SUMMARY
A population-based insurance claims database was used to examine cellulitis incidence, anatomical sites of infection, complicating diagnoses, source of health service, and recurrence rates. Insurance claim files were searched for cellulitis ICD-9-CM codes 681.0–682.9. Complications of cellulitis including erysipelas, lymphadenitis, lymphangitis, and necrotizing fasciitis were also identified by ICD-9-CM codes. We found a cellulitis incidence rate of 24·6/1000 person-years, with a higher incidence among males and individuals aged 45–64 years. The most common site of infection was the lower extremity (39·9%). The majority of patients were seen in an outpatient setting (73·8%), and most (82·0%) had only one episode of cellulitis during the 5-year period studied. There was a very low incidence of cellulitis complications, including necrotizing fasciitis. Cellulitis is fairly common, usually treated in outpatient settings, and is infrequently complicated by erysipelas, lymphadenitis, lymphangitis, or necrotizing fasciitis.
INTRODUCTION
Cellulitis is a commonly occurring infection of the skin and subcutaneous tissues that can lead to life-threatening complications [1–8]. Cellulitis is seen in both primary-care settings and in hospital emergency departments, but little is known about its impact on health-care resources. It has been suggested that cellulitis patients comprise 1–14% of emergency room visits [1] and 4–7% of hospital admissions [2, 9] among select populations such as HIV patients, intravenous drug users, and Medicare patients. Because the clinical presentation and severity can vary greatly from patient to patient, there appears to be a conflict in the way physicians perceive the typical case of cellulitis. In general, hospital-based physicians see more severe, potentially life-threatening cases of cellulitis [5], while community-based providers see uncomplicated cases that respond rapidly to treatment. The resolution of these differing views has not occurred, probably due to a lack of population-based data.
Despite the common occurrence of cellulitis, we found only two incidence-based studies of this disease in the literature. Both are government publications with an 80-fold difference in the reported cellulitis incidence rates. The first was a study of select UK physician practices serving National Health Service patients of all ages between 1 September 1991 and 31 August 1992. The UK study estimated a cellulitis incidence rate of 16·4/1000 person-years [10]. The second study was of US military personnel aged 18–40 years, and estimated a cellulitis incidence rate of 0·2/1000 person-years among active-duty service members between 1 January 1998 and 31 December 2001 [11]. Most studies of cellulitis have emphasized clinical treatment of the disease and have been limited to highly selective adult populations that cannot be generalized [2, 8, 9, 12]. These studies cannot provide estimates of cellulitis incidence. In addition, we found no published data about the proportion of cellulitis cases with severe complications, source of health care utilized, or recurrence patterns in a general population.
In this study, we estimated the rates of cellulitis incidence using regional data from a national health insurance claims database and report incidence rates by age, sex, and month of year. Cases were stratified by anatomical site of infection and source of health care. In addition, we report on the recurrence of cellulitis; the incidence of lymphangitis, erysipelas, and necrotizing fasciitis; and the proportion of cellulitis cases complicated by lymphadenitis.
METHODS
Study population
This study was approved by the University of Utah’s Institutional Review Board as low risk. Data from the Deseret Mutual Benefit Administration (DMBA) medical insurance claims database were used to estimate the incidence of cellulitis. The DMBA insurance company was established in 1970 to provide health insurance and retirement income to employees of The Church of Jesus Christ of Latter-day Saints (Latter-day Saints or Mormons) and their families. Electronic recording of data began in 1995 and was completed by the end of 1996. This study used data beginning in 1997, the first year that complete electronic records were made available, to the end of 2002. The DMBA insurance claims database is comprised of a stable group with ∼61 000 enrollees per year, including nearly equal numbers of males and females. There are slightly more males in the 0–24 years age group, and slightly more females in the 25–64 years age group. The cohort has little employment turnover, estimated at <5% per year. The majority of turnover occurs among young adults who lose eligibility for coverage as dependants of their parents and elderly individuals who become eligible for Medicare. Due to religious proscription, nearly all enrollees abstain from alcohol, tobacco, and illicit drugs, all of which may be risk factors for cellulitis [2, 12–14], making this a uniquely healthy population. The age-adjusted all-causes mortality rate is 52% less than that of the United States.
