SUMMARY
Mucormycosis is a fairly rare fungal infection caused by ubiquitous fungi of the order Mucorales and primarily affects immunocompromised hosts. A series of 16 cases of invasive mucormycosis admitted to three referral centres in Beirut, Lebanon between 1981 and 1999 is described. It includes 12 patients with rhinocerebral, three with cutaneous, and one with pulmonary infection. Onset of symptoms occurred in the summer and autumn in 15 out of 16 patients, showing a statistically significant seasonal variation (P=0·007) A recent report of 19 patients from Tel Aviv describes a strikingly similar seasonal pattern. Studies on atmospheric concentration of Mucorales spores in the Eastern Mediterranean are lacking. Weather pattern analysis in Beirut revealed clustering of onset of invasive mucormycosis at the end of a dry, warm period, which begins around May and ends in October. Mucormycosis incidence appears to be seasonal in the Eastern Mediterranean.
Mucormycosis, also known as zygomycosis, is the third leading cause of invasive fungal infection after Candidiasis and Aspergillosis [1]. It is, nevertheless, a rare fungal infection that affects immunocompromised individuals. It is not known to have a seasonal pattern. Its histopathological hallmark is the invasion of blood vessels, vasculotropism, leading to tissue infarction [1, 2].
Species causing infection in humans belong to genera of the Mucoraceae family in the order Mucorales of the class Zygomycetes [2, 3] and these include: Absidia, Rhizopus, Mucor, Rhizomucor and Apophysomyces [2]. They are ubiquitous in nature, being found in soil, plants, and decaying material. They can also be airborne [2, 3].
The fungus may affect one of several organ systems, most commonly the paranasal sinuses and brain [1]. In addition to rhinocerebral mucormycosis, the commonest and most studied form [4], pulmonary, primary cutaneous, gastrointestinal as well as disseminated forms have been described [2, 3]. Susceptible populations include diabetics, renal failure patients, victims of trauma and burns and patients with haematological malignancies. Rare cases of Apophysomyces elegans causing mucormycosis in otherwise healthy individuals have been reported [5–8].
Seasonal variation in atmospheric concentration of fungal spores has been documented for some moulds in several geographical locations. Atmospheric concentration of Aspergillus spores peaked in autumn and early winter in the Western Mediterranean basin (Cordoba, Spain); Cardiff, Wales; St. Louis, MO, USA; and other parts of northern Europe [9–11]. A study of dog skin biota demonstrated peak prevalence of Rhizopus spores in summer, and of Mucor in autumn [12]. However, to our knowledge, there have been no reports on atmospheric concentration of Mucorales spores in the Eastern Mediterranean. Mucormycosis have been reported from Greece [13, 14], Turkey [15, 16] and Israel [17–21]. Only one study by Talmi et al. [21] of 19 patients, reported dates of onset of symptoms and those appeared to vary seasonally, but did not establish statistical significance. A similar seasonal pattern of infection in a series of 16 mucormycosis cases from Beirut, Lebanon is reported here.
Medical records at three major care centres: the American University of Beirut Medical Center, Rizk Clinic and St George’s Hospital were searched for all documented cases of mucormycosis between January 1981 and December 1999. Sixteen cases were identified based on the discharge diagnosis (Table). Data concerning patient gender, age at onset of disease, date of symptom onset, symptoms and signs, diagnostic tests, treatment modalities, and outcome were analysed retrospectively.
Table.
Cases of mucormycosis
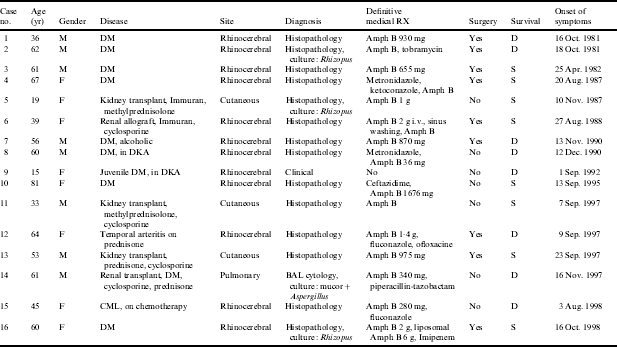
M, Male; F, female; DM, diabetes mellitus; DKA, diabetic ketoacidosis; CML, chronic myelogenous leukaemia; BAL, bronchoalveolar lavage; Amph B, amphotericin B; S, survived; D, died.
The series consisted of eight females and eight males. Their ages ranged between 15 and 81 years with a mean of 50·7 years. Twelve patients had rhinocerebral mucormycosis (Table). Overall survival for the rhinocerebral form was 42%, slightly lower than the reported figures of 50–85% [2, 3]. Nine of these patients were diabetics, two of whom presented in diabetic ketoacidosis. Risk factors in the remaining three patients were: chronic myelogenous leukaemia, immunosuppression for renal transplant, and hyperglycaemia induced by corticosteroid used for temporal arteritis.
The diagnosis was confirmed in 14 cases by histopathological identification of large non-septate hyphae with right angle branching invading blood vessels and tissue in biopsy material from the affected site (Table). In three of these patients cultures grew Rhizopus species. In addition, bronchoalveolar lavage from one patient with pulmonary mucormycosis grew Rhizopus and Aspergillus. Cytology of the fluid confirmed the presence of hyphi. In patients who did not undergo surgery, biopsies were obtained from nasal or palatine ulcers or the affected sinus. Tissue material recovered intraoperatively from debrided sinuses and orbits was examined in patients who underwent surgery. Wound biopsies were obtained from patients with the cutaneous form.
Ten of the 12 patients with rhinocerebral mucormycosis were treated with regular amphotericin B and one was treated with liposomal amphotericin B. Eight patients underwent surgical debridement and four survived. All patients who did not undergo surgery died. One patient (case no. 9, Table) succumbed within hours of presentation prior to receiving any therapy or obtaining any biopsies. Eleven of the 12 rhinocerebral cases (91·6%) had onset of symptoms between 23 August and 12 December.
Three renal allograft recipients on immunosuppressive therapy had cutaneous mucormycosis, with extension of infection into the transplanted kidney in one of them. One patient with pulmonary mucormycosis was a diabetic as well as a kidney transplant recipient. The cutaneous category had the best prognosis, as expected [2, 3].
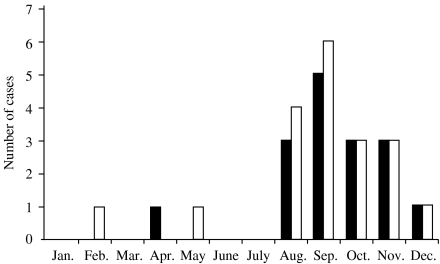
Onset of symptoms in 15 out of 16 patients (87·5%) was between August and December. In the Eastern Mediterranean the weather pattern is characterized by four seasons: a mild spring, a hot dry summer, an initially dry and later wet cooler autumn, and a cold rainy winter. Formally spring, summer, autumn, and winter begin 21 March, 21 June, 22 September, and 21 December respectively. To investigate the case distribution over the seasons a χ2 goodness-of-fit test of the null hypothesis, that case onset is evenly dispersed across seasons, was constructed by classifying each case as follows: 21 March to 20 June (n=1); 21 June to 21 September (n=7); 22 September to 20 December (n=8); or 21 December to 20 March (n=0). Assuming equal expected frequencies in the four intervals (χ2=12·5 and d.f.=3). The method of Radlow & Alf [22] was used to compute an exact P value of 0·007. Thus, the case distribution was statistically significantly variable by season. Although this is a small series, it may be representative of the disease in the Eastern Mediterranean. Moreover, it is in striking agreement with the findings of Talmi et al. [21] whereby 17 out of 19 (84·4%) rhinocerebral mucormycosis cases had onset of infection between August and December. Both this series and that of Talmi et al. report a total of 35 cases from the Eastern Mediterranean region with a total of 32 out of 35 cases presenting with symptoms between August and December (Fig. 1).
Fig. 1.
Distribution of Lebanese ( ) and Israeli (
) and Israeli ( ) cases by month of year at onset of symptoms.
) cases by month of year at onset of symptoms.
Since the pathogenesis of rhinocerebral and pulmonary mucormycosis is thought to be due to inhalation of spores by a susceptible patient [3], it would be reasonable to hypothesize that seasonal variation of the disease may either be due to varying atmospheric concentration of Mucorales spores, or a seasonal variation of underlying predisposing illnesses. To date, however, no seasonal variation of diabetic ketoacidosis incidence, or other immunosuppression state associated with mucormycosis, has been reported.
The clinical course of zygomycosis is characterized by extremely rapid progression. In diabetic animal models inhalation of Mucorales spores resulted in rapidly progressive pulmonary mucormycosis and death within 1–4 days [23]. This suggests that onset of symptoms rapidly follows the inhalation of fresh spores in susceptible hosts. Hence, the seasonal pattern of onset of symptoms may be due to fresh deposition of spores in affected tissue with an inoculum size proportional to peak atmospheric concentration of airborne Mucorales spores during the late summer and autumn seasons in the Eastern Mediterranean.
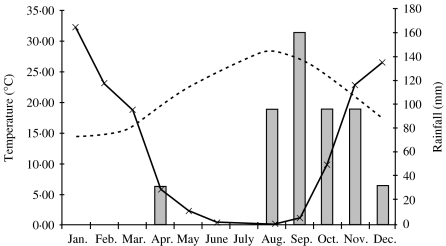
In the absence of data on atmospheric spore concentration of Mucorales species in the Greater Beirut area the seasonal weather pattern was investigated. Late summer and early autumn seasons in the Eastern Mediterranean are fairly dry, rather warm and windy with much organic decay from deciduous plants (August to October; Fig. 2). These conditions are conducive to proliferation and aerosolation of thermophilic moulds.
Fig. 2.
Frequency of Lebanese cases by month ( ). Dotted line represents monthly average of the mean of daily maximal and minimal temperatures in °C. Continuous line represents the average monthly rainfall (in mm) over the years 1980–1999.
). Dotted line represents monthly average of the mean of daily maximal and minimal temperatures in °C. Continuous line represents the average monthly rainfall (in mm) over the years 1980–1999.
Maximal and minimal daily temperatures in the Greater Beirut Area for the years 1998 and 1999 were obtained from Beirut International Airport (BIA) and the data was averaged by month (Fig. 2). Data from BIA shows that these monthly temperature averages have been consistent since 1954. Most species belonging to Rhizopus, Rhizomucor and Absidia are thermophilic and grow best at 40–50°C. [24] Despite comparable average ambient temperatures between May and November, June and October, July and September respectively, cases tended to manifest in September to November rather than May to July. Temperature variation alone does not explain the clustering of cases. Subsequently, rainfall data from BIA were obtained from recordings (taken since 1954) of cumulative monthly rainfall into a well 15 m above sea level at BIA. Data between 1980 and 1999 were averaged by month. It was noted that onset of symptoms in mucormycosis cases clustered at the end of a dry period, which consistently extended from late May to September or October. This seasonal rainfall pattern has been consistent since 1932 according to BIA records of daily rainfall. It coincides with the late summer and early autumn. Twelve of our 16 cases occurred following a period of 4 months where monthly rainfall averaged over the years 1980–1999 never exceeded 25 mm per month. Clusters of cases in certain years occurred, but there was no relation between the number of cases and the cumulative rainfall in those specific years.
This seasonal pattern seems to be a regional finding rather than a worldwide phenomenon, since similar clusters were not reported by mycologists even in areas of the United States where weather patterns similar to the Eastern Mediterranean basin prevail such as California.
Factors other than rainfall and temperature variation are likely to be involved as rainfall does not explain yearly clusters of cases. Investigating atmospheric Mucorales spore concentration in the Eastern Mediterranean and correlating such data with rainfall, ambient humidity, wind activity, CO2 concentration and temperature may clarify further this seasonal pattern.
In their literature review of mucormycosis in renal transplant recipients, Morduchowicz et al. [20], noted as early as 1986 that there may be increased incidence of mucormycosis in the Eastern Mediterranean. Lately Petrikkos et al. [14] reported an increase in reported incidence of mucormycosis in Greece. Neither reports, however, reported statistical evidence of their anecdotal observations. At this point no evidence exists that mucomycosis has increased prevalence in the Eastern Mediterranean nor that a similar seasonal pattern is reported elsewhere in the world.
Further research on invasive mucormycosis is needed to answer these questions.
ACKNOWLEDGEMENTS
Thanks are due to Beirut International Airport Weather Observatory, Riad El-Khudary, Leila El-Merhebi, and Maher Al-Ajam, M.B.E.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Eucker J, Sezer O, Graf B, Possinger K. Mucormycosis. Mycoses. 2000;44:253–260. [PubMed] [Google Scholar]
- 2.Meyers B, Gurtman A., Gorbach SL, Bartlett JG, Blacklow NR. Infectious diseases. 2nd edn. Philadelphia: W. B. Saunders; 1998. Phycomycetes; pp. 2382–2387. [Google Scholar]
- 3.Sugar A., Mandell G, Bennette JE, Gordon R, Dolin R. Principles and practice of infectious disease. 4th edn. New York: Churchill Livingstone; 1995. Agents of Mucormycosis and related species; pp. 2311–2321. [Google Scholar]
- 4.Blitzer A, Lawson W, Meyers BR, Biller HF. Patient survival factors in paranasal mucormycosis. Laryngoscope. 1980;90:635–648. doi: 10.1288/00005537-198004000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Brown SR, Shah IA, Grinstead M. Rhinocerebral mucormycosis caused by Apophysomyces elegans. Am J Rhinol. 1998;12:289–292. doi: 10.2500/105065898781389994. [DOI] [PubMed] [Google Scholar]
- 6.Radner AB, Witt MD, Edwards JE., Jr. Acute invasive rhinocerebral mucormycosis in an otherwise healthy patient: case report and review. Clin Infect Dis. 1995;20:163–166. doi: 10.1093/clinids/20.1.163. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharyya AK, Deshpande AR, Nayak SR, Kirtane MV, Ingle MV, Vora IM. Rhinocerebral mucormycosis: an unusual case presentation. J Laryngol Otol. 1992;106:48–49. doi: 10.1017/s0022215100118584. [DOI] [PubMed] [Google Scholar]
- 8.Quattrocolo G, Pignatta P, imanico U, Tarenzi L, Baggiore P. Rhinocerebral mucormycosis and internal carotid artery thrombosis in a previously healthy patient. Acta Neurol Belg. 1990;90:20–26. [PubMed] [Google Scholar]
- 9.Trujillo J, Infante G, Galan S, Dominguez V. Seasonal and daily variation of Aspergillus Mich. ex Fr. spores in the atmophere of Cordoba (Spain) Allergol Immunopathol (Madr) 1990;18:167–173. [PubMed] [Google Scholar]
- 10.Beaumont F, Kauffman HF, Van-der Mark TH, Sluiter HJ, De Vries K. Volumetric aerobiological survey of conidial fungi in the north-east Netherlands. I. Seasonal patterns and the influence of metereological variables. Allergy. 1985;40:173–180. doi: 10.1111/j.1398-9995.1985.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 11.Mullins J, Hutcheson PS, Slavin RG. Aspergillus fumigatus spore concentration in the outside air: Cardiff and St. Louis compared. Clin Allergy. 1984;14:351–354. doi: 10.1111/j.1365-2222.1984.tb02215.x. [DOI] [PubMed] [Google Scholar]
- 12.Cabnes FJ, Abarca ML, Bragulat MR et al. Seasonal study of the fungal biota of the fur of dogs. Mycopathologia. 1996;133:1–7. doi: 10.1007/BF00437092. [DOI] [PubMed] [Google Scholar]
- 13.Economopoulou P, Laskaris G, Ferekidis E, Kanelis N. Rhinocerebral mucormycosis with severe oral lesions: a case report. J Oral Maxillofac Surg. 1995;53:215–217. doi: 10.1016/0278-2391(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 14.Petrikkos G, Skiada A, Sambatakou H et al. Mucormycosis: ten-year experience at a tertiary-care center in Greece. Eur J Clin Microbiol Infect Dis. 2003;22:753–756. doi: 10.1007/s10096-003-1035-y. [DOI] [PubMed] [Google Scholar]
- 15.Onerci M, Gursel B, Hosal S, Gulekon N, Gokoz A. Rhinocerebral mucormycosis with extension to the cavernous sinus. A case report. Rhinology. 1991;29:321–324. [PubMed] [Google Scholar]
- 16.Saltoglu N, Tasova Y, Zorludemir S, Dundar IH. Rhinocerbral zygormycosis treated with liposomal amphotericin B and surgery. Mycoses. 1998;41:45–49. doi: 10.1111/j.1439-0507.1998.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 17.Bentur Y, Shupak A, Ramon Y et al. Hyperbaric oxygen therapy for cutaneous soft tissue zygomycosis complicating diabetes mellitus. Plast Reconstr Surg. 1998;102:822–824. doi: 10.1097/00006534-199809030-00030. [DOI] [PubMed] [Google Scholar]
- 18.Moses AE, Rahav G, Barenholz Y et al. Rhinocerebral mucormycosis treated with amphotericin B colloidal despersion in three patients. Clin Infect Dis. 1998;26:1430–1433. doi: 10.1086/516349. [DOI] [PubMed] [Google Scholar]
- 19.Linder N, Keller N, Hury C, Kuint J, Goldshmidt-Reuven A, Barzilai A. Primary cutaneous mucormycosis in a premature infant: case report and review of the literature. Am J Perinatol. 1998;15:35–38. doi: 10.1055/s-2007-993895. [DOI] [PubMed] [Google Scholar]
- 20.Morduchowicz G, Shmueli D, Shapira Z et al. Rhinocerebral mucormycosis in renal ransplant recipients: report of three cases and review of the literature. Rev Infect Dis. 1986;8:441–446. doi: 10.1093/clinids/8.3.441. [DOI] [PubMed] [Google Scholar]
- 21.Talmi YP, Goldschmied-Reouven A, Bakon M et al. Rhino-orbital and rhino-orbito-cerebral mucormycosis. Otolaryngol Head Neck Surg. 2002;127:22–31. doi: 10.1067/mhn.2002.126587. [DOI] [PubMed] [Google Scholar]
- 22.Radlow R, Alf EF. An alternate multinomial assessment of the accuracy of the chi-square test of goodness of fit. J Am Statist Assoc. 1975;70:811–813. [Google Scholar]
- 23.Waldorf AR, Ruderman N, Diamond RD. Specific susceptibility to mucormycosis in murine diabetes and bronchoalveolar macrophage defense against Rhizopus. J Clin Invest. 1984;74:150–160. doi: 10.1172/JCI111395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larone DH. Medically important fungi – a guide to identification. 3rd edn. Washington, DC: ASM Press; 1995. [Google Scholar]