SUMMARY
This report describes an outbreak of gastroenteritis of 5 months’ duration in a farming family, associated with the consumption of unpasteurized cows’ milk, where Campylobacter jejuni was implicated. A total of six individuals in the family acquired the illness, and two had several episodes of diarrhoea within the 5-month period. Identical PFGE genotypes of C. jejuni were isolated from human and bovine faeces, and bulk tank milk samples. Incompletely sealed rubber liners fitted to a milking machine shortly before the outbreak started was the probable reason, allowing faecal material to contaminate the milk over the period concerned.
Many outbreaks of Campylobacter jejuni have been caused by the consumption of unpasteurized or inadequately pasteurized milk [1–4]. In two surveys, 8·05% and 9·2% of raw cows’ milk were found to be contaminated with campylobacter [5, 6]. Most commonly the source of campylobacter in raw milk is faecal contamination from cattle that frequently have campylobacter as commensals in their gastrointestinal tract [7, 8]. A few cases of direct milk excretion of C. jejuni have also been described [9, 10]. In the United Kingdom, wild birds pecking milk-bottle tops have also been associated with milk-borne transmission of campylobacter infection in humans [11]. In the present study we describe a long-lasting outbreak of C. jejuni in a farming family who had used raw milk from their own farm, housing 52 dairy cows.
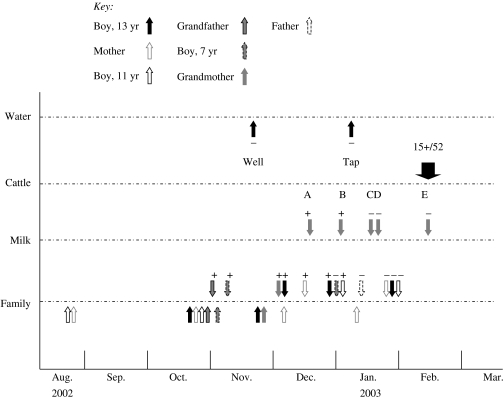
Six people were involved in the outbreak; the mother, three children aged 7–13 years, as well as the grandfather and grandmother who often visited the family. The outbreak probably started in August 2002, when the mother and the 11-year-old child had occasional episodes of diarrhoea. On 21 October 2002, the 13-year-old child fell ill with diarrhoea, abdominal pain, vomiting and fever (Fig. 1). The child was hospitalized for a few days, but no faecal sample was analysed. After that, over a few days, the mother, the 11-year-old child, the grandfather and the 7-year-old child all fell ill with diarrhoea and fever. C. jejuni was first isolated from a stool specimen collected from the grandfather on 29 October (Fig. 1). This result was available on 4 November. Subsequent faecal samples from other symptomatic family members were analysed in November and December 2002, when campylobacter was isolated from all these individuals, including the grandmother, who had had abdominal pain for a period without diarrhoea. The grandfather, the grandmother, the 11-year-old child and the mother were treated with erythromycin and azithromycin, after which the mother and the 11-year-old child still had bouts of illness, the mother having her last illness, and clarithromycin treatment, in January 2003. The only campylobacter isolates available at that time for further studies were those from the 13- and 11-year-old children, identified as C. jejuni susceptible to erythromycin and ciprofloxacin. The faecal sample of the asymptomatic father in January 2003 was campylobacter negative (Fig. 1).
Fig. 1.
Time-course of events of the outbreak from August 2002, showing specific markers associated with the outbreak and its investigation in family members (Family), dairy milk (Milk), cattle faeces (Cattle) and water (Water). For Family, arrows below the line indicate when individuals were symptomatic; arrows above the line show when faecal specimens were collected and if they were positive (+) or negative (−) for campylobacter culture. Bulk milk tank samples A and B were positive (+), while samples C, D, E were negative (−).