Data collection
Medical claims records from 1 January 1997 to 31 December 2002 were examined to determine incidence rates for cellulitis in this cohort. To rule out prevalent cases, any claims by an individual occurring within the first 28 days after enrolment were excluded. To separate follow-up care from disease recurrence, each case’s treatment history in 7-day intervals (from 7 days to 35 days) was examined in order to assess the duration of typical cases. It was determined that after 28 days, 97·5% of individuals with cellulitis had been accounted for, and we concluded that most patients had completed treatment within this time frame. The cellulitis literature reports that nearly all patients are treated successfully within 5–14 days [2, 13]. However, we found that 5% of cellulitis patient undergoing follow-up care were counted as recurrent cases when using a 14-day criterion as opposed to a 28-day criterion. Thus, to be conservative in our estimates of incidence, the 28-day criterion was used to separate follow-up care from recurrence. Cases were examined to determine incidence, seasonal trend, source of health care utilized, and recurrence patterns.
Definitions
Approximately 70% of DMBA enrollees reside in Utah, Idaho, and ‘Other Mountain’ states (Arizona, Colorado, Montana, Nevada, New Mexico, and Wyoming) although there are enrollees throughout the United States. This study was limited to individuals from these regions, which comprise ∼50 000 enrollees per year (Table 1). The vast majority of those included reside in Utah (82%).
Table 1.
Age- and sex-specific person-years for Deseret Mutual Benefit Administration enrollees, Intermountain West*, 1997–2002
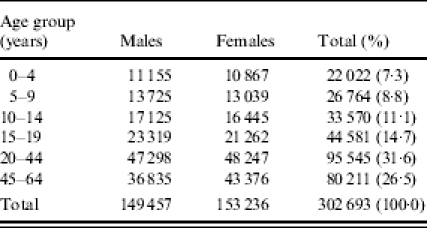
Arizona, Colorado, Idaho, Montana, Nevada, New Mexico, Utah, and Wyoming.
Cellulitis was defined using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) [14] codes 681.0–682.9, which included all cutaneous cellulitis. We excluded ICD-9-CM codes for pelvic, orbital, larynx/pharynx, and oral cellulitis. All medical care must have occurred between 1 January 1997 and 31 December 2002. The DMBA claims database relies on ICD-9-CM coded forms submitted by medical providers for reimbursement and collects a maximum of two ICD-9-CM codes per visit.
We examined the incidence of complications of cellulitis including lymphangitis (ICD-9-CM 457.2), erysipelas (ICD-9-CM 035), and necrotizing fasciitis (ICD-9-CM 728.86). The majority of cases with these complications were not concurrently coded as cellulitis cases. Thus, an additional incidence rate is reported, which combines the codes for cellulitis with those for lymphangitis, erysipelas, and necrotizing fasciitis. Because each DMBA claim was limited to two diagnostic codes, we assumed that individuals with these complications had a concurrent diagnosis of cellulitis which was not captured in the database. Lymphadenitis (ICD-9-CM 683) also occurs as a complication of cellulitis and may occur independently, and so the proportion of cases having a lymphadenitis diagnosis within 14 days of the cellulitis diagnosis was examined.
Anatomical site of infection was grouped into five categories using ICD-9-CM codes: upper extremity (681.0, 682.3, 682.4), lower extremity (681.1, 681.6, 681.7), trunk (682.2, 682.5), head/face/neck (682.0, 682.1, 682.8), and other/unspecified (681.9, 682.9). Age categories were created using the following categories: 0–4, 5–9, 10–14, 15–19, 20–44, and 45–64 years. Individuals aged ⩾65 years were excluded, as most use Medicare as their primary insurance. To calculate seasonal trends, the proportion of cellulitis cases occurring during each month of the year was determined.
Health-care settings for each encounter are included in the DMBA database. Health-care setting utilization was assessed by examining the proportion of cellulitis cases treated in in-patient hospital settings, acute care, and outpatient settings. Utilization was determined using a hierarchical system and coding to the highest level of care accessed at any time (in-patient, acute care, and outpatient respectively) for the treatment of each case.
Cellulitis recurrence was examined by determining the number of individual patients who were seen again for cellulitis within 12 months, 24 months, or >2 years after their initial cellulitis diagnosis. These categories are mutually exclusive, and an individual patient must have been seen for cellulitis at least 29 days after the initial cellulitis diagnosis to be counted as a recurrent case. We also examined the proportion of individuals who were seen for cellulitis once, twice, three times, or four or more times within the 5-year time period.
Data analyses
The denominators used in these analyses were determined by counting the number of person-years contained within the database annually on 1 July between 1997 and 2002. Incidence rates were calculated for each age group as defined above. After adjusting the incidence rates to the 2000 US Standard Million population, no changes were found in the incidence of cellulitis from 1997 to 2002, so all 6 years were reported as one time period. Rate ratios (RR) and confidence intervals (CI) were calculated for age and sex, anatomical site of infection and sex, and season [15].
RESULTS
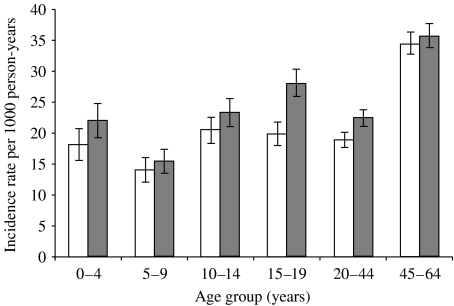
A total of 7438 new cases of cellulitis occurred between 1 January 1997 and 31 December 2002, resulting in an overall incidence rate of 24·6/1000 person-years. Including the codes for lymphangitis, erysipelas, and necrotizing fasciitis, the overall incidence rate increased to 24·8/1000. The incidence was highest in both females and males aged 45–64 years, with females having an incidence of 34·5/1000 person-years and males having an incidence of 35·7/1000 person-years (Fig. 1 and Table 2). The incidence of cellulitis was highest among males in all age groups; however, this male/female difference was only statistically significant in the 15–19 (RR 1·42, 95% CI 1·26–1·61) and 20–44 years age groups (RR 1·19, 95% CI 1·09–1·30).
Fig. 1.
Age- and sex-specific cellulitis (ICD-9 codes 681–682.9) incidence rates per 1000 person-years, 1997–2002. 95% confidence intervals represented by error bars. □, Females; , males.
, males.
Table 2.
Age- and sex-specific and total cellulitis* incidence rates per 1000 person-years, 1997–2002
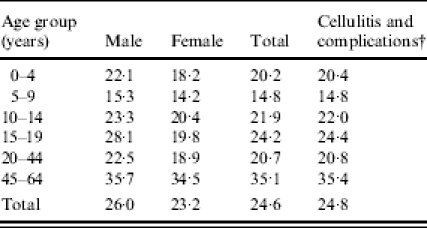
Cellulitis (ICD-9 codes 681.0–682.9).
sCellulitis (ICD-9 codes 681.0–682.9), erysipelas (ICD-9 code 035), lymphangitis (ICD-9 code 457.2), and necrotizing fasciitis (ICD-9 code 728.86).
Overall, the most common anatomical site of cellulitis infection was the lower extremity, which comprised 39·9% (n=2970) of cases, followed by other/unspecified (32·8%, n=2441), upper extremity (14·0%, n=1046), head/face/neck (8·7%, n=647), and trunk (4·5%, n=334). Females had an increased risk of cellulitis of the trunk (1·6, 95% CI 1·29–1·99) and head/face/neck (1·26, 95% CI 1·08–1·47), while males were at an increased risk for lower extremity cellulitis (1·16, 95% CI 1·08–1·25) (Table 3).
Table 3.
Proportion of age- and sex-specific cellulitis cases (ICD-9 codes 681.0–682.9) by anatomic site of infection, 1997–2002
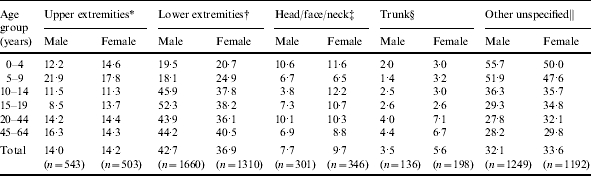
Upper extremities: finger (681.0), upper arm and forearm (682.3), hand (682.4).
Lower extremities: toe (681.1), leg (682.6), foot (682.7).
Head/face/neck: head and scalp (682.8), face (682.0), neck (682.1).
Trunk: trunk (682.2), buttock (682.5).
Other unspecified: unspecified digit (681.9), unspecified site (682.9).
The incidence rate for erysipelas was 0·09/1000 person-years, lymphangitis, 0·16/1000 person-years, and necrotizing fasciitis, 0·04/1000 person-years. In addition, we found that 0·16% (n=12) of cellulitis cases were complicated by lymphadenitis within 14 days of the initial cellulitis diagnosis.
Most cellulitis cases (73·8%) were seen in outpatient health-care settings (doctors’ offices, ambulatory surgery centres, homes, other clinics), while 20·5% were seen in acute care settings (emergency departments, outpatient hospitals), and 5·7% in in-patient hospital settings (Table 4).
Table 4.
Distribution of cellulitis cases (ICD-9 codes 681–682) by source of health care service, 1997–2002

In-patient care facilities include in-patient hospital and in-patient psychiatric facility.
Among the 5780 individual patients diagnosed with cellulitis in the DMBA database, 11·1% (n=640) were seen again for cellulitis within 1 year, 3·6% (n=208) for cellulitis within 2 years, and 3·3% (n=192) >2 years. Most patients with cellulitis (82·0%, n=4740) had only one incident episode from 1997 to 2002, while 12·9% (n=745) had two, 2·9% (n=167) had three, and 2·2% (n=128) had four or more incident episodes.
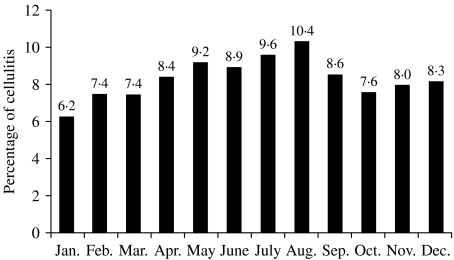
Cellulitis was most common during the summer months of June, July, and August and least common during the winter months of December, January, and February (Fig. 2). The relative risk of cellulitis was 1·32 times greater (95% CI 1·24–1·41) during the summer months than during the winter months.
Fig. 2.
Percentage of cellulitis cases by month of year.
DISCUSSION
This study found a cellulitis incidence rate of 24·6/1000 person-years, a higher incidence of cellulitis in males of all ages, an increasing incidence of cellulitis with increasing age, and that most cellulitis cases (78%) received treatment in outpatient settings. This was a higher incidence rate than other population-based studies [10, 11]. Less than one-fifth of patients in our study developed a recurring infection or required care for longer than 28 days. Additionally, there was a low incidence of lymphangitis, lymphadenitis, erysipelas, and necrotizing fasciitis among enrollees and a higher relative risk of cellultis during summer months compared to winter months. We also found that the lower extremity was the most common site of cellulitis among both males and females, although this finding is limited by the large number of cases where the body location is not stated.
Our study included approximately equal numbers of males and females in a stable population, ranging in age from birth to 64 years. We did not exclude anyone for health reasons, so our population contained both healthy and ill individuals. By using the DMBA claims database, the study population was limited to insured, predominately Latter-day Saint individuals. As previously mentioned, these individuals generally abstain from alcohol, tobacco, and illicit drugs, making them healthier than the general US population. We also excluded enrollees aged ⩾65 years. Based on the trends in the data, we believe that the cellulitis incidence rate would have been higher had this age group been included.
Insurance claims data are likely to be more accurate than voluntary reports of illness because physicians rely on ICD-9-CM coding for reimbursement. However, using an insurance claims database to evaluate incidence rates has inherent limitations. The rates we report are not verifiable as incident cases by chart review, and instead reflect the incidence of insurance claims for cellulitis. Yet the use of insurance claims data in this study is similar to the methods used by others to define cellulitis incidence [10, 11].
There is also a possibility of data entry or coding errors, and over- or underreporting when using insurance claims data. Because the DMBA database allows only two ICD-9-CM codes per patient visit, physicians are more likely to code more severe illnesses first. Thus, they may fail to code cellulitis when more serious conditions are also present. This would cause our estimates of incidence to be underestimated by an unknown factor. However, cellulitis is a disease which worsens rapidly if untreated, making underreporting unlikely. We believe that overreporting is more likely than underreporting in this dataset.
We found only two articles, both in government publications, describing epidemiological patterns of cellulitis in population-based cohorts [10, 11]. The majority of cellulitis research has focused on clinical treatment of the disease, while a small number of case series have focused on hospitalized adult patient populations [1, 5, 13, 16] or atypical populations such as intravenous drug users and HIV patients [2, 12]. The two government studies of cellulitis incidence reported quite different rates. The first study, in the United Kingdom, involved selected physician practices serving national health care recipients and reported an incidence rate of 16·4/1000 person-years [10]. Physicians voluntarily enrolled their practices in this study and were compensated for the costs of study participation. Incidence rates were based on physician-reported ‘first and new’ cellulitis cases using ICD-9-CM codes 681.0–682.9 and included males and females of all ages. However, rates were based on voluntary physician participation and reporting, which may have resulted in lower incidence rates.
The second study, from the US Army, reported a cellulitis incidence rate of 0·2/1000 person-years between 1998 and 2001 [11]. This study was limited to active-duty service members aged 18–40 years, and only 14% were female. Military recruits must pass fitness tests and physical examinations before being allowed to join the military, making active military personnel more physically fit than the general US population. This may be a partial explanation for the low incidence rates reported here.
We examined incidence rates among 18- to 40-year-old males and females in the DMBA claims database, and found rates of 23·2/1000 person-years for males and 21·2/1000 person-years for males and females combined. The peak incidence of cellulitis occurred among those aged 60–64 years. The US Army report found that the incidence of cellulitis was higher among service members <20 years of age, compared with older service members [11]. We came to a similar conclusion when analysing rates among 18- to 40-year-olds, with a peak incidence at age 18.
Many studies have found a higher prevalence of lower extremity cellulitis infections than upper extremity infections [1, 5, 16]. Among hospitalized patients, Carratala et al. found that 70% of cases involved lower extremities, 16% involved upper extremities, and 4% of cases involved more than one area [5]. Our study partially validated these findings, although only 39·9% of cases involved lower extremities. However, among hospitalized patients, we found that 42·4% of cellulitis cases involved the lower extremities and 15·5% the upper extremities. The high percentage of cellulitis cases with ‘other/unspecified’ sites of infection in our study (32·8%) may help to account for this difference.
The perception that cellulitis often leads to serious complications is not supported by this study. No deaths were associated with cellulitis or its complications including necrotizing fasciitis. In addition, there was a low incidence of lymphangitis, erysipelas, and necrotizing fasciitis. Less than 1% of cellulitis cases in our study were complicated by lymphadenitis.
A search of the literature did not reveal any population-based studies examining the source of health-care treatment utilized by cellulitis patients. The US Army study found that 97% of cellulitis cases were diagnosed in ambulatory clinics [11], but they did not examine hospitalization rates or sources of health care after diagnosis. We found that the vast majority of cellulitis cases were treated in an outpatient setting. This is significant because it shows that cellulitis treatment outside of the acute care or in-patient setting is effective for the majority of patients and supports the suggestion of Dong et al. [1] that most cellulitis cases can be treated at an outpatient clinic in order to decongest the emergency department.
Cellulitis is relatively common, frequently treated in outpatient settings, and in most cases, uncomplicated by lymphadenitis, lymphangitis, erysipelas, or necrotizing fasciitis. These findings may help resolve conflicting health-care provider views of the infection. In addition, the vast majority of cellulitis cases did not require emergency room or in-patient treatment. This information can be used by both hospitals and community health clinics for health services planning. Further research is needed in order to understand how comorbid conditions may predispose individuals to cellulitis infection and cellulitis recurrence.
REFERENCES
- 1.Dong SL, Kelly KD, Oland RC, Holroyd BR, Rowe BH. ED management of cellulitis: a review of five urban centers. Am J Emerg Med. 2001;19:535–540. doi: 10.1053/ajem.2001.28330. [DOI] [PubMed] [Google Scholar]
- 2.Manfredi R, Calza L, Chiodo F. Epidemiology and microbiology of cellulitis and bacterial soft tissue infection during HIV disease: a 10-year survey. J Cutan Pathol. 2002;29:168–172. doi: 10.1034/j.1600-0560.2002.290307.x. [DOI] [PubMed] [Google Scholar]
- 3.Bisno AL, Stevens DL. Streptococcal infections of skin and soft tissues. N Engl J Med. 1996;334:240–245. doi: 10.1056/NEJM199601253340407. [DOI] [PubMed] [Google Scholar]
- 4.Stevens DL, Musher DM, Watson DA et al. Spontaneous, nontraumatic gangrene due to Clostridium septicum. Rev Infect Dis. 1990;12:286–296. doi: 10.1093/clinids/12.2.286. [DOI] [PubMed] [Google Scholar]
- 5.Carratala J, Roson B, Fernandez-Sabe N et al. Factors associated with complications and mortality in adult patients hospitalized for infectious cellulitis. Eur J Clin Microbiol Infect Dis. 2003;22:151–157. doi: 10.1007/s10096-003-0902-x. [DOI] [PubMed] [Google Scholar]
- 6.Headley AJ. Necrotizing soft tissue infections: a primary care review. Am Family Physician. 2003;68:323–328. [PubMed] [Google Scholar]
- 7.Majeski JA, John JF., Jr. Necrotizing soft tissue infections: a guide to early diagnosis and initial therapy. South Med J. 2003;96:900–905. doi: 10.1097/01.SMJ.0000066658.35160.A1. [DOI] [PubMed] [Google Scholar]
- 8.Swartz MN. Clinical practice. Cellulitis. N Engl J Med. 2004;350:904–912. doi: 10.1056/NEJMcp031807. [DOI] [PubMed] [Google Scholar]
- 9.McCall N, Harlow J, Dayhoff D. Rates of hospitalization for ambulatory care sensitive conditions in the Medicare+Choice population. Health Care Financing Rev. 2001;22:127–145. [PMC free article] [PubMed] [Google Scholar]
- 10.Office of Population Censuses and Surveys. Morbidity statistics from general practice Fourth National Study. 1992. Her Majesty’s Stationery Office Series MB5(272) [Google Scholar]
- 11.Cellulitis among active duty service members. US Armed Forces, 1998–2001. Med Surveill Monthly Rep. 2002;8:6–9. [Google Scholar]
- 12.Binswanger IA, Kral AH, Bluthenthal RN, Rybold DJ, Edlin BR. High prevalence of abscesses and cellulitis among community-recruited injection drug users in San Francisco. Clin Infect Dis. 2000;30:579–581. doi: 10.1086/313703. [DOI] [PubMed] [Google Scholar]
- 13.Ginsberg MB. Cellulitis: analysis of 101 cases and review of the literature. South Med J. 1981;74:530–533. [PubMed] [Google Scholar]
- 14.American Medical Association. International classification of diseases, 9th revision, Clinical modification. American Medical Association Press; 2002. [Google Scholar]
- 15.Selvin S. Epidemiologic analysis: a case oriented approach. Oxford University Press; 2001. pp. 31–33. [Google Scholar]
- 16.Aly AA, Roberts NM, Seipol KS, MacLellan DG. Case survey of management of cellulitis in a tertiary teaching hospital. Med J Aust. 1996;165:553–556. doi: 10.5694/j.1326-5377.1996.tb138641.x. [DOI] [PubMed] [Google Scholar]