Campbell DM, Curnow J, Ramsay CN. Environmental risk factors for sporadic Escherichia coli O157 infection in Scotland: results of a descriptive epidemiology study. J Infect. 1998;36:317–321. doi: 10.1016/s0163-4453(98)94423-1. [DOI] [PubMed] [Google Scholar]
- 8.Tozzi AE, Caprioli A, Minelli F et al. Hemolytic Uremic Syndrome Study Group. Shiga toxin-producing Escherichia coli infections associated with hemolytic uremic syndrome, Italy, 1988–2000. Emerg Infect Dis. 2003;9:106–108. doi: 10.3201/eid0901.020266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caprioli A, Luzzi I, Rosmini F et al. Hemolytic-uremic syndrome and verocytotoxin-producing Escherichia coli infection in Italy. J Infect Dis. 1992;166:154–158. doi: 10.1093/infdis/166.1.154. [DOI] [PubMed] [Google Scholar]
- 10.Conedera G, Dalvit P, Martini M et al. Verocytotoxin-producing Escherichia coli O157 in minced beef and dairy products in Italy. Int J Food Microbiol. doi: 10.1016/j.ijfoodmicro.2004.03.010. (in press). [DOI] [PubMed] [Google Scholar]
- 11.Vinjé J, Koopmans MPG. Molecular detection and epidemiology of small round-structured viruses in outbreaks of gastroenteritis in the Netherlands. J Infect Dis. 1996;174:610–615. doi: 10.1093/infdis/174.3.610. [DOI] [PubMed] [Google Scholar]
- 12.Vinjé J, Deijl H, van der Heide R et al. Molecular detection and epidemiology of Sapporo-like viruses. J Clin Microbiol. 2000;38:530–536. doi: 10.1128/jcm.38.2.530-536.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caprioli A, Luzzi I, Rosmini F et al. Community-wide outbreak of hemolytic-uremic syndrome associated with non-O157 verocytotoxin-producing Escherichia coli. J Infect Dis. 1994;169:208–211. doi: 10.1093/infdis/169.1.208. [DOI] [PubMed] [Google Scholar]
- 14.Luzzi I, Tozzi AE, Rizzoni G et al. Detection of serum antibodies to the lipopolysaccharide of Escherichia coli O103 in patients with hemolytic-uremic syndrome. J Infect Dis. 1995;171:514–515. doi: 10.1093/infdis/171.2.514. [DOI] [PubMed] [Google Scholar]
- 15.Besser RE, Lett SM, Weber JT et al. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. J Am Med Assoc. 1993;5:2217–2220. [PubMed] [Google Scholar]
- 16.Mahon BE, Griffin PM, Mead PS, Tauxe RV. Hemolytic-uremic syndrome surveillance to monitor trends in infection with Escherichia coli O157:H7 and other Shiga toxin-producing E. coli. Emerg Infect Dis. 1997;3:409–412. doi: 10.3201/eid0303.970329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrett TJ, Green JH, Griffin PM, Pavia AT, Ostroff SM, Wachsmuth IK. Enzyme-linked immunosorbent assays for detecting antibodies to Shiga-like toxin I, Shiga-like toxin II, and Escherichia coli O157:H7 lipopolysaccharide in human serum. Curr Microbiol. 1991;23:189–195. [Google Scholar]
- 18.Decludt B, Bouvet P, Mariani-Kurkdjian P et al. Haemolytic uraemic syndrome and Shiga toxin-producing Escherichia coli infection in children in France. Epidemiol Infect. 2000;124:215–220. doi: 10.1017/s0950268899003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mead PS, Griffin PM. Escherichia coli O157:H7. Lancet. 1998;352:1207–1212. doi: 10.1016/S0140-6736(98)01267-7. [DOI] [PubMed] [Google Scholar]
- 20.Locking ME, O’Brien SJ, Reilly WJ et al. Risk factors for sporadic cases of Escherichia coli O157 infection: the importance of contact with animal excreta. Epidemiol Infect. 2001;127:215–220. doi: 10.1017/s0950268801006045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien SJ, Adak GK, Gilham C. Contact with farming environment as a major risk factor for Shiga toxin (Vero cytotoxin)-producing Escherichia coli O157 infection in humans. Emerg Infect Dis. 2001;7:1049–1051. doi: 10.3201/eid0706.010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Payne CJ, Petrovic M, Roberts RJ et al. Vero cytotoxin-producing Escherichia coli O157 gastroenteritis in farm visitors, North Wales. Emerg Infect Dis. 2003;9:526–530. doi: 10.3201/eid0905.020237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson JB, Clarke RC, Renwick SA et al. Vero cytotoxigenic Escherichia coli infection in dairy farm families. J Infect Dis. 1996;174:1021–1027. doi: 10.1093/infdis/174.5.1021. [DOI] [PubMed] [Google Scholar]
- 24.Silvestro L, Caputo M, Blancato S et al. Asymptomatic carriage of Verocytotoxin-producing Escherichia coli O157 in farm workers in Northern Italy. Epidemiol Infect. doi: 10.1017/s0950268804002390. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall JA, Goulding JS, Bean NH, Tauxe RV, Hedberg CW. Epidemiologic profiling: evaluating foodborne outbreaks for which no pathogen was isolated by routine laboratory testing: United States, 1982–9. Epidemiol Infect. 2001;127:381–387. doi: 10.1017/s0950268801006161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caprioli A, Pezzella C, Morelli R et al. Enteropathogens associated with childhood diarrhea in Italy. Pediatr Infect Dis J. 1996;15:876–883. doi: 10.1097/00006454-199610000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Giorgi Rossi P, Faustini A, Perucci CA. Regional foodborne disease surveillance group. Validation of guidelines for investigating foodborne disease outbreaks: the experience of the Lazio region, Italy. J Food Prot. 2003;66:2343–2348. [PubMed] [Google Scholar]
- 28.Roberts L, Jorm L, Patel M, Smith W, Douglas RM, McGilchrist C. Effect of infection control measures on the frequency of diarrheal episodes in child care: a randomized, controlled trial. Pediatrics. 2000;105:743–746. doi: 10.1542/peds.105.4.743. [DOI] [PubMed] [Google Scholar]
- 29.Jiang X, Dai X, Goldblatt S et al. Pathogen transmission in child care settings studied by using a cauliflower virus DNA as a surrogate marker. J Infect Dis. 1998;177:881–888. doi: 10.1086/515253. [DOI] [PubMed] [Google Scholar]