After the first campylobacter-positive family member was identified, studies to trace the source of the organism were initiated. Water samples were taken from the well of the farm in November 2002 and from the kitchen tap of the farm in January 2003 by the local health authority. These were examined for the presence of campylobacter and faecal indicator organisms using a filtration technique. An 8-l water sample was filtered and enriched in a Campylobacter enrichment broth (Lab M, Bury, Lancashire, UK) with cefoperazone (8·0 mg/l), teicoplanin (4·0 mg/l) and amphotericin B (10·0 mg/l) (CAT selective supplement, Oxoid, Basingstoke, Hampshire, UK) and 7% of blood. Incubation took place in a mixture of 5% O2, 10% CO2, 3% H2 and 82% N2 for 2 days at 37°C, followed by subculture on mCAT medium, which consisted of a Campylobacter blood-free selective agar base (Oxoid) with cefoperazone (8·0 mg/l), teicoplanin (4·0 mg/l) and amphotericin B (10·0 mg/l) (CAT selective supplement) and 7% of blood. Plates were incubated under microaerophilic conditions at 37°C for 2 days. Faecal indicator bacteria were studied from a 2-l sample of water by a membrane filtration technique on Chromocult¯ coliform agar (Merck, Darmstadt, Germany), followed by incubation for 24 h at 37°C. Coliforms and Escherichia coli were counted as described by the manufacturer.
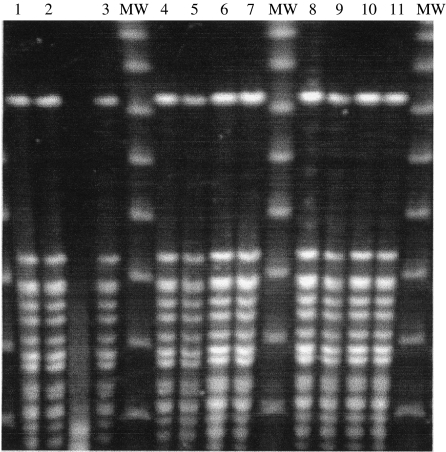
Examination for potential source(s) of the outbreak continued into December 2002. Bulk tank milk samples of the farm were collected five times between 16 December 2002 and 18 February 2003 (Fig. 1). Bulk tank milk samples were examined for campylobacter using the enrichment procedure. In addition, individual bulk milk samples (10 ml) from cows (n=47) were examined for the presence of campylobacter by using both a direct culture and the enrichment procedures. Individual faecal samples (n=52) from the herd were taken from the rectum by swab and transported in Probact transport medium (Technical Service Consultants Ltd, Heywood, Lancashire, UK) in order to test for the presence of campylobacter. The media used were the same as that for the water samples. Suspect colonies were identified as campylobacter by their characteristic appearance on Gram-staining, positive catalase test, microaerophilic growth and as C. jejuni by a positive hippurate hydrolysis test. Antimicrobial susceptibility profiles to erythromycin and ciprofloxacin were done by disk diffusion. Campylobacter isolates from two family members, two bulk tank milk samples and 10 bovine faecal samples were examined by pulsed-field gel electrophoresis (PFGE) as previously described [12] and the SmaI and KpnI patterns of the isolates were compared (Fig. 2).
Fig. 2.
PGFE profiles of KpnI-digested DNA of Campylobacter jejuni. Lanes 1–3, Isolates from bulk tank milk sampled in December 2002 [1, 2] and January 2003 [3]; lanes 4, 5, isolates from family members, 7-year-old [4] and 13-year-old [5]; lanes 6–11, isolates from cattle faeces. MW, Molecular-weight markers.
Campylobacter and coliforms were not detected in the well water or the municipal main network water. However, C. jejuni was detected twice both in 10 ml and 200 ml of bulk tank milk over a period of 3 weeks from December 2002 to January 2003 (Fig. 1). Bulk milk samples taken from mid-January onwards did not grow campylobacter. While samples of milk from 47 individual cattle were free of the organism, it was grown from 15/52 bovine faecal specimens collected in February 2003 (Fig. 1). All the isolates were C. jejuni. Two bovine faecal isolates were susceptible to erythromycin and ciprofloxacin. Examination of two human, two milk and 10 bovine faecal C. jejuni isolates by PFGE showed all had identical pattern in SmaI and KpnI digest. An example of the PFGE patterns of isolates from two family members, milk and six bovine faeces is shown in Figure 2.
This family outbreak lasted ∼5 months, probably starting in August 2002 and ending in January 2003 after the family had stopped using unpasteurized milk from the farm. The estimated consumption of raw milk by all family members was 2–5 glasses daily. The grandfather and grandmother had also consumed this milk while visiting the family. This suggested that the raw milk had been contaminated on several occasions by bovine faecal material, even if no clear breaches in hygiene in the milking procedure were detected. Detection of C. jejuni twice within a period of 3 weeks from the bulk tank milk further supported the contamination of bulk tank milk. It became obvious that the source of the human campylobacter outbreak was bovine faecal contamination of the bulk tank milk, supported by the identical PFGE genotypes of C. jejuni isolates in milk, human and bovine faeces (Fig. 2). At least eight out of 10 campylobacter-positive cattle excreted the PFGE genotype associated with the outbreak. The most probable site for contamination of the raw milk was in the milking process, as the rubber liners of the milking machine were changed in August 2002, a few weeks before the first episodes occurred in the family. These liners were subsequently found to fit poorly, and air drained into the milk during milking. The liners were changed in January 2003, and after that no campylobacter was detected in milk (Fig. 1). It is obvious that even with good milking hygiene a small amount of bovine faecal bacteria may contaminate bulk tank milk. Of bulk tank milk, 26·7% has been shown to contain one or more species of pathogenic bacteria including C. jejuni, shiga-toxin producing E. coli, Listeria monocytogenes, Salmonella spp. and Yersinia enterocolitica [6]. However, good milking hygiene and properly working milking machines probably reduce the degree of faecal contamination of raw milk. Although some reports have suggested, that campylobacter has the potential to cause bovine mastitis [13] and be excreted directly into milk [9, 10], we did not find support for this hypothesis.
Drinking raw milk is always a risk factor for enteric diseases. In other outbreaks contaminated unpasteurized milk has been identified as the point source, when this has been consumed, for example during a meal or farm visit [2, 4], or when consumption of unpasteurized milk has been associated with an increase in cases of diarrhoea in a certain area [1, 3]. Small family outbreaks caused by long-lasting contamination of bulk milk may be more common than reported because patients with diarrhoea do not always seek medical care, and consequently faecal samples are not collected for study of enteric pathogens. In addition, viral gastroenteritis, such as that associated with noroviruses, spread as epidemics in a region, misleading medical doctors into not suspecting other causes of diarrhoea. This was the situation in autumn 2002, when the 13-year-old child was hospitalized, but no faecal sample was collected for examination.
The illness lasted in the mother and one child for many months with several episodes of diarrhoea. Both of them fell ill again after the course of antimicrobial therapy with macrolides. Because the isolates were tested as being susceptible to erythromycin, it may be assumed that the patients were re-infected several times, possibly from the raw milk, and no protective immunity developed. When the potential source was identified as the raw milk in late 2002, the family started to use retail milk. However, since May 2003, they have returned to using unpasteurized bulk tank milk without any enteric symptoms.
An interesting fact is that despite using the bulk tank milk, the father of the family and his brother and parents who lived in the same neighbourhood did not get ill. One explanation is that the father, who was mostly responsible for the milking process, may have had repeated exposure to campylobacter, which may have induced immunity [14]. It is also possible that the likelihood of illness developing depends on the amount of milk consumed. It has been shown that there is a significant dose–response relationship between the amount of raw milk consumed and the risk of illness [2]. Here all the affected members of the family and the father consumed several glasses of milk daily, identifying a similar opportunity for exposure through milk. The bacterium was isolated from 10 ml of milk indicating that the number of bacteria in a glass of milk (∼200 ml) would potentially be high enough to cause infection. Susceptibility among family members may vary depending on the previous occupational exposure to campylobacter.
In conclusion, small family outbreaks at milk-producing farms may be more common than recognized from official reports. In the Finnish National Registry for Infectious Diseases for 2002, 31 individuals with campylobacter infection were reported for October, November and December in the entire area where the family was living, representing a population of ∼350 000 people. Thus, six cases in this family accounted for a significant proportion of the total number of the reported cases. The prolonged nature of this outbreak underlines the importance of analysing stool specimens at the earliest opportunity, so that an offending pathogen can be identified. This along with a risk assessment for the likely source at the earliest opportunity is a recurrent public health message.
ACKNOWLEDGEMENTS
The study was supported by grants from the Academy of Finland and the Walter Ehrstrom Foundation. The Oulu Regional Public Health Laboratory and Food and Environment Laboratory are acknowledged for human isolates and a milk strain respectively.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Fahey T, Morgan D, Gunneburg C, Adak GK, Majid F, Kaczmarski E. An outbreak of Campylobacter jejuni enteritis associated with failed milk pasteurisation. J Infect. 1995;31:137–143. doi: 10.1016/s0163-4453(95)92160-5. [DOI] [PubMed] [Google Scholar]
- 2.Evans MR, Roberts RJ, Ribeiro CD, Gardner D, Kembrey D. A milk-borne campylobacter outbreak following an educational farm visit. Epidemiol Infect. 1996;117:457–462. doi: 10.1017/s0950268800059112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrington P, Archer J, Davis JP, Croft DR, Varma JK. Outbreak of Campylobacter jejuni infections associated with drinking unpasteurized milk produced through a cow-leasing program – Wisconsin, 2001. Morb Mortal Wkly Rep. 2002;51:548–549. [PubMed] [Google Scholar]
- 4.Peterson MC. Campylobacter jejuni enteritis associated with consumption of raw milk. J Environ Health. 2003;65:20–21. [PubMed] [Google Scholar]
- 5.Uraz G, Yucel N. The isolation of certain pathogenic micro-organisms from raw milk. Eur J Public Health. 1999;7:145–148. [PubMed] [Google Scholar]
- 6.Jayarao BM, Henning DR. Prevalence of foodborne pathogens in bulk tank milk. J Dairy Sci. 2001;84:2157–2162. doi: 10.3168/jds.S0022-0302(01)74661-9. [DOI] [PubMed] [Google Scholar]
- 7.Waterman SC, Park RW, Bramley AJ. A search for the source of Campylobacter jejuni in milk. J Hyg (Lond) 1984;93:333–337. doi: 10.1017/s0022172400064871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humphrey TJ, Beckett P. Campylobacter jejuni in dairy cows and raw milk. Epidemiol Infect. 1987;98:263–269. doi: 10.1017/s0950268800062014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutchinson DN, Bolton FJ, Hinchliffe PM, Dawkins HC. Evidence of udder excretion of Campylobacter jejuni as the cause of milk-borne campylobacter outbreak. J Hyg (Lond) 1985;94:205–215. doi: 10.1017/s0022172400061416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orr KE, Lightfoot NF, Sisson PR et al. Direct milk excretion of Campylobacter jejuni in a dairy cow causing cases of human enteritis. Epidemiol Infect. 1995;114:15–24. doi: 10.1017/s0950268800051876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neal KR, Slack RCB. Diabetes mellitus, anti-secretory drugs and other risk factors for campylobacter gastro-enteritis in adults: a case-control study. Epidemiol Infect. 1997;119:307–311. doi: 10.1017/s0950268897008224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kärenlampi R, Rautelin H, Hakkinen M, Hänninen M-L. Temporal and geographical distribution and overlap of Penner heat-stable serotypes and pulsed-field gel electrophoresis genotypes of Campylobacter jejuni isolates collected from humans and chickens in Finland during a seasonal peak. J Clin Microbiol. 2003;41:4870–4872. doi: 10.1128/JCM.41.10.4870-4872.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gudmundson J, Chirino-Trejo JM. A case of bovine mastitis caused by Campylobacter jejuni. J Vet Med B. 1993;40:326–328. doi: 10.1111/j.1439-0450.1993.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 14.Jones DM, Robinson DA, Eldridge J. Serological studies in two outbreaks of Campylobacter jejuni infection. J Hyg (Lond) 1981;87:163–170. doi: 10.1017/s0022172400069369. [DOI] [PMC free article] [PubMed] [Google Scholar